2022 Volume 45 Issue 10 Pages 1531-1536
2022 Volume 45 Issue 10 Pages 1531-1536
Acne-like eruption caused by anti-epidermal growth factor receptor (EGFR) antibodies such as panitumumab reduces treatment adherence and patient QOL; an alternative therapy is desired. Meanwhile, the usefulness of oral Non-steroidal Anti-inflammatory Drugs (NSAIDs) for acne-like eruptions caused by low-molecular-weight EGFR inhibitors such as erlotinib has been reported in the treatment of lung cancer. This study aimed to investigate whether the combined use of oral NSAIDs and panitumumab for colorectal cancer patients helps prevent acne-like eruption. We retrospectively investigated 167 colorectal cancer patients who had been treated with panitumumab for three cycles or more. The observation period was set from the start of panitumumab treatment to the end of three cycles. Within this period, the incidence and severity of acne-like eruptions were compared. A total of 59 and 108 patients were in the NSAIDs use and non-use groups, respectively, showing differences in the incidence of acne-like eruption rates (78.0 vs. 90.7%, respectively; p = 0.033). In the use group, eruption severity grades 0, 1, 2, and 3 were observed in 13, 33, 13, and 0 patients, respectively; the corresponding values in the non-use group were 10, 60, 36, and 2, respectively (p = 0.007). Oral NSAIDs may help prevent acne-like eruptions caused by panitumumab.
Numerous studies have been conducted on the role of the epidermal growth factor (EGF) in cancer development. Panitumumab1,2) and cetuximab3) are anti-epidermal growth factor receptor (EGFR) antibodies used against KRAS wild-type colorectal cancer. The low-molecular-weight EGFR inhibitors, gefitinib, erlotinib, afatinib, osimertinib, and dacomitinib, are used for EGFR mutation-positive non-small cell lung cancer.4)
Panitumumab and cetuximab can be used as both first- and second-line treatments. They are effective when used alone and in combination with cytocidal anticancer drugs. Although the therapeutic effects of both drugs are comparable, panitumumab is a fully human monoclonal antibody. It has a lower incidence of infusion reaction than cetuximab, a human-mouse chimeric monoclonal antibody, and is used as a key drug for the treatment of colorectal cancer.5) A common side effect of panitumumab and cetuximab is acne-like eruption, which reduces treatment adherence rates and QOL of the patients. To prevent acne-like eruptions, external moisturizing agents that include heparinoids, tetracycline antibiotics, and agents with UV protection properties have been used and their effects have been examined.6,7) However, these supportive therapies do not suppress acne-like eruptions; therefore, a more effective preventive therapy is required.
Meanwhile, acne-like eruptions caused by low-molecular-weight EGFR inhibitors may be alleviated by oral administration of non-steroidal anti-inflammatory drugs (NSAIDs).8,9) EGFR inhibition increases the expression of cyclooxygenase (COX)-2 via the peroxisome proliferator-activated receptor γ (PPARγ) and induces the production of inflammatory cytokine tumor necrosis factor α (TNFα), which may trigger acne-like eruptions.10) NSAIDs may suppress the action of COX-2 expressed by this mechanism and may thus prevent the development of acne-like eruptions. Therefore, this study aimed to investigate whether the concurrent use of oral NSAIDs and panitumumab in colorectal cancer patients may help prevent acne-like eruptions.
We conducted a retrospective survey of the electronic medical records of patients with intractable unresectable colorectal cancer having a wild-type KRAS gene who received at least 3 cycles of 6 mg/kg panitumumab for 2 weeks at the Shizuoka Cancer Center between April 2010 and March 2021. The duration of panitumumab-derived skin toxicity is 1 to 2 months. The observation period was therefore defined as 6 weeks (three cycles), as in previous reports (the STEPP and J-STEPP studies)6,7) that investigated preventive care but excluded oral NSAIDs. The observation period was from the start of panitumumab treatment to the end of three cycles, and the participants were divided into the NSAIDs use group and non-use group. The NSAIDs combination group was defined as cases prescribed daily NSAIDs (loxoprofen, diclofenac, naproxen, meloxicam, celecoxib, aspirin) from the start of panitumumab treatment until the end of the observation period. The NSAIDs non-use group was defined as cases that did not receive NSAID during the observation period.
Patients who were prescribed NSAIDs for single use or for a part of the study period, those who had been treated with cetuximab, and those who had participated in other prospective clinical trials were excluded from this study.
Patient CharacteristicsThe patients’ baseline characteristics of interest included: type, dose, and purpose of NSAIDs; age (≤ 74 or ≥ 75 years); sex; performance status (PS: ≤ 1 or ≥ 2); renal dysfunction; hepatic dysfunction; cytocidal anticancer drug (regimen) combination use; treatment line; minocycline use; panitumumab dose reduction, and treatment postponement. Based on Common Terminology Criteria for Adverse Events (CTCAE ver. 4.0), elevated serum creatinine level (≥1.04 mg/dL for men and ≥ 0.79 mg/dL for women) was defined as renal dysfunction, and elevated blood serum aspartate aminotransferase (AST) or alanine aminotransferase (ALT) levels (either value ≥ 40 U/L) were defined as hepatic dysfunction. “Minocycline use” referred to cases who were prescribed minocycline during the entire observation period to prevent acne-like eruption from the start of panitumumab treatment. The patients’ baseline characteristics were compared between the NSAIDs use and non-use groups using Fisher’s exact test.
Analysis of Acne-Like Rash OnsetWe investigated the incidence, onset timing, and severity of acne-like eruption. The primary outcome was the incidence of grade ≥1 acne-like eruption during the observation period. Between-group comparisons of the incidence and patients’ baseline characteristics were performed using Fisher’s exact test. Severity was the secondary outcome; severity grades were compared between the groups using Cochran–Armitage analysis. Sub-group analyses compared participants who used both NSAIDs and minocycline and those who used neither agent; the incidence and severity of eruptions were compared in both groups.
To analyze factors associated with the onset of acne-like eruption, univariate logistic regression analysis was performed with the onset of acne-like eruption as the dependent variable. The independent variables were NSAID use, age (≥ 75 years), male sex, PS of ≥ 2, renal dysfunction, hepatic dysfunction, cytocidal anticancer drug combination use, treatment line (beyond second line), and minocycline combination use. Furthermore, baseline characteristics associated with p-values of < 0.200 in the univariate logistic regression analysis were included in the multivariate logistic regression analysis. Since age has been shown to be a risk factor for cetuximab-derived skin rash in previous reports,11) and since it has been suggested that a decreased general condition can lead to worsening of acne-like skin rash and pressure ulceration due to increased sitting and lying time and increased pressure on certain areas,12) age (≥ 75 years) and PS of ≥ 2 were considered as factors in the multivariate analysis, even if the p value in the univariate analysis was ≥ 0.200. The significance level was set at 5%, and Bell Curve for Excel (Social Survey Research Information Co., Ltd.) was used for the analysis.
Ethical ConsiderationsThis study adhered to the Declaration of Helsinki and the Ethical Guidelines for Medical and Biological Research Involving Human Subjects. The study was approved by the Shizuoka Cancer Center Ethics Review Board (Approval No. J2021-129-2021-1-3). The informed consent in this study was obtained by opt-out by the Shizuoka Cancer Center due to the observational nature of this study.
Between April 2010 and March 2021, 349 patients received panitumumab. Of those, 292 patients received more than three cycles (Fig. 1). Overall, this study included 167 participants; among them, 59 and 108 participants were included in the NSAIDs use and non-use groups, respectively. In addition, the 167 patients received moisturizing therapy (topical heparinoid) to prevent acne-like eruption.

The participants’ characteristics are presented in Table 1. There were fewer patients aged ≥75 years in the NSAIDs use group than in NSAIDs non-use group (p = 0.041). In addition, two patients used aspirin for antithrombotic therapy, and 57 patients used other NSAIDs for cancer pain relief.
| NSAIDs | p | ||
|---|---|---|---|
| Combination group (n = 59) | Non-combination group (n = 108) | ||
| Types of NSAIDs: dose/d (loxoprofen: 60–180 mg, diclofenac: 37.5–100 mg, naproxen: 300 mg, meloxicam: 10 mg, celecoxib: 100–400 mg, aspirin: 100 mg) | 48, 5, 1, 1, 2, and 2 | — | — |
| Age (≤74/ ≥ 75 years old) | 55/4 | 88/20 | 0.041* |
| Sex (male/female) | 45/14 | 68/40 | 0.086* |
| Performance status (≤1/ ≥ 2) | 47/12 | 92/16 | 0.391* |
| Renal dysfunction (yes/no) | 5/54 | 16/92 | 0.330* |
| Hepatic dysfunction (yes/no) | 18/41 | 27/81 | 0.469* |
| Cytocidal anticancer drug combination use (FOLFIRI or FOLFOX or irinotecan combination use/panitumumab single dose) | 38/21 | 71/37 | 0.867* |
| Treatment line (1/ ≥ 2) | 13/46 | 32/76 | 0.362* |
| 100 to 200 mg/d minocycline use (yes/no) | 30/29 | 69/39 | 0.138* |
| Panitumumab dose reduction (yes/no) | 3/56 | 8/100 | 0.748* |
| Panitumumab postponement (yes/no) | 7/52 | 17/91 | 0.646* |
*Fisher’s exact test. NSAIDs; Non-steroidal Anti-inflammatory Drugs.
There were 11 cases of panitumumab dose reduction due to acne-like eruption (n = 5), diarrhea (n = 2), malaise (n = 1), and stomatitis (n = 3). Panitumumab treatment was postponed in 24 cases due to acne-line eruption (n = 2), diarrhea (n = 2), stomatitis (n = 4), malaise (n = 3), nausea (n = 1), neutropenia (n = 5), thrombocytopenia (n = 1), hypomagnesemia (n = 1), fever (n = 1), thrombus formation (n = 1), surgery and treatment (n = 2), and patient choice (n = 1). Dose reduction and treatment postponement were performed in six cases. In addition, no case of adverse events due to NSAIDs, postponement of panitumumab treatment, or discontinuation of panitumumab treatment due to NSAID use was observed during the study period.
Incidence of Acne-Like EruptionThe incidence of acne-like eruption differed between NSAIDs use and non-use groups (78.0 vs. 90.7%, respectively; p = 0.033). The timing of onset and cumulative incidence rates of acne-like eruptions in both groups are presented in Fig. 2. In the use group, severity grades 0, 1, 2, and 3 were observed in 13, 33, 13, and 0 patients, respectively; the corresponding values for the non-use group were 10, 60, 36, and 2, respectively. These values differed between the groups (p = 0.007).

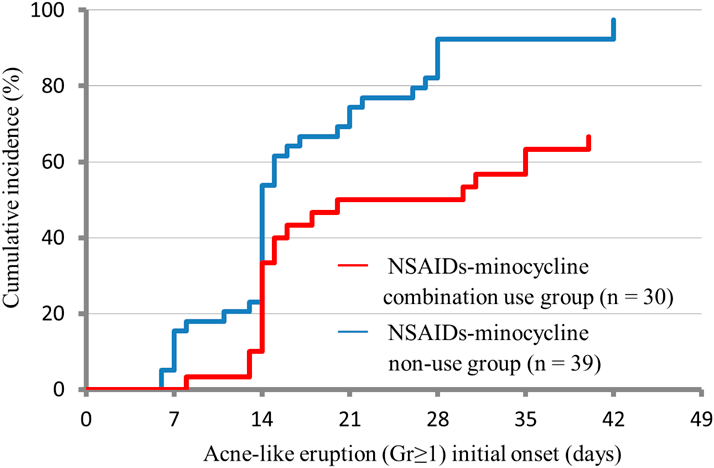
Thirty patients used both NSAIDs and minocycline, and 39 did not use either agent. A significant difference in the incidence of acne-like eruption was observed between the NSAIDs-minocycline combination use group and NSAIDs-minocycline non-use group (66.7 vs. 97.4%, respectively; p < 0.001). In addition, Fig. 3 shows the timing of onset and cumulative incidence rates of acne-like eruptions in the NSAIDs-minocycline combination use group vs. NSAIDs-minocycline non-use group. In the combination use group, severity grades 0, 1, 2, and 3 were observed in 10, 16, 4, and 0 patients, respectively; the corresponding values in the NSAIDs-minocycline non-use group were 1, 21, 15, and 2, respectively. These values differed between the two groups (p < 0.001).
Baseline characteristics that were statistically significant in univariate analysis included NSAIDs use, male sex, treatment line greater than two, and minocycline use (Table 2). Multivariate logistic regression analysis including these factors, age ≥75 years, and PS of ≥2 revealed significant negative correlations with the onset of acne-like eruption in NSAIDs use (odds ratio (OR): 0.2, 95% confidence interval (CI): 0.05–0.5, p = 0.001) and minocycline use (OR: 0.1, 95% CI: 0.04–0.5, p = 0.004). A significant positive correlation with the onset of acne-like eruption was found in males (OR: 4.2, 95% CI: 1.4–12.6, p = 0.009) (Table 3).
| Eruption (n = 144) | No eruption (n = 23) | Odds ratio | 95% CI | p | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | ||||
| NSAIDs use | 46 | 31.9 | 13 | 56.5 | 0.4 | 0.1–0.9 | 0.026 |
| Aged ≥75 years | 19 | 13.2 | 5 | 21.7 | 0.5 | 0.2–1.6 | 0.283 |
| Male | 102 | 70.8 | 11 | 47.8 | 2.6 | 1.1–6.5 | 0.032 |
| Performance status ≥2 | 22 | 15.3 | 6 | 26.1 | 0.5 | 0.2–1.4 | 0.204 |
| Renal dysfunction | 17 | 11.8 | 4 | 17.4 | 0.6 | 0.2–2.1 | 0.456 |
| Hepatic dysfunction | 40 | 27.8 | 5 | 21.7 | 1.4 | 0.5–4.0 | 0.546 |
| Cytocidal anticancer drug combination use | 93 | 64.6 | 16 | 69.6 | 0.8 | 0.3–2.1 | 0.641 |
| Treatment line ≥2 | 108 | 75.0 | 14 | 60.9 | 1.9 | 0.8–4.8 | 0.161 |
| Minocycline use | 80 | 55.6 | 19 | 82.6 | 0.3 | 0.1–0.8 | 0.020 |
NSAIDs; Non-steroidal Anti-inflammatory Drugs.
| Eruption (n = 144) | No eruption (n = 23) | Odds ratio | 95% CI | p | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | ||||
| NSAIDs use | 46 | 31.9 | 13 | 56.5 | 0.2 | 0.05–0.5 | 0.001 |
| Aged ≥75 years | 19 | 13.2 | 5 | 21.7 | 0.4 | 0.1–1.3 | 0.121 |
| Male | 102 | 70.8 | 11 | 47.8 | 4.2 | 1.4–12.6 | 0.009 |
| Performance status ≥2 | 22 | 15.3 | 6 | 26.1 | 0.6 | 0.2–1.8 | 0.346 |
| Treatment line ≥2 | 108 | 75.0 | 14 | 60.9 | 1.6 | 0.6–4.7 | 0.371 |
| Minocycline use | 80 | 55.6 | 19 | 82.6 | 0.1 | 0.04–0.5 | 0.004 |
NSAIDs; Non-steroidal Anti-inflammatory Drugs.
These findings suggest that the use of NSAIDs can help prevent acne-like eruptions caused by panitumumab and that NSAIDs-minocycline combination has good efficacy. The efficacy of preventive skin care (external moisturizing agents, including heparinoids, tetracycline antibiotics, and agents with UV protective properties), excluding the administration of NSAIDs, was evaluated in the previous studies.6,7) The STEPP6) and the J-STEPP7) studies reported a 6-week incidence rate of acne-like skin rash (Gr 2 or higher) of 27.1% and a 6-week skin toxicity rate of 21.3%, respectively. Although skin toxicity in the J-STEPP study included onychomycosis, skin dryness, and pruritus in addition to acne-like skin rash, the incidence of acne-like skin rash of Gr 2 or higher at 6 weeks in the NSAIDs-minocycline combination group in the current study (13.3%) indicated a tendency toward a reduction in this rate. However, the combined use of NSAIDs and minocycline does not completely suppress the eruption (incidence rate of Gr ≥1 of 66.7%), suggesting a need for alternative therapies.
Consistent with previous study findings on low-molecular-weight EGFR inhibitors,8,9) the present findings suggest that oral NSAIDs may suppress acne-like eruptions caused by anti-EGFR antibodies. Based on this evidence, acne-like eruption caused by the anti-EGFR antibody may be affected by the expression of COX-2. Other mechanisms may involve the nuclear factor-kappa B (NFκB)–TNFα pathway that is not related to COX-210) and that may be active alongside the PPARγ–COX-2–TNFα-mediated pathway. Consequently, COX-2 inhibition by NSAIDs alone may be insufficient to prevent acne-like eruptions.
Several mechanisms for minocycline eruption suppression have been proposed. They include inactivation of the NFκB–TNFα pathway and direct inhibition of the TNFα-converting enzyme, which may supplement the anti-inflammatory effects of NSAIDs.13–15) A study that included patients with acne vulgaris, which is not caused by anticancer drugs, reported that the combined use of ibuprofen and tetracycline improved outcomes compared to placebo.16) Individual effects were not observed for either of these agents; thus, the combined use is considered beneficial. The same study reported similar rates of adverse events associated with the use of placebo, ibuprofen alone, tetracycline alone, and ibuprofen-tetracycline combination. Since NSAIDs may increase the risk of gastrointestinal bleeding,17,18) the prophylactic use of NSAIDs for patients treated with anti-EGFR antibodies or EGFR inhibitors is not recommended; the use of NSAIDs should be tailored according to the patient’s condition.
The results of logistic regression analysis suggest that men are at increased risk of acne-like eruption; this finding is consistent with those of previous studies. Arai et al. proposed that the levels of sex hormones such as testosterone and estrogen affect EGF and EGFR expression. In addition, men are less likely than women to use creams for UV protection or moisturizers.19,20) These explanations may account for the present findings.
This study has some limitations. First, treatment adherence rates were unclear in this retrospective study. Second, the severity of acne-like eruptions may have been inaccurately assessed by the attending care providers. Third, the number of patients aged ≥ 75 years was smaller in the NSAIDs combination group than in the non-combination group. This finding may be associated with physician reluctance to use NSAIDs for older adults due to the associated risk of reduced renal function.21) Jatoi et al. reported a risk factor of age < 70 years in their analysis of 933 patients with cetuximab-derived skin rash.11) The present study also indicated a trend toward a negative correlation in patients older than 75 years, although the difference was not significant due to differences in power of detection caused by the small number of cases (OR: 0.4, 95% CI: 0.1–1.3, p = 0.121). If we consider that panitumumab also predisposes young patients to an acne-like skin rash, it is possible that the incidence of acne-like skin rash was higher in the NSAIDs-use group that consisted of a large number of young patients. The confounding of age and skin disorders may have multiple causes, as examined in regorafenib-induced hand-foot syndrome, but it has been reported that younger patients with occupations are less adherent to prophylactic topical care.22) There is some evidence of confounding in the opposite direction, with the tendency of elderly patients to develop skin frailty,23) suggesting that the relationship between age and skin disorders may not be consistent in actual clinical practice. In addition, the preventive effect on acne-like eruptions may differ depending on the strength of COX inhibitory effect and dose of NSAIDs. Since loxoprofen was the most used NSAID in this study, future studies should examine the selectivity and dose of COX-2-based agents that are most effective in this context.
The additional use of oral NSAIDs may help prevent acne-like eruptions caused by panitumumab.
The authors declare no conflict of interest.