2021 Volume 44 Issue 2 Pages 188-196
2021 Volume 44 Issue 2 Pages 188-196
ONO-4641, 1-({6-[(2-methoxy-4-propylbenzyl)oxy]-1-methyl-3,4-dihydronaphthalen-2-yl}methyl)azetidine-3-carboxylic acid (ceralifimod), is a second-generation sphingosine 1-phosphate receptor agonist selective for sphingosine 1-phosphate receptors 1 and 5, and has clinical effects in multiple sclerosis. The objective of the present study was to explore other potential indications for ONO-4641 based on its immunomodulatory effects. ONO-4641 was tested in non-obese diabetic (NOD) mice, an animal model of spontaneous type 1 diabetes mellitus, an autoimmune disease with unmet medical needs. ONO-4641 at a dose of 0.1 mg/kg prevented the onset of diabetes mellitus in NOD mice. Furthermore, ONO-4641 at doses of 0.03 and 0.1 mg/kg decreased diabetic prevalence in NOD mice after the onset of diabetes mellitus in a dose-dependent manner. Histopathological analysis demonstrated that insulin-positive areas in the islets of mice administered 0.03 and 0.1 mg/kg ONO-4641 showed a tendency of high values although they were not significantly different from the Control group, which was treated with vehicle. These observations suggest ONO-4641 may delay the onset and progression of type 1 diabetes mellitus.
Sphingosine 1-phosphate (S1P) mediates physiological functions by signaling through S1P receptors, S1P1–S1P5.1) Fingolimod (FTY720), a S1P receptor agonist, is approved for the prevention of relapsing forms of multiple sclerosis (MS).2) FTY720 is phosphorylated by sphingosine kinase 2 to the active metabolite FTY720-phosphate,3) a nonselective S1P receptor agonist for four S1P receptors: S1P1, S1P3, S1P4, and S1P5.4,5) Previous studies with S1P1-deficient mice demonstrated S1P1 is required for lymphocyte recirculation into the blood.6,7) S1P is present in high concentrations (100–400 nM) in the blood1,8) and its concentration gradient induces the migration of lymphocytes expressing S1P1 from lymphoid tissues into the blood.9,10) S1P5 is highly expressed in the central nervous system, particularly on oligodendrocytes and brain endothelium. Activation of S1P5 on brain endothelial cells enhanced barrier integrity and reduced the transendothelial migration of monocytes in vitro.11) The therapeutic effects of FTY720 are thought to be mediated by S1P1 and S1P5. However, rodent studies suggested S1P3 activity was associated with the cardiovascular side effects of FTY720 including heart rate reduction.12,13) Then, we hypothesized that S1P receptor agonists that lack S1P3 activity would be useful to reduce the risk of cardiovascular side effects. In the course of identifying S1P receptor agonists without S1P3 activity, we developed a second-generation S1P receptor agonist selective for S1P1 and S1P5, termed ONO-4641 (1-({6-[(2-methoxy-4-propylbenzyl)oxy]-1-methyl-3,4-dihydronaphthalen-2-yl}methyl)azetidine-3-carboxylic acid; ceralifimod), as a potential treatment for lymphocyte-mediated diseases.14) The effectiveness of ONO-4641 was demonstrated in a phase II clinical trial for the treatment of MS.15) Since MS is characterized by lymphocyte infiltration, ONO-4641 was suggested to be also effective against other lymphocyte-mediated diseases.
Type 1 diabetes mellitus (T1DM) is a tissue-specific chronic autoimmune disease. Because β-cell loss causes a lack of insulin and diabetes mellitus, major research efforts are needed for its early diagnosis, prevention of β-cell loss, and development of better treatment options to improve the QOL and prognosis of those affected. Because insulin administration, the current therapy for T1DM, has a limited control of blood glucose, novel therapies are required. Although the etiology of T1DM is not completely understood, its pathogenesis is thought to involve the T cell-mediated destruction of β-cells.16) Reportedly, FTY720 shows efficacy in non-obese diabetic (NOD) mice, an animal model of T1DM.17) We speculated that ONO-4641, which has improved selectivity against S1P3, would show similar efficacy to that of FTY720.
In this article, we report preclinical efficacy data of ONO-4641 for T1DM in NOD mice. Our results support the therapeutic benefits of ONO-4641 for T1DM as a second-generation S1P receptor agonist.
ONO-4641 was provided by Ono Pharmaceutical Co., Ltd. (Osaka, Japan). FTY720 was obtained from Cayman Chemical (MI, U.S.A.).
Experimental AnimalsFemale NOD/ShiJcl mice were obtained from CLEA Japan Inc. (Tokyo, Japan). Based on the national regulations and guidelines, animal experiments were reviewed by the Institutional Animal Care and Use Committee and finally approved by the director of the research institution. The animal experiments were performed in accordance with Regulations for Animal Experiments of Ono Pharmaceutical Co., Ltd.
Assessment of Preventive EffectsThe preventive effects of ONO-4641 were evaluated by incidence of diabetes, the rate of mice with diabetes mellitus. The onset of diabetes mellitus was defined as a point when the plasma glucose level was ≥200 mg/dL at two timepoints. NOD mice were treated with 0.5% (w/v) Methyl Cellulose 400 cP Solution (0.5%MC; WAKO, Osaka, Japan) as a vehicle (Control), ONO-4641 or FTY720 once a day from a grouping date (19 weeks of age) to 43 weeks of age. ONO-4641 (0.01, 0.03, and 0.1 mg/kg), FTY720 (1 mg/kg) or 0.5% MC was orally administered once daily for 24 weeks. The incidence rates of diabetes mellitus are shown as survival curves generated by the Kaplan–Meier method, and were compared between the Control group and each treatment group by the log-rank test, with a p value of less than 5% considered statistically significant.
Assessment of Therapeutic EffectsThe therapeutic effects of ONO-4641 were evaluated by the prevalence rate of diabetes mellitus, the percentage of mice with a plasma glucose level of ≥200 mg/dL. NOD mice with an onset of diabetes mellitus were treated with 0.5% MC as a vehicle (Control), ONO-4641 or FTY720 once daily from the onset of disease. Mice at 20 to 26 weeks of age that had achieved initial onset were selected and grouped, and ONO-4641 (0.01, 0.03, or 0.1 mg/kg), FTY720 (3 mg/kg) or 0.5% MC was administered orally daily for 12 weeks. The prevalence rate of diabetes mellitus at 12 weeks after administration was compared between the Control group and each treatment group by Fisher’s exact test, with a p value of less than 5% considered statistically significant. Dead animals whose plasma glucose level had reached ≥200 mg/dL were handled as the mice with diabetes mellitus even after the death.
Blood Glucose and Insulin MeasurementAt least 20 µL of blood was collected from the tail vein using a heparinized capillary tube once a week in the morning from 18 to 43 weeks of age. During the administration period of the test substance, blood was collected before administration on the day of blood collection. The collected blood was centrifuged to prepare plasma, which was stored on ice. Plasma glucose concentrations were measured with a Glucose CII Test Wako kit (Wako), and the remaining plasma was stored at −80 °C. In the assessment of therapeutic effects, insulin concentration in the plasma collected at grouping date and 12 weeks after administration was measured using LBIS Mouse Insulin enzyme-linked immunosorbent assay Kit (Wako). For the samples with a plasma insulin concentration below the limit of quantitation (<0.156 ng/mL), the data are presented as this limit value (0.156 ng/mL). Residual insulin secretion ability was determined as the ratio of plasma insulin concentration at 12 weeks after administration to that on the day of grouping.
Preparation of Immunostained SpecimensThe pancreas was removed from mice in all groups and from mice without onset (No onset group) and a day after onset (Immediately after onset group) and fixed in IHC Zinc Fixative solution (BD Bioscience, CA, U.S.A.). Paraffin blocks of the fixed pancreas were prepared and sliced in three consecutive sections in the long axis direction. The first section was immunostained with anti-insulin antibody (DAKO, Glostrup, Denmark) and hematoxylin (Polysciences, PA, U.S.A.) stained (hereinafter, anti-insulin antibody-stained specimen). The second section was immunostained with an anti-CD3 antibody (BD, NJ, U.S.A.) and then hematoxylin stained (hereinafter, anti-CD3 antibody-stained specimen). The remaining section was subjected to hematoxylin and eosin (Polysciences) staining (hereinafter, H&E-stained specimen).
Analysis of Immunostained SpecimensThe insulin-positive area (mm2) in an enlarged image of each islet of the anti-insulin antibody-stained specimen was calculated using image analysis software (Win RooF Ver. 5.6).
We investigated the disease-preventive effect of ONO-4641 in NOD mice. ONO-4641 showed a dose-dependent peripheral blood lymphopenic effect at 24 h after oral administration, and approximately 80% lymphopenic activity was observed at a dose of 0.1 mg/kg.18) Based on these data, three doses of ONO-4641, 0.03, 0.01 and 0.1 mg/kg, were used for administration. FTY720 was previously reported to completely prevent the onset of diabetes mellitus in NOD mice at 1 mg/kg; therefore, a dose of 1 mg/kg was used for FTY720 administration.17)
The incidence of diabetes in the Control group was 0% by 7 weeks but gradually increased to 40% by 24 weeks after administration whereas that in the ONO-4641 groups remained low even at 24 weeks after administration with a value of 11, 10 and 0% at 0.01, 0.03 and 0.1 mg/kg, respectively (Fig. 1). The log rank test showed a significant preventive effect of ONO-4641 at a dose of 0.1 mg/kg compared with the Control group (Fig. 1). FTY720, the positive control, at a dose of 1 mg/kg, also showed a significant preventive effect.

Non-diabetic non-obese diabetic mice were treated with 0.5% methyl cellulose (Control), ONO-4641, or FTY720 once a day for 24 weeks from a grouping date (19 weeks of age) to 43 weeks of age. ONO-4641 (0.01, 0.03 and 0.1 mg/kg), FTY720 (1 mg/kg) or 0.5% methyl cellulose was orally administered once daily from the grouping date. The abscissa indicates the number of weeks after the initiation of administration, and incidence of diabetes curves for the rate of mice with onset of diabetes mellitus (onset was defined as the timepoint when the plasma glucose level was ≥200 mg/dL at two timepoints) were generated by the Kaplan–Meier method. Control (○) (n = 10), ONO-4641 0.01 mg/kg (●) (n = 9), 0.03 mg/kg (▲) (n = 10), 0.1 mg/kg (△) (n = 10), and FTY720 1 mg/kg (+) (n = 9). The curves for the Control group and the treatment groups were compared by the log-rank test, with a p-value of less than 5% indicating significance. * p < 0.05.
The plasma glucose concentration in the Control group was increased from 8 weeks after administration, fluctuated, and gradually increased to 255.3 ± 73.65 mg/dL at 24 weeks after administration (Table 1). The plasma glucose concentration in the ONO-4641 0.01 mg/kg group also increased from 13 weeks after administration to 206.4 ± 75.44 mg/dL at 24 weeks after administration. In contrast, plasma glucose concentrations in the ONO-4641 0.03 and 0.1 mg/kg groups were maintained lower than that in the Control group, at 135.0 ± 9.21 mg/dL and 116.9 ± 6.71 mg/dL, respectively, 24 weeks after administration. However, there was no significant difference between each ONO-4641 group and the Control group. Plasma glucose concentrations in the FTY720 1 mg/kg group were also maintained lower than in the Control group, at 122.4 ± 6.07 mg/dL, 24 weeks after administration. There was no significant difference in plasma glucose concentrations between the FTY720 group and the Control group. The individual transition of plasma glucose concentration is shown in Fig. 2. Two of 10 mice in the Control group and one of 10 mice in the ONO-4641 0.01 mg/kg group had a plasma glucose concentration of ≥600 mg/dL. In contrast, there was no significant increase in plasma glucose concentration in the ONO-4641 0.03 mg/kg group, even though one mouse showed the onset of disease. In the ONO-4641 0.1 mg/kg and FTY720 1 mg/kg groups, the onset of disease was completely suppressed and plasma glucose concentrations did not reach ≥200 mg/dL.
| Group | Grouping date | 1 week | 2 weeks | 3 weeks | 4 weeks | 5 weeks | 6 weeks | 7 weeks | 8 weeks |
|---|---|---|---|---|---|---|---|---|---|
| Control | 118.3±4.68 | 118.0±3.65 | 118.2 ±4.71 | 123.1±5.67 | 117.2±4.78 | 114.0±3.79 | 116.8±5.02 | 124.0±9.35 | 143.6±35.38 |
| ONO-4641 0.01 mg/kg | 116.3±4.27 | 115.4±2.83 | 120.4±5.02 | 120.3±4.95 | 121.7±4.96 | 112.4±3.99 | 122.1±5.70 | 115.6±3.80 | 119.5±5.68 |
| ONO-4641 0.03 mg/kg | 117.6±4.24 | 116.8±5.86 | 116.4±3.54 | 133.2±17.96 | 122.0±9.26 | 115.3±4.55 | 122.4±7.24 | 117.1±6.77 | 118.1±6.86 |
| ONO-4641 0.1 mg/kg | 116.3±3.18 | 114.9±4.68 | 117.3±2.83 | 119.5±7.36 | 113.5±6.04 | 109.9±5.20 | 109.3±3.81 | 103.0±4.30 | 109.0±5.04 |
| FTY720 1 mg/kg | 116.1±4.79 | 118.6±2.86 | 113.9±4.48 | 120.2±5.73 | 114.8±4.50 | 113.8±4.12 | 120.1±3.39 | 111.2±2.40 | 115.4±2.85 |
| Group | 9 weeks | 10 weeks | 11 weeks | 12 weeks | 13 weeks | 14 weeks | 15 weeks | 16 weeks | |
| Control | 153.0±30.40 | 123.5±15.92 | 132.1±20.53 | 146.9±23.98 | 147.5±27.81 | 182.7±58.01 | 172.3±53.09 | 206.3±78.02 | |
| ONO-4641 0.01 mg/kg | 127.9±4.39 | 112.1±5.58 | 123.4±10.23 | 125.7±7.78 | 128.6±15.71 | 182.2±46.49 | 187.8±53.42 | 195.4±66.79 | |
| ONO-4641 0.03 mg/kg | 126.3±5.27 | 111.4±3.77 | 125.9±7.01 | 126.9±6.87 | 123.3±5.35 | 120.6±5.74 | 130.8±7.27 | 124.4±6.32 | |
| ONO-4641 0.1 mg/kg | 115.6±3.26 | 105.4±8.24 | 111.5±8.09 | 111.6±7.80 | 106.3±4.53 | 110.0±7.73 | 108.3±4.63 | 109.9±7.95 | |
| FTY720 1 mg/kg | 124.3±4.47 | 119.3±4.38 | 103.1±2.28 | 116.4±4.09 | 117.6±2.75 | 109.6±3.11 | 110.7±5.03 | 101.0±4.07 | |
| Group | 17 weeks | 18 weeks | 19 weeks | 20 weeks | 21 weeks | 22 weeks | 23 weeks | 24 weeks | |
| Control | 194.3±64.38 | 177.6±55.60 | 185.2±58.72 | 233.8±78.89 | 227.2±60.01† | 225.7±74.76 | 246.6±90.23 | 255.3±73.65 | |
| ONO-4641 0.01 mg/kg | 194.9±71.68 | 201.2±75.11 | 208.5±72.05 | 201.8±80.21 | 196.1±67.78 | 196.9±60.68 | 206.5±73.73 | 206.4±75.44 | |
| ONO-4641 0.03 mg/kg | 126.0±7.89 | 127.1±8.74 | 139.0±9.84 | 139.9±9.48 | 137.1±10.03 | 142.4±11.61 | 145.9±7.95 | 135.0±9.21 | |
| ONO-4641 0.1 mg/kg | 117.3±3.98 | 115.2±6.56 | 113.6±8.49 | 122.3±5.98 | 116.9±5.51 | 116.3±4.53 | 117.3±3.67 | 116.9±6.71 | |
| FTY720 1 mg/kg | 111.1±3.95 | 113.9±1.55 | 114.5±3.39 | 116.2±4.25 | 118.4±3.61 | 114.9±1.59 | 115.6±4.42 | 122.4±6.07 |
Non-obese diabetic mice without diabetes mellitus were treated with 0.5% methyl cellulose (Control), ONO-4641, or FTY720 once a day for 24 weeks from a grouping date (19 weeks of age) to 43 weeks of age. Plasma glucose concentrations (mg/dL) in the Control group (n = 10), ONO-4641 0.01 mg/kg group (n = 9), ONO-4641 0.03 mg/kg group (n = 10), ONO-4641 0.1 mg/kg group (n = 10) and the FTY720 1 mg/kg group (n = 9) are expressed as the mean ± standard error († represents data obtained the last week before death). Plasma glucose concentrations in Control and ONO-4641 groups were compared by Dunnett’s test at each timepoint. Plasma glucose concentrations were compared between Control and FTY720 groups by the Student’s t-test at each timepoint. A two-sided test was performed and the significance level was 5% in both tests, but there were no significant differences between groups for all tests.
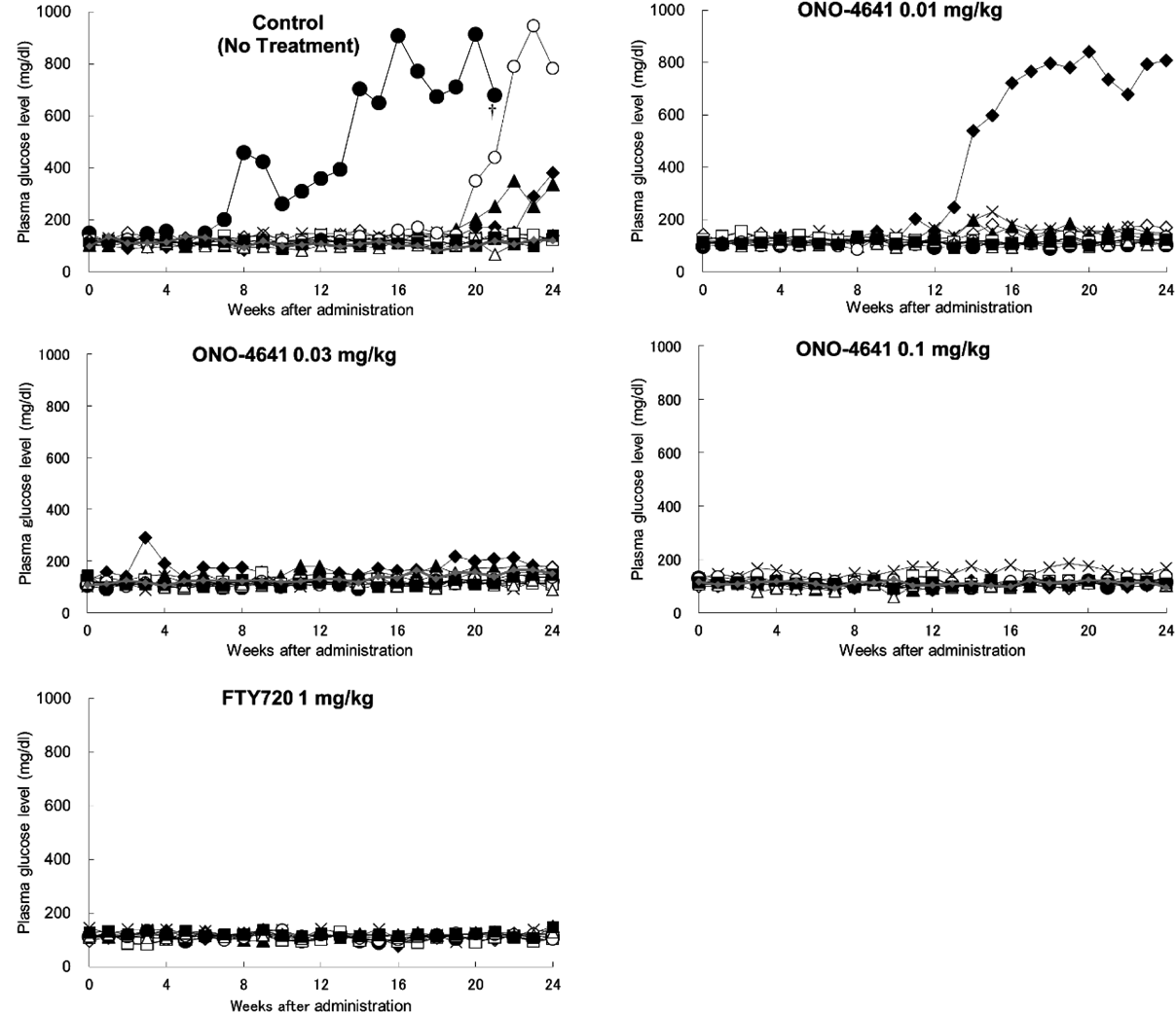
Non-obese diabetic mice without onset of diabetes mellitus were treated with 0.5% methyl cellulose (Control), ONO-4641, or FTY720 once a day for 24 weeks from a grouping date (19 weeks of age) to 43 weeks of age. The abscissa indicates the number of weeks after the initiation of administration and the ordinate shows the plasma glucose concentration (mg/dL) for each animal (†: dead).
The therapeutic effect of ONO-4641 was evaluated in diabetic mice. The same doses of ONO-4641 were used as in the previous experiments. FTY720 at a dose of 3 mg/kg was reported to have a therapeutic effect on diabetes mellitus in NOD mice; therefore, a dose of 3 mg/kg FTY720 was used in this study.17)
The prevalence rate of diabetes mellitus in the Control group decreased temporarily, 67% at 1 week after administration, but increased thereafter, and returned to 100% at 8 weeks where it remained until the end of the experiment (Fig. 3). The plasma glucose concentration in each group is shown in Table 2. In the ONO-4641 0.01 mg/kg group, the prevalence rate of diabetes mellitus was similar to that in the Control group. In contrast to these groups, the rates of diabetes mellitus in the ONO-4641 0.03 and 0.1 mg/kg groups decreased from 1 to 7 weeks after administration, and were maintained lower than that in the Control group. The prevalence rate of diabetes mellitus in mice at 12 weeks after administration was 100% in the Control group. In contrast, the prevalence rates of diabetes mellitus were 100, 50, and 13% in the ONO-4641 0.01, 0.03, and 0.1 mg/kg groups, respectively. These rates in the ONO-4641 0.3 and 0.1 mg/kg groups were significantly lower than in the Control group, demonstrating ONO-4641 has therapeutic effects. The prevalence rate of diabetes mellitus in the FTY720 3 mg/kg group at 12 weeks after administration was significantly lower than that of the Control group, showing that FTY720 also has a therapeutic effect.
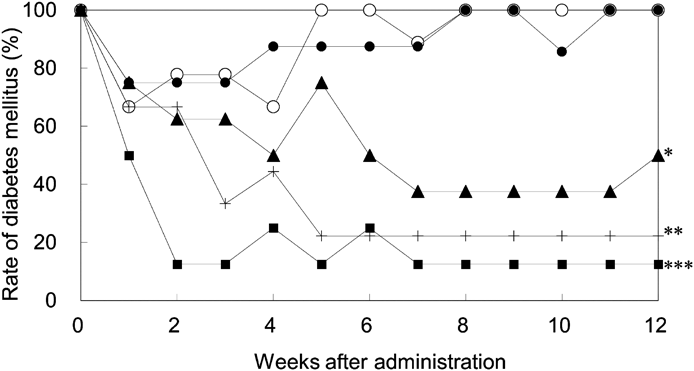
Non-obese diabetic mice with diabetes mellitus were orally administered 0.5% methyl cellulose (Control), ONO-4641 or FTY720 once daily for 12 weeks. The abscissa shows the number of weeks after the initiation of the administration and the ordinate shows the rate of diabetes mellitus (percentage of individuals with a plasma glucose level ≥200 mg/dL). Control (○) (n = 9), ONO-4641 0.01 mg/kg (●) (n = 8), 0.03 mg/kg (▲) (n = 8), 0.1 mg/kg (■) (n = 8), and FTY720 3 mg/kg (+) (n = 9). The rate of diabetes mellitus at 12 weeks after administration was compared between the Control group and each treatment group by Fisher’s exact test, with a p-value of less than 5% indicating significance. * p < 0.05, ** p < 0.01, *** p < 0.001.
| Group | Grouping date | 1 week | 2 weeks | 3 weeks | 4 weeks | 5 weeks | 6 weeks |
|---|---|---|---|---|---|---|---|
| Control | 290.2±21.61 | 296.1±47.26 | 368.7±73.89 | 373.0±66.70 | 413.8±73.74 | 483.9±73.53 | 464.4±78.52 |
| ONO-4641 0.01 mg/kg | 302.8±29.22 | 360.9±62.68 | 425.4±80.99 | 496.1±85.72 | 595.1±75.88 | 623.5±82.90 | 653.3±77.63 |
| ONO-4641 0.03 mg/kg | 314.1±32.79 | 369.7±46.93 | 325.9±69.73 | 367.8±100.20 | 363.1±99.80 | 432.1±109.88 | 372.5±105.50 |
| ONO-4641 0.1 mg/kg | 274.6±25.11 | 247.5±24.70 | 184.3±34.35 | 186.8±24.07 | 222.5±51.47 | 195.1±38.11* | 206.1±44.33 |
| FTY720 3 mg/kg | 295.8±25.32 | 274.0±41.34 | 289.4±61.84 | 263.8±71.64 | 278.8±71.36 | 282.0±81.17 | 259.6±67.84 |
| Group | 7 weeks | 8 weeks | 9 weeks | 10 weeks | 11 weeks | 12 weeks | |
| Control | 506.4±91.32 | 596.4±96.98† | 546.6±87.38 | 527.6±80.75† | 531.5±76.80 | 525.9±96.95 | |
| ONO-4641 0.01 mg/kg | 720.5±89.10 | 669.7±59.03† | 792.5±112.06 | 677.7±93.28 | 734.8±87.01†† | 788.3±36.87 | |
| ONO-4641 0.03 mg/kg | 379.2±111.92 | 416.2±123.02 | 409.3±121.73 | 395.4±115.59 | 405.1±125.22 | 376.6±99.34 | |
| ONO-4641 0.1 mg/kg | 199.0±50.08 | 198.3±53.56** | 221.0±69.42 | 231.8±77.21 | 246.9±88.80 | 236.9±85.42 | |
| FTY720 3 mg/kg | 261.3±68.41# | 296.4±97.31# | 274.0±86.35# | 306.7±103.04 | 297.2±96.68 | 280.7±96.62 |
Non-obese diabetic mice with diabetes mellitus were orally administered with 0.5% methyl cellulose (Control), ONO-4641 or FTY720 once daily for 12 weeks. Plasma glucose concentrations (mg/dL) in the Control group (n = 9), ONO-4641 0.01 mg/kg group (n = 8), ONO-4641 0.03 mg/kg group (n = 8), ONO-4641 0.1 mg/kg group (n = 8), and FTY720 3 mg/kg group (n = 9) are expressed as the mean ± standard error at weeks after dosing (†: 1 death, ††: 2 deaths). Plasma glucose concentrations were compared between Control and ONO-4641 groups by Dunnett’s test at each timepoint (* p < 0.05, ** p < 0.01). Plasma glucose concentrations were compared between Control and FTY720 groups by the Student’s t-test at each timepoint (# p < 0.05).
The individual transition of the plasma glucose concentration was shown in Fig. 4. In the Control group, the plasma glucose concentrations increased steadily with a fluctuating pattern. In the ONO-4641 0.03, 0.1 mg/kg and FTY720 3 mg/kg groups, the plasma glucose concentrations in most mice were <200 mg/dL. In some mice, however, the concentrations increased steadily and eventually exceeded 600 mg/dL. An individual difference in the efficacy was observed.
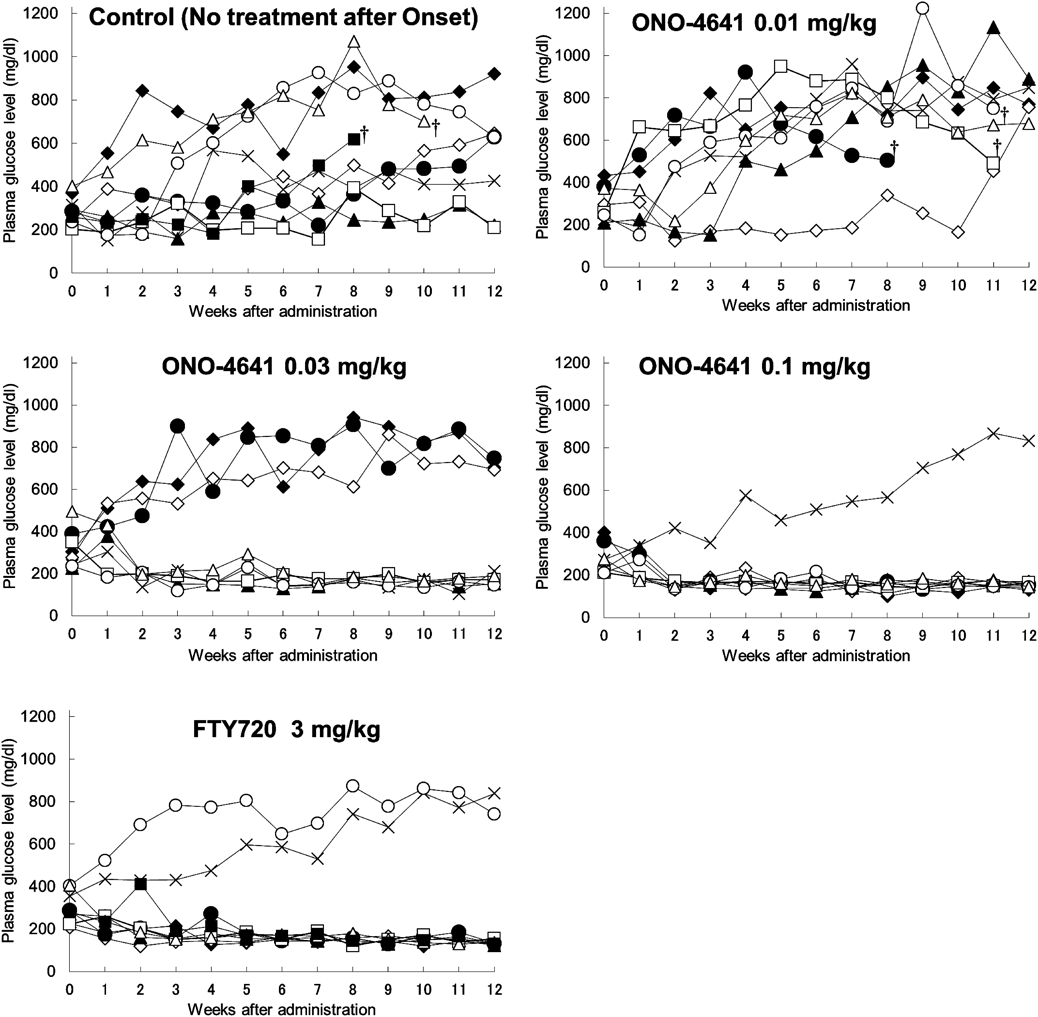
Non-obese diabetic mice with diabetes mellitus were orally administered 0.5% methyl cellulose (Control), ONO-4641 or FTY720 once daily for 12 weeks. The abscissa shows the number of weeks after the initiation of administration and the ordinate shows the plasma glucose level (mg/dL) of each animal (†: dead).
The insulin-positive area in each group was compared in Fig. 5. The insulin-positive area in the No onset group was 0.128 ± 0.0472 mm2. The insulin-positive area in the Immediately after onset group (0.074 ± 0.0325 mm2) was lower than that in the No onset group. The insulin-positive area in the Control group was 0.015 ± 0.0075 mm2, which was significantly lower than that in the No onset group but not the Immediately after onset group. The insulin-positive area in the ONO-4641 0.01 mg/kg group was 0.002 ± 0.0011 mm2, which was lower than that in the Control group. However, the insulin-positive areas in the ONO-4641 0.03 and 0.1 mg/kg groups as well as the FTY720 3 mg/kg group were 0.027 ± 0.0174, 0.039 ± 0.0352, and 0.04 ± 0.0225 mm2, respectively, which were higher than in the Control group. Additionally, residual insulin secretion ability, a ratio of plasma insulin concentration at 12 weeks after administration to that on the day of grouping, is compared among the Control group, ONO-4641 and FTY720 groups in Fig. S1. The residual insulin secretion ability in the ONO-4641 0.01 mg/kg group was 4%, which was lower than that in the Control group by 13 points. Meanwhile, the residual insulin secretion ability in the ONO-4641 0.03 and 0.1 mg/kg groups and the FTY720 3 mg/kg group were higher than that in the Control group with the values of 33, 53, and 33%, respectively. Insulin level tended to be maintained higher in the higher dosing groups. This tendency is similar to that of the insulin positive areas in the islets shown in Fig. 5. Individual plasma insulin and glucose concentrations at 12 weeks after administration are also shown in Fig. S2. All of the mice with diabetic remission (plasma glucose level of <200 mg/dL) maintained plasma insulin level at 0.7 ng/mL or higher. On the other hand, all of the mice with a low insulin level (lower limit of quantitation: < 0.156 ng/mL) had diabetes mellitus with a plasma glucose level higher than 600 mg/dL.

Non-obese diabetic mice with diabetes mellitus (defined as plasma glucose concentrations ≥200 mg/dL at two timepoints) were assigned to the study groups and Immediately after onset group, respectively. We randomly assigned the mice without diabetes mellitus to the Immediately after onset group and the No onset group. In another group, non-obese diabetic mice with diabetes mellitus were orally administered 0.5% methyl cellulose (Control), ONO-4641 or FTY720 once daily for 12 weeks. Mice pancreases were removed from the No onset group and Immediately after onset group on the day of grouping, and the day after the last dose for the other groups. The insulin-positive area (mm2) of each specimen was calculated by imaging analysis and expressed as the mean ± standard error. Dunnett’s test was used to compare the insulin-positive area between the Control and ONO-4641 groups. Comparisons of the insulin-positive areas between the Control group and the FTY720 group, the No onset group, the Immediately after onset group, and between the No onset group and the Immediately after onset group were performed by the Student’s t-test. Significant differences were observed between the No onset group and the Control group, and there were no significant differences between the other groups (#p < 0.05 vs. No onset group).
Typical individual staining images of the insulin or CD3-positive areas of the pancreas in each group are shown in Fig. 6. The infiltration of CD3-positive cells was observed around the insulin-positive area in all groups, and there were no clear differences among CD3-positive cells infiltrating the pancreas in each group.

Pancreases from the No onset group, Immediately after onset group, Control group, ONO-4641 groups, and FTY720 group were removed and various histopathological specimens with CD3 immunostaining, insulin immunostaining or H&E staining were prepared. An image of staining around the pancreatic islet is shown for each group.
In this study, we compared the in vivo efficacy of ONO-4641 and FTY720 in the T1DM model. ONO-4641, a selective agonist for S1P1 and S1P5 is different from FTY720, which is selective for S1P1/S1P5 and S1P3.4,18) ONO-4641 suppressed the onset of disease in a dose-dependent manner in the T1DM model, and completely prevented the onset at a dose of 0.1 mg/kg. ONO-4641 at doses of 0.01, 0.03, and 0.1 mg/kg reduced the number of lymphocytes in the peripheral blood of NOD mice by about 20, 60, and 80%, respectively, at 24 h after a single dose.18) The plasma glucose concentration at 24 weeks after administration was highest in the Control group, followed by the ONO-4641 0.01, 0.03, and 0.1 mg/kg groups. Taken together with the significant preventive effect of ONO-4641 0.1 mg/kg, the reduction of plasma glucose concentration suggests that ONO-4641 reduces the number of peripheral blood lymphocytes, which prevents the onset of diabetes mellitus. Since ONO-4641 prevented the disease progression in a mouse MS model by reducing peripheral blood lymphocytes and preventing their infiltration into the affected area,18) we surmise that ONO-4641 decreased the lymphocytes infiltrating into the islets also in this study, which seems similar to the preventive effect of FTY720 in a previous study.19)
Histopathological analysis revealed the infiltration of CD3-positive cells (T cells) around islets in all groups. This insulitis is a characteristic of NOD mice, and the involvement of T cells in the pathogenesis of T1DM was confirmed in this study. Lymphocyte infiltration around islets was observed in mice at 3 to 4 weeks of age before the onset of disease in NOD mice,20) indicating that T cell infiltration around islet occurs prior to disease onset. Reportedly, the T cell subsets infiltrating in the islet include activated T cell, helper T cell and cytotoxic suppressor T cell and are common in human T1DM and NOD mice.21) Therefore, decrease of T cells in the islet plays an important role in the onset of T1DM. A previous report suggested that lymphocytes that had already infiltrated into the islets of NOD mice were not removed following the administration of FTY720 and that the preventive effects of FTY720 were related to suppressing the infiltration of new lymphocytes into the islets.19) In this study, T cell infiltration was also observed around islets during diabetic remission in the ONO-4641 and FTY720 groups, indicating ONO-4641 and FTY720 induced their therapeutic effects by suppressing the further infiltration of lymphocytes into the islets. In this assessment of therapeutic effects, no clear difference was observed in the extent of T cell infiltration between ONO-4641 and FTY720 groups. Penaranda et al. analyzed T cell subsets infiltrating into the pancreas of NOD mice in a study evaluating the preventive effect of FTY720.19) In the study, FTY720 slightly decreased helper T cells but tended to increase regulatory T cells, suggesting the limitations in detecting the change in the composition of all CD3-positive cells in the islets. Reportedly, levels of cytokines such as tumor necrosis factor-α and interferon-γ increase in pancreatic islets.22) Therefore, with its mechanism of action, ONO-4641 is expected to inhibit the further infiltration of new T cells into the islets, thereby decreasing the level of interferon-γ. However, further research needs to be carried out to validate the suppression of cytokine production in the islets by ONO-4641, and T cell subsets infiltrating into the pancreas should be clarified. Further, when ONO-4641 was therapeutically administered at doses of 0.03 and 0.1 mg/kg, the insulin-positive area and the insulin prevention rate were higher than that in the Control group, though the differences were not statistically significant. Additionally, we compared the individual plasma insulin level against plasma glucose level at the end of the experiment (Supplementary Materials). The mice with low glucose, i.e., diabetic remission, maintained insulin concentration at 0.7 ng/mL or higher in the peripheral blood, but the insulin concentration of mice with high glucose, i.e., diabetes mellitus, was barely detectable. It implicates a potential therapeutic effect of ONO-4641 based on the retention of insulin release from the preserved islet cells. These results suggest that ONO-4641 decreased the number of peripheral lymphocytes infiltrating into the islets, thereby protecting the insulin-positive cells in the islets. Therefore, suppression of T cell infiltration into the islets might be involved in the potential mechanism related to the effectiveness of ONO-4641 in the T1DM model.
People with T1DM are at risk for cardiovascular disease and death, which is two-to-eightfold higher than that in non-T1DM people. The complications of chronic diabetes mellitus are subdivided into microvascular (nephropathy, neuropathy, and retinopathy, which are specific to diabetes mellitus) and macrovascular (coronary heart disease, cerebrovascular disease and peripheral artery disease) forms. Macrovascular disease is more aggressive in individuals with T1DM than in those who do not have diabetes mellitus. Evidence is accumulating that T1DM confers a very high risk of developing heart failure. Cardiovascular disease in T1DM is predominantly coronary heart disease, which reflects an accelerated atherosclerotic process. The risk of congenital heart disease in patients with T1DM is roughly threefold in men and sevenfold in women relative to the general population. The risk of stroke is also increased.16) FTY720 has efficacy in the T1DM model,17) but it can induce hypertension, reported as an adverse event in a clinical study.23,24) Because hypertension is a risk factor for the development of complications in T1DM, and risks of the complications such as heart disease and stroke in patients with T1DM are increased relative to the general population, side effects that induce hypertension should be avoided. ONO-4641 might not affect circulatory organs because it lacks S1P3 activity. The results of this study show that ONO-4641, which is selective for S1P1/S1P5, may be more beneficial than insulin administration or nonselective S1P receptor agonists for the therapy of T1DM. However, clinical trials for direct comparison need to be carried out to validate the differences of the cardiotoxicity between ONO-4641 and the existing therapies in the future.
ONO-4641 can maintain a reduction of peripheral blood lymphocyte circulation during administration and is safer than typical immunosuppressive agents because the reduction in peripheral blood lymphocytes by ONO-4641 was shown to be reversible, and the lymphocyte count recovered over time after stopping the administration. In a previous study, we confirmed that the peripheral blood lymphocyte count of rats orally received 0.1 mg/kg ONO-4641 as the maximum dose recovered at 96 h after administration.18) Additionally, for cellular functions of human T cells and B cells in vitro, we assessed the effects of ONO-4641 on T cell proliferation and interferon-γ production in response to anti-CD3 plus anti-CD28 antibodies and on B cell proliferation and immunoglobulin G production in response to anti-immunoglobulin M plus interleukin-4 treatment. ONO-4641 showed no obvious effects on T cell and B cell functions even at 1000 nM,18) which is at least 1000 times higher than the half maximal effective concentrations of in vitro Ca2+ influx assay or cAMP accumulation assay toward S1P1.14) These observations suggest that ONO-4641 inhibits lymphocyte infiltration into the disease lesions by regulating lymphocyte recirculation without affecting their functions and exerts immunomodulatory actions.
In conclusion, ONO-4641, a potent and selective S1P1/S1P5 agonist, demonstrated clear therapeutic benefits in a mouse T1DM model. Considering these preclinical results, it is expected that ONO-4641 will have therapeutic effects in patients with T1DM. Furthermore, ONO-4641 may represent a new therapeutic option for the treatment of lymphocyte-mediated tissue inflammation such as that in autoimmune diseases.
The authors would like to thank Dr. Shinji Asano and Dr. Masafumi Nakayama of Ritsumeikan University for a critical review of the manuscript and helpful advice. We acknowledge the editorial support of Tamami Arai for preparation of the manuscript.
The authors are employees of Ono Pharmaceutical Co., Ltd.
The online version of this article contains supplementary materials.