Abstract
Background:
Both clinical data and basic science studies suggest that advanced oxidation protein products (AOPPs) may contribute to the progression of atherosclerosis. The aim of this study was to investigate the effects of AOPPs on ATP-binding cassette transporter (ABC) A1 and ABCG1 expression, lipid accumulation and atherosclerotic lesions in apolipoprotein E knockout (apoE-KO) mice.
Methods and Results:
Male 8-week-old apoE-KO mice were fed a high-fat/high-cholesterol diet. Mice received intraperitoneal injections of AOPPs (5 mg/kg) and/or Janus Kinase (JAK) inhibitor AG-490 (5 mg/kg) once every other day for 8 weeks. As shown in our data, AOPPs increased lipid levels of plasma, and promoted advanced lesions in the aortic regions in apoE-KO mice. The ABCA1, ABCG1 and liver X receptor alpha (LXRα) expression were downregulated in apoE-KO mice treated with AOPPs, whereas the lesions in the aortas were decreased, and the ABCA1, ABCG1 and LXRα expression were upregulated in mice treated with AOPPs plus AG-490, compared to the mice treated with AOPPs only. The ABCA1 and LXRα expressions of aortas, liver and intestine were downregulated in the AOPPs group, while the expressions were upregulated in the AOPPs-plus-AG-490 group when compared to the AOPPs group. The same results can be also observed in peritoneal macrophages.
Conclusions:
AOPPs increase accumulation of lipids and exacerbate atherosclerosis through downregulation of ABCA1 and ABCG1 expression, and the JAK-LXRα signaling pathway in apoE-KO mice. (Circ J 2014; 78: 2760–2770)
Atherosclerosis is a chronic disease process that takes place in the intima-media layer of the arterial wall. A variety of cellular elements are involved in this process including endothelial cells, macrophages and platelets, as well as a host of compounds produced by them, such as cytokines, adhesion molecules, oxidative and glycative modifications of lipoproteins and other proteins.1–3
The particular importance in this context may be the recent observation made by Liu et al4
that advanced oxidation protein products (AOPPs) significantly accelerated atherosclerosis through promoting oxidative stress and inflammation.
Advanced oxidation protein products can be formed in vitro by exposure of serum albumin to hypochlorous acid (HOCl). In vivo, plasma AOPPs are mainly carried by albumin, and their concentrations are closely correlated with the levels of dityrosine. AOPPs result from the effects of free radicals on proteins, which might act as inflammatory mediators triggering the oxidative “ignition” of neutrophils, monocytes and T-lymphocytes, thus leading to upregulation and excessive stimulation of dendritic cells.5
These processes might account for immune disorders in atherosclerosis. More interestingly, it was found that AOPPs are likely to be related to atherosclerotic cardiovascular events.6
Increased levels of AOPPs were also found in diabetic and non-uremic subjects with coronary artery disease, suggesting that AOPPs might be relevant in atherosclerosis.7
Although the observational studies suggest a close relationship between AOPPs and atherosclerosis, there is little evidence to show that AOPPs contribute to the occurrence and/or progression of atherosclerosis.
ATP-binding cassette transporter A1 (ABCA1) is an ATP-binding cassette protein that promotes efflux of cholesterol and phospholipids from intracellular compartments to extracellular cholesterol acceptors. ABCA1 is regulated by the liver X receptor (LXR). Our previous studies demonstrated that oleate8
and interferon (IFN)-γ9
reduce the level of ABCA1 and impair ABCA1-dependent cholesterol efflux in THP-1 cells. A LXR agonist promoted ABCA1 expression in apolipoprotein E knockout (apoE-KO) mice.10
More importantly, our studies indicated that AOPPs might first downregulate the expression of LXRα and ABCA1 through activation of the Janus kinase/signal transducers and activators of the transcription (JAK/STAT) signal pathway and then inhibit cholesterol efflux in the THP-1 macrophage.11
However, the effects and mechanism of AOPPs remain to be further investigated in vivo.
The present study was designed to investigate the effects of AOPPs on ABCA1, ABCG1 expression and cholesterol efflux, and further determine the possible mechanism in vivo. In the present study, apoE-KO mice were fed a high-fat/high-cholesterol diet (HFHC), and received intraperitoneal injections of AOPPs and/or the JAK inhibitor, AG-490. The results have suggested that AOPPs might play a causal role in cholesterol efflux and the development of atherosclerosis through the regulation of ABCA1 and ABCG1 expression, and the JAK-LXRα signaling pathway in apoE-KO mice.
Methods
Preparation of Advanced Oxidation Protein Products-Bovine Serum Albumin (AOPPs-BSA)
AOPPs-BSA was prepared in vitro as described previously.12,13
Briefly, fatty acid-free BSA was exposed to 200 mmol/L HOCl for 30 min and then dialyzed overnight against phosphate-buffered saline (PBS) to remove any free HOCl. AOPP concentrations were measured by a spectrophotometric assay, as described previously.12,14
The content of AOPPs was 62.2±13.5 nmol/mg protein in prepared AOPPs-BSA.
Animal Model
Male 8-week-old apoE-KO mice (purchased from the Laboratory Animal Center of Peking University, Beijing, China) were randomly divided into 4 groups (n=10). All of the mice were fed a HFHC diet (15% fat wt/wt, 0.25% cholesterol wt/wt). The AOPP group received intraperitoneal injections of AOPPs (5 mg/kg) once every other day; the AG-490 group received intraperitoneal injections of AG-490 (5 mg/kg) once every other day; the AOPP and AG-490 group received the same doses of AOPPs and AG-490 once every other day; the control animals received intraperitoneal injections of the same volume of normal saline. All mice were killed after 8 weeks for evaluation of the atherosclerotic lesions, with the tissues collected for further analysis. All animal experiments were done in accordance with the Institutional Animal Ethics Committee and the University of South China Animal Care Guidelines for Use of Experimental Animals. All protocols and procedures were approved by the Institutional Animal Care and Use Committee.
Biochemical Variables in Terminal Plasma Analysis
Mice were fasted overnight and euthanized, and blood samples were obtained from the retro-orbital plexus. Triglyceride (TG), total cholesterol (TC) and high-density lipoprotein cholesterol (HDL-C) were determined by commercial enzymatic methods (Test kits, Shanghai Rongsheng Biotech Inc, Shanghai, China). Plasma levels of AOPPs were determined by spectrophotometry as previously described.15
The assay was calibrated using chloramine-T. The absorbance was read at 340 nm and AOPP concentrations were expressed as μmol/L of chloramine-T equivalents. The levels of tumor necrosis factor-alpha (TNF-α) and interleukin-1beta (IL-1β) in plasma were measured using ELISA kits strictly according to the manufacturer’s instructions.
Histology and Morphometric Analyses
Right after removal of the upper portion of the heart and proximal aorta, the pieces of aorta and liver tissues were immersed in Optimal Cutting Temperature solution on dry ice and stored at –80℃. Serial 10-µm-thick cryosections of aorta starting from the aortic root were collected at a distance of 400 µm. The aorta sections were stained by Oil red O, Hematoxylin-Eosin (H&E) and Masson’s Trichrome (MT) as described previously.16,17
The liver sections were stained only by Oil red O and H&E. Collagen content was assessed by MT staining of consecutive slides from serial sections. Image-Pro Plus image analysis software (Media Cybernetics, Shanghai, China) was used for all quantifications. The macrophage accumulation in atherosclerotic lesions was evaluated by CD68 immunohistochemistry staining. The lipid accumulation of the liver was quantified using the hepatic lipid accumulation assay kit (Abcam Trading (Shanghai) Company, Shanghai, China) according the kit instruction. The dye was eluted by adding 100% isopropanol and the extracts were determined by measuring the absorbance at 490 nm. Protein oxidation was assayed using a nitrotyrosine ELISA kit (Abcam) according to the manufacturer’s instructions, as described previously.18
Macrophage-Specific Reverse Cholesterol Transport (RCT) In Vivo
To assay macrophage-specific reverse cholesterol transport in vivo, the radiolabeled cholesterol from prepared macrophages were used as described previously.19,20
J774 macrophages were cholesterol-loaded with oxidized low-density lipoprotein (ox-LDL) to become foam cells and labeled with [3H]-cholesterol. Then, the macrophages were injected intraperitoneally into apoE-KO mice, which were treated by AOPPs (5 mg/kg) and/or AG-490 (5 mg/kg). At 48 h, the blood, collected via the retro-orbital plexus from anesthetized mice, was centrifuged for plasma supernatant. The liver and gall bladder were isolated. Feces were collected continuously over 48 h. [3H]-cholesterol levels were measured by liquid scintillation counting. [3H]-labeled counts in the plasma, liver, bile and feces were expressed as a percentage of total [3H]-cholesterol injected.
Peritoneal Macrophage (PM) Isolation and Culture
Elicited PMs were collected 4 days after injection of 1 ml of 10% thioglycolate into the peritoneal cavity of 9–15-week-old mice as described previously.21
PMs were suspended in Roswell Park Memorial Institute (RPMI) 1640 medium and plated on tissue culture plates. Two hours later, non-adherent cells were removed by washing with PBS, and adherent macrophages were used for the studies.
Cellular Cholesterol Content Detection and Cholesterol Efflux Experiments
Cellular cholesterol content was detected by using high performance liquid chromatography (HPLC) as described previously.11
Cells were labeled with [3H]-cholesterol (0.2 µCi/ml) for the experiments of cellular cholesterol efflux. Medium and cell-associated [3H]-cholesterol was then measured by liquid scintillation counting. Percent efflux was calculated by using the following equation: (total media counts/(total cellular counts+total media counts))×100.
Measurement of Cellular Phospholipids and TGs
Ceramides (CM), sphingomyelins (SM) and phosphatidylcholines (PC) were measured in lipid extracts as described previously.22,23
In brief, cells were labeled with 2 μCi/ml 14[C]serine or 0.5 μCi/ml 14[C]choline. Lipids in the samples were extracted, and phospholipid subclasses were analyzed by thin-layer chromatography (TLC). The spots were scraped, and their radioactivity was quantitated in a liquid scintillation counter. The phospholipids content was normalized by cell protein (dpm/mg cell protein). TG levels were measured by Glycerol Phosphate Oxidase-Peroxidase method using a commercial assay kit (BioSino Biotechnology & Science Inc, Beijing, China) The TG content was normalized by cell protein (mg/g cell protein).
Western Immunoblotting
Protein (20 μg from lysates) was loaded on a 8% SDS-polyacrylamide electrophoresis gel and was electrophoresed for 2 h at 100 V in buffer, and transferred to polyvinylidene fluoride (PVDF) membranes. Immunoreactivity was detected by using the enhanced chemiluminescence (ECL) test. Protein content was calculated by densitometry using Labwords analysis software (Shenteng, Shanghai, China).
RNA Isolation and Real-Time Quantitative Polymerase Chain Reaction Analysis (qPCR)
Total RNA was extracted using TRIzol reagent. qPCR using SYBR Green detection chemistry, was performed on a Roche light Cycler Run 5.32 Real-Time PCR System (Roche, Mannheim, Germany). The sequences of the real-time PCR primers are as follows: ABCA1, forward primer 5’-GATTGGCTTCAGGATGTCCATG TTGGAA-3’, reverse primer 5’-GTATTTTTGCAAGGCTACCAGTTACATTTGACAA-3’; LXRα, forward primer 5’-AGCGTCCACTCAGAGCAAGT-3’, reverse primer 5’-GGGGACAGAACAGTC ATTCG-3’; ABCG1, forward primer 5’-GATTGGCTTCAGGATGTCCATGTTGGAA-3’, reverse primer 5’-GTATTTTTGCAAGGCTACCAGTTACATTTGACAA-3’. Melt curve analyses of all real-time PCR products were performed and shown to produce a single DNA duplex. Quantitative measurements were determined using the ∆∆Ct method and the expression of β-actin was used as the internal control.
Small Interference RNA (siRNA) Transfection
Predesigned siRNA targeting JAK and control non-silencing siRNA were synthesized by Biology Engineering Corporation (Shanghai, China). Cells were transfected with siRNA for JAK or control siRNA using Lipofectamine 2000 (Invitrogen, CA, USA) and the efficacy of gene silencing was evaluated by Western blotting.
Statistical Analysis
All data are presented as mean±SD. Results were analyzed by using one-way ANOVA and Student’s t-test using SPSS 16.0 software. A probability value <0.05 was regarded as significant.
Results
Regulation of Plasma Lipids and Inflammatory Factors by AOPPs and AG490 in apoE-KO Mice
We determined the efficacy of AOPP and AG-490 treatments by measuring circulating lipid and inflammatory response levels in apoE-KO mice fed a HFHC diet. All animals survived through the course of the experiment. We examined the terminal levels of plasma lipids and inflammatory factors in all experimental mice. As shown in
Table 1, AOPPs increased the levels of plasma lipids (TC, TG, low-density lipoprotein cholesterol (LDL-C) and HDL-C) and inflammatory factors (TNF-α and IL-1β), while AG490 attenuated the effects of AOPPs in apoE-KO mice fed a HFHC diet. Additionally, no difference was found in body weight between the groups at the end of experiments.
Table 1.
Biochemical Variables in Terminal Plasma of apoE-KO Mice
| |
Control |
AOPPs |
AG-490 |
AOPPs and AG-490 |
| Body weight (g) |
27.90±3.32 |
28.6±2.69 |
27.5±2.96 |
29.2±3.04 |
| TG (mmol/L) |
1.96±0.56 |
2.49±0.65* |
2.01±0.49 |
2.25±0.55# |
| TC (mmol/L) |
15.20±3.43 |
19.63±4.11* |
15.31±3.7 |
18.33±3.98# |
| HDL-C (mmol/L) |
3.36±1.02 |
4.25±0.89* |
3.50±0.81 |
4.90±0.77 |
| LDL-C (mmol/L) |
11.62±2.06 |
15.20±2.90* |
12.12±2.19 |
13.23±2.56# |
| AOPPs (μmol/L) |
36.38±4.51 |
73.86±9.55* |
39.27±5.33 |
51.67±8.25# |
| TNF-α (pg/ml) |
10.22±1.19 |
27.56±3.75* |
12.06±2.03 |
20.54±3.51# |
| IL-1β (pg/ml) |
6.58±1.03 |
15.24±2.09* |
7.37±1.35 |
12.59±2.05# |
apoE-KO, apolipoprotein E knockout; AOPPs, advanced oxidation protein products; HDL-C, high-density lipoprotein cholesterol; IL-1β, interleukin-1β; LDL-C, low-density lipoprotein cholesterol; TG, triglyceride; TC, total cholesterol; TNF-α, tumor necrosis factorα.
All mice were fed a high-fat/high-cholesterol diet (15% fat wt/wt, 0.25% cholesterol wt/wt). The AOPP group received intraperitoneal injection of AOPPs (5 mg/kg) once every other day. The AG-490 group received intraperitoneal injection of AG-490 (5 mg/kg) once every other day. The AOPP and AG-490 group received the same volume of AOPPs and AG-490 once every other day. The control animals received an intraperitoneal injection of the same volumes of normal saline. The data are expressed as mean±SD, n=10. *P<0.01 vs. the control group; #P<0.05 vs. the AOPP group.
Continuous accumulation of atherosclerotic plaque is one of the major risk factors for cardiovascular disease.24
We examined the development of atherosclerotic lesions by analysis of cross-sections of the aortic roots of mice. We found that atherosclerotic lesion areas and necrotic core areas were significantly increased in the aortic roots of mice injected with AOPPs, compared with those in control groups. We also found that atherosclerotic lesions and necrotic areas were reduced in the aortic roots of mice injected with AOPPs and AG-490 compared with those in the groups injected with AOPPs alone (Figures 1A,B). Further analysis of atherosclerotic lesions revealed that AOPPs increased the fibrous cap areas, macrophage accumulation and protein oxidation, while AG490 attenuated the effects of AOPPs in apoE-KO mice fed a HFHC diet (Figures 1C,D,J).
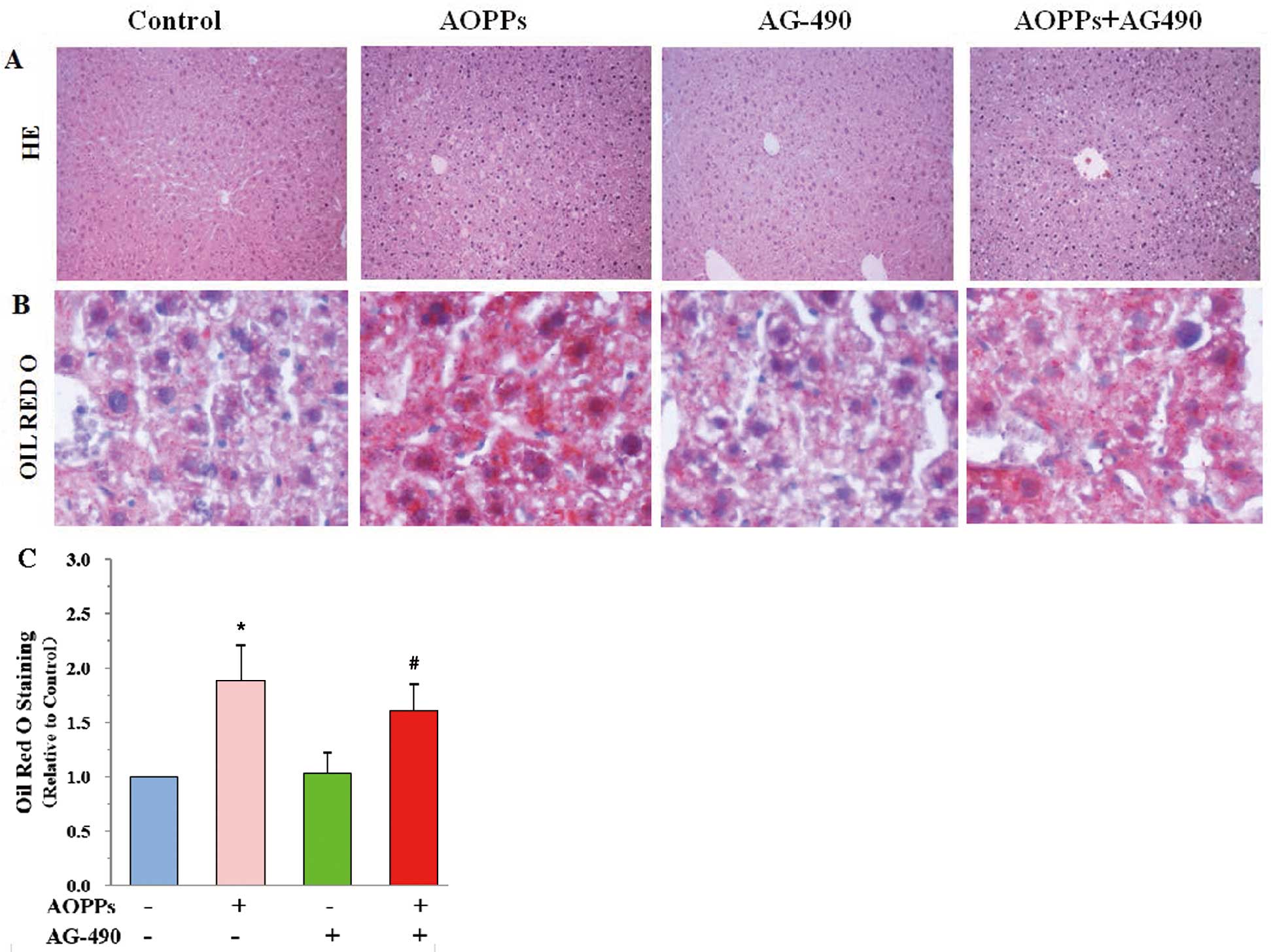
Effects of AOPPs and AG-490 on Hepatic Injury and Lipids Accumulation in the Livers of apoE-KO Mice
In the current view of macrophage RCT, macrophage-derived cholesterol is delivered to the liver because the liver plays an important role in RCT.25
We determined hepatic injury and lipid accumulation by H&E (Figure 2A) and Oil red O staining (Figure 2B). We found that the liver structure was damaged, as indicated by liver lobule structure disorder and increased lipid droplet (Figure 2C) in AOPP groups, but those liver damages were alleviated in mice injected with both AOPPs and AG-490.
AOPPs Decrease the Expression of ABCA1, ABCG1 and LXRα in apoE-KO Mouse Aorta, Liver and Small Intestine
We determined the efficacy of the AOPP and/or AG-490 treatments by measuring the expression of ABCA1, ABCG1 and LXRα in mouse aorta, liver and small intestine. Quantitative PCR and Western blot analysis revealed that the above tissues from apoE-KO mice showed significant downregulation of ABCA1, ABCG1 and LXRα expression in the AOPPs-treated group, but not in the group treated with both the JAK inhibitor AG-490 and AOPPs. Instead, the expression of ABCA1, ABCG1 and LXRα was upregulated in mice treated with both AG-490 and AOPPs, compared with the group treated with AOPPs alone (Figure 3).
Effects of AOPPs and AG-490 on Macrophage-Specific RCT In Vivo
The effects of AOPPs and AG-490 on [3H]-cholesterol movement from intraperitoneal macrophages into the plasma were determined by analysis of plasma [3H]-labelled counts. The results showed a reduction in plasma counts in AOPPs-treated mice compared with the control mice. While compared with mice treated with AOPPs only, plasma [3H]-labeled counts increased in mice treated with both AOPPs and AG-490 (Figure 4A).
We analyzed the [3H]-labeled counts of hepatic, bile and feces for reserving secretion from the liver to bile and feces. Compared with control mice, hepatic [3H]-labeled counts revealed an increase and bile counts were markedly reduced in mice treated with AOPPs. A reduction in fecal counts was also observed in AOPPs-treated mice. Compared to mice treated with AOPPs alone, hepatic [3H]-labeled counts were shown to increase (Figure 4B), but bile and fecal counts revealed a decrease in mice treated with both AOPPs and AG-490 (Figures 4C,D).
Effects of AOPPs and AG-490 on ABCA1 and ABCG1 Expression, and Cholesterol Efflux in PMs
The effects of AOPPs on ABCA1 and ABCG1 expression were examined by real-time quantitative PCR and Western blot assays in PMs. AOPPs downregulated ABCA1, ABCG1, LXRα mRNA and protein expression (Figures 5A,B). In the PMs treated with AG-490 and AOPPs, however, the expression of ABCA1, ABCG1 and LXRα was upregulated when compared with the AOPPs only group (Figures 5A,B). Furthermore, cellular cholesterol efflux was decreased in cells treated with AOPPs, but co-treatment with AOPPs and AG-490 increased the cellular cholesterol efflux (Figure 5E). Meanwhile, AOPPs increased cellular cholesterol, TG and phospholipid (CM, SM and PC) contents, but it was decreased those in mice co-treatment with AG-490 and AOPPs in comparison of AOPPs treating alone (Tables 2,3).

Table 2.
Cholesterol Content in PMs of apoE-KO Mice (mg/g Protein)
| |
Control |
AOPPs |
AG-490 |
AOPPs and AG-490 |
| TC |
205.2±34.3 |
373.6±40.1* |
192.8±28.7 |
309.83±31.9# |
| FC |
71.6±11.2 |
133.7±20.1* |
69.4±10.5 |
108.7±17.7# |
| CE |
136.2±20.6 |
243.7±30.7* |
126.9±18.9 |
198.5±25.6# |
CE, cholesterol ester; FC, free cholesterol; PMs, peritoneal macrophages. Other abbreviations as in Table 1.
The PMs of apoE-KO mice were divided into 4 groups and cultured in medium at 37℃ containing AOPPs and/or AG-490 for 24 h, respectively. Cellular cholesterol and cholesterol ester were extracted, as described in the Methods. High performance liquid chromatography was performed to determine cellular TC, FC and CE. The results are expressed as the mean±SD of 3 independent experiments, each performed in triplicate. *P<0.05 vs. the control group; #P<0.05 vs. the AOPP group.
Table 3.
Phospholipid and TG Contents in PMs of apoE-KO Mice
| |
Control |
AOPPs |
AG-490 |
AOPPs and AG-490 |
| CM (dpm/mg) |
(8.6±0.3)×105 |
(14.9±0.2)×105* |
(8.7±0.4)×105 |
(11.5±0.4)×105# |
| SM (dpm/mg) |
(9.7±1.6)×106 |
(19.4±3.8)×106* |
(10.2±1.3)×106 |
(15.2±3.1)×106# |
| PC (dpm/mg) |
(22.5±3.4)×106 |
(39.2±6.9)×106* |
(20.3±2.5)×106 |
(30.4±5.7)×106# |
| TG (mg/g) |
260.3±35.1 |
367.5±40.4* |
255.0±30.8 |
311.8±296# |
CM, ceramides; PC, phosphatidylcholines; PG, phosphatidyl glycerol; SM, sphingomyelins. Other abbreviations as in Table 1.
The PMs of apoE-KO mice were divided into 4 groups and cultured in medium at 37℃ containing AOPPs and/or AG-490 for 24 h, respectively. Cellular phospholipids were extracted, as described in the Methods. Thin-layer chromatography was performed to determine cellular CM, SM and PC. TG contents were measured using a commercial assay kit. The results are expressed as mean±SD of 3 independent experiments, each performed in triplicate. *P<0.05 vs. the control group; #P<0.05 vs. the AOPP group.
In addition, cells were transfected with JAK siRNA and/or treated with AOPPs (100 μmol/L), respectively. In the cells treated with AOPPs-puls-JAK siRNA, however, the expressions of ABCA1, ABCG1 and LXRα were upregulated in comparison with the AOPPs alone (Figures 5C,D). Then, we further examined the cholesterol efflux in PMs by using liquid scintillation count. Cellular cholesterol efflux was decreased in cells treated with AOPPs, then it was increased in cells treated with AOPPs-puls-JAK siRNA (Figure 5F).
Discussion
Advanced oxidation protein products have emerged as a novel class of renal pathogenic mediators.26
Plasma AOPPs are mainly carried by albumin, and their concentration closely correlates with the level of dityrosine, a hallmark of oxidized protein. Therefore, AOPPs have been considered as the markers of oxidant-mediated protein damage.14
AOPPs are also involved in the further development of oxidative stress and inflammation by the activation of immune cells.27,28
Accumulation of AOPPs has been reported in various pathologies and is also associated with impaired carbohydrate metabolism.29,30
The lipid metabolism plays a key role in atherogenesis and contributes to atherosclerotic plaque formation in vivo. The previous studies demonstrate a strong relationship between levels of plasma lipids and the incidence of atherosclerotic cardiovascular disease.24,31
In the present study, we also evaluated the effects of AOPPs on the plasma lipid profiles in the atherosclerosis-prone apoE-KO mice fed an atherogenic diet. The levels of plasma lipids were shown in
Table 1. Significant increases were found in TC, TG and LDL-C of apoE-KO mice treated with AOPPs, compared with the control mice. Meanwhile, AOPPs promoted the increase in lipids accumulation in the aortic sinus plaques and livers in apoE-KO mice, as shown in
Figures 1A,2B
by Oil red O staining. Additionally, we found that AOPPs exacerbated the atherosclerotic lesions in the aortic sinus, as shown in
Figure 1. These findings suggest that there are significant correlations between changes of atherosclerotic lesions and plasma lipid levels in AOPPs-treated mice. AOPPs accelerate the progression of atherosclerosis, through a mechanism that might involve increasing the plasma levels of TG, TC and LDL-C in apoE-KO mice fed an atherogenic diet.
Both ABCA1 and ABCG1 play a pivotal role in the removal of excess intracellular cholesterol and phospholipids to lipid-poor apolipoprotein acceptors in the initial step of RCT.32
In vitro studies from several laboratories have shown that ABCA1 plays an important role in the removal of excess cholesterol from macrophages.33
The development of foam cells, resulting from the accumulation of excess cholesterol in macrophages, leads to the formation of fatty streaks, complex lesions, and eventually plaque rupture.34
In the current study, we found that the ABCA1 and ABCG1 expression was significantly downregulated in AOPPs-treated apoE-KO mice. Thus, we speculate that AOPPs exacerbate lipid accumulation and atherosclerosis of apoE-KO mice most likely through downregulating the expression of ABCA1 and ABCG1.
Macrophages are the key players in the pathogenesis of atherosclerosis. Stimulation of macrophage cholesterol efflux by enhancing ABCA1 and ABCG1 expression would be predicted to inhibit foam cell formation and consequently reduce atherosclerosis.35
To directly test the potential role and the mechanism of macrophage efflux in progression of atherosclerotic lesions induced by AOPPs, we isolated and cultured PMs of apoE-KO mice for further experiments in the current study. We found that the cellular cholesterol, cholesterol ester, TG and phospholipid (CM, SM and PC) contents were increased, and the cellular cholesterol efflux was decreased in PMs of AOPPs-treated mice compared with the control (as shown in
Tables 2,3
and
Figure 5C). Meanwhile, the expressions of ABCA1 and ABCG1 were downregulated in mice treated with AOPPs, as shown in
Figures 5A,B. These findings suggest that AOPPs inhibited cholesterol efflux and increased cellular lipid contents in PMs. The role of AOPPs might be induced by the downregulation of ABCA1 and ABCG1 expression. According to the above findings in the present study it suggest that AOPPs accelerate the progression of atherosclerosis through increasing the plasma lipid levels and impairing the macrophage cholesterol efflux.
In addition, both vascular smooth muscle cells (vSMCs) and endothelial cells (vECs) are important in the progression of atherosclerosis. Therefore, we analyzed the effects of AOPPs on ABCA1 and ABCG1 expression in vSMCs and vECs. We found that AOPPs can downregulate the expression of ABCA1 and ABCG1 in vSMCs, but not significantly in vECs, compared with the control (data are shown in
Supplementary File 1). Previous studies have shown that both smooth muscle cells (SMCs) and macrophages produce proteinases to facilitate cell migration, but they might also thin the fibrous cap by degrading the matrix in atherosclerotic plaque.36,37
Liu et al4
also found that AOPPs can induce SMCs proliferation in atherosclerotic plaque. AOPPs treatment increases the fibrous cap, which might be related to SMCs migration and proliferation. Another study demonstrated that activation of the signaling pathway by the AOPPs-vECs interaction resulted in the overexpression of vascular cell adhesion molecule-1 (VCAM-1) and inter-cellular adhesion molecule 1 (ICAM-1) at both gene and protein levels,13
suggesting that overexpression of adhesion molecules is the pro-inflammatory pathway of AOPPs in vECs.
Reverse cholesterol transport is a complex process ensuring the efflux of cholesterol from peripheral cells and its transport back to the liver for its metabolism and biliary excretion. The abnormalities of the various components of RCT accelerate atherogenesis.38
Therefore, we assayed the macrophage-specific RCT in vivo and found that AOPPs can decrease the [3H]-cholesterol counts in plasma, liver, bile and feces of apoE-KO mice. These results suggest that AOPPs impair the RCT at multiple steps in the RCT pathway, including the macrophage cholesterol efflux and the cholesterol flux through the liver to bile and feces. The impairment of RCT might contribute to the accumulation of lipids and exacerbation of atherosclerosis induced by treatment with AOPPs in apoE-KO mice.
To gain further insights into the relationship between AOPPs and ABCA1 in modulation of atherosclerosis and cholesterol efflux of cells, we evaluated the mechanism underlying the effects of AOPPs in apoE-KO mice. LXR forms a heterodimer with the retinoid X receptor (RXR), which binds to the LXR response element in the promoter sequence of ABCA1.39
LXR can upregulate ABCA1 expression through forming heterodimers with RXR. In the current study, we found that the LXRα expression was significantly downregulated in AOPPs-treated apoE-KO mice and PMs. These results suggested that LXRα was involved in the downregulation of ABCA1 and ABCG1 induced by AOPPs.
The JAK/STAT pathway is considered a stress-responsive signaling cascade that transduces signals from cell surface receptors to the nucleus, thereby modulating gene expression as the compensatory mechanism. To date, various vascular stress factors are described linking activation of the JAK/STAT signaling pathway to vascular diseases.40
Our previous data showed that IFN-γ might decrease the expression of ABCA1 through the JAK/STAT1 signaling pathway in THP-1 macrophage-derived foam cells.9
In the study, we investigated the effects of the JAK inhibitor (AG-490) on ABCA1 and ABCG1 expression and atherosclerosis in AOPPs-treated apoE-KO mice. We found that the plasma lipid levels were decreased, the ABCA1, ABCG1 and LXRα expressions in the aorta were upregulated, the lesions in the aortas were decreased, and the lipid content in aortic sinus plaques and livers were decreased in the AOPPs and AG-490 group mice in comparison with the AOPP group, suggesting a role of the JAK/STAT signaling pathway in the effects of AOPPs on ABCA1 and ABCG1 expression and lipid accumulation.
In summary, our study showed that AOPPs can exacerbate lipid accumulation and atherosclerosis in apoE-KO mice, downregulate the ABCA1 and ABCG1 expression, and inhibit the cholesterol efflux in PMs. Expression of LXRα is also inhibited by AOPPs, which can be reversed by a JAK inhibitor, AG-490. The main conclusion that can be drawn from our studies is that there is an interrelationship between AOPPs and cholesterol efflux, in which AOPPs might first downregulate the expression of LXRα through the JAK/STAT signaling pathway and then decrease the ABCA1 and ABCG1 expression and cholesterol efflux. These data suggest that treatment with pharmacological agents that decrease the AOPP levels may be an effective approach for reducing atherogenic risk in humans.
Acknowledgments
This work was supported by financial support from the National Natural Sciences Foundation of China (81100211, 81070220, 81370377 and 81170278) and the Science and Technology Plan Projects of Hunan Province (NO.2013SK3114), the Aid Program for Science and Technology Innovative Research Team in Higher Educational Institutions (2008-244) of Hunan Province, and the construct program of the key discipline in Hunan Province, China (Basic Medicine Sciences in University of South China, Xiangjiaofa NO. [2011]76).
Supplementary Files
Supplementary File 1
Methods
Results
Figure S1. SR-BI, SREBP-1 and CETP expression in the aorta and peritoneal macrophages (PMs) of apolipoprotein E knockout (apoEKO) mice.
Figure S2. The role of advanced oxidation protein products (AOPPs) on ATP-binding cassette transporter A1 (ABCA1) and ATP-binding cassette transporter G1 (ABCG1) expression in vascular smooth muscle cells (vSMCs) and vascular endothelial cells (vECs).
Figure S3. Effect of advanced oxidation protein products (AOPPs) on cholesterol efflux in peritoneal macrophages (PMs) treated with small interference RNA (siRNA).
Please find supplementary file(s);
http://dx.doi.org/10.1253/circj.CJ-14-0193
References
- 1.
Woollard KJ. Immunological aspects of atherosclerosis. Clin Sci (Lond) 2013; 125: 221–235.
- 2.
Wildgruber M, Swirski FK, Zernecke A. Molecular imaging of inflammation in atherosclerosis. Theranostics 2013; 3: 865–884.
- 3.
Shimada K. Diversity and plasticity of monocyte subsets: Tipping the delicate balance involved in the pathogenesis of atherosclerosis. Circ J 2012; 76: 2331–2332.
- 4.
Liu SX, Hou FF, Guo ZJ, Nagai R, Zhang WR, Liu ZQ, et al. Advanced oxidation protein products accelerate atherosclerosis through promoting oxidative stress and inflammation. Arterioscler Thromb Vasc Biol 2006; 26: 1156–1162.
- 5.
Alderman CJ, Shah S, Foreman JC, Chain BM, Katz DR. The role of advanced oxidation protein products in regulation of dendritic cell function. Free Radic Biol Med 2002; 32: 377–385.
- 6.
Descamps-Latscha B, Witko-Sarsat V, Nguyen-Khoa T, Nguyen AT, Gausson V, Mothu N, et al. Advanced oxidation protein products as risk factors for atherosclerotic cardiovascular events in nondiabetic predialysis patients. Am J Kidney Dis 2005; 45: 39–47.
- 7.
Martin-Gallan P, Carrascosa A, Gussinye M, Dominguez C. Biomarkers of diabetes-associated oxidative stress and antioxidant status in young diabetic patients with or without subclinical complications. Free Radic Biol Med 2003; 34: 1563–1574.
- 8.
Tang CK, Yang JH, Yi GH, Wang Z, Liu LS, Wan ZY, et al. Effects of oleate on ATP binding cassette transporter A1 expression and cholesterol efflux in THP-1 macrophage-derived foam cells. Sheng Wu Hua Xue Yu Sheng Wu Wu Li Xue Bao (Shanghai) 2003; 35: 1077–1082 (in Chinese).
- 9.
Hao XR, Cao DL, Hu YW, Li XX, Liu XH, Xiao J, et al. IFN-gamma down-regulates ABCA1 expression by inhibiting LXRalpha in a JAK/STAT signaling pathway-dependent manner. Atherosclerosis 2009; 203: 417–428.
- 10.
Dai XY, Ou X, Hao XR, Cao DL, Tang YL, Hu YW, et al. The effect of T0901317 on ATP-binding cassette transporter A1 and Niemann-Pick type C1 in apoE–/– mice. J Cardiovasc Pharmacol 2008; 51: 467–475.
- 11.
Mo ZC, Xiao J, Liu XH, Hu YW, Li XX, Yi GH, et al. AOPPs inhibits cholesterol efflux by down-regulating ABCA1 expression in a JAK/STAT signaling pathway-dependent manner. J Atheroscler Thromb 2011; 18: 796–807.
- 12.
Witko-Sarsat V, Gausson V, Nguyen AT, Touam M, Drueke T, Santangelo F, et al. AOPP-induced activation of human neutrophil and monocyte oxidative metabolism: A potential target for N-acetylcysteine treatment in dialysis patients. Kidney Int 2003; 64: 82–91.
- 13.
Guo ZJ, Niu HX, Hou FF, Zhang L, Fu N, Nagai R, et al. Advanced oxidation protein products activate vascular endothelial cells via a RAGE-mediated signaling pathway. Antioxid Redox Signal 2008; 10: 1699–1712.
- 14.
Witko-Sarsat V, Friedlander M, Capeillere-Blandin C, Nguyen-Khoa T, Nguyen AT, Zingraff J, et al. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int 1996; 49: 1304–1313.
- 15.
Servettaz A, Guilpain P, Goulvestre C, Chereau C, Hercend C, Nicco C, et al. Radical oxygen species production induced by advanced oxidation protein products predicts clinical evolution and response to treatment in systemic sclerosis. Ann Rheum Dis 2007; 66: 1202–1209.
- 16.
Rotllan N, Ramirez CM, Aryal B, Esau CC, Fernandez-Hernando C. Therapeutic silencing of microRNA-33 inhibits the progression of atherosclerosis in Ldlr–/– mice: Brief report. Arterioscler Thromb Vasc Biol 2013; 33: 1973–1977.
- 17.
Higuchi K, Nakaoka Y, Shioyama W, Arita Y, Hashimoto T, Yasui T, et al. Endothelial Gab1 deletion accelerates angiotensin II-dependent vascular inflammation and atherosclerosis in apolipoprotein E knockout mice. Circ J 2012; 76: 2031–2040.
- 18.
Tarry-Adkins JL, Martin-Gronert MS, Fernandez-Twinn DS, Hargreaves I, Alfaradhi MZ, Land JM, et al. Poor maternal nutrition followed by accelerated postnatal growth leads to alterations in DNA damage and repair, oxidative and nitrosative stress, and oxidative defense capacity in rat heart. FASEB J 2013; 27: 379–390.
- 19.
Briand F, Treguier M, Andre A, Grillot D, Issandou M, Ouguerram K, et al. Liver X receptor activation promotes macrophage-to-feces reverse cholesterol transport in a dyslipidemic hamster model. J Lipid Res 2010; 51: 763–770.
- 20.
McGillicuddy FC, de la Llera Moya M, Hinkle CC, Joshi MR, Chiquoine EH, Billheimer JT, et al. Inflammation impairs reverse cholesterol transport in vivo. Circulation 2009; 119: 1135–1145.
- 21.
Thorp E, Li Y, Bao L, Yao PM, Kuriakose G, Rong J, et al. Brief report: Increased apoptosis in advanced atherosclerotic lesions of Apoe–/– mice lacking macrophage Bcl-2. Arterioscler Thromb Vasc Biol 2009; 29: 169–172.
- 22.
Lin CY, Duan H, Mazzone T. Apolipoprotein E-dependent cholesterol efflux from macrophages: Kinetic study and divergent mechanisms for endogenous versus exogenous apolipoprotein E. J Lipid Res 1999; 40: 1618–1627.
- 23.
Lucic D, Huang ZH, Gu D, Subbaiah PV, Mazzone T. Cellular sphingolipids regulate macrophage apolipoprotein E secretion. Biochemistry 2007; 46: 11196–11204.
- 24.
Nohara R, Daida H, Hata M, Kaku K, Kawamori R, Kishimoto J, et al. Effect of long-term intensive lipid-lowering therapy with rosuvastatin on progression of carotid intima-media thickness: Justification for Atherosclerosis Regression Treatment (JART) extension study. Circ J 2013; 77: 1526–1533.
- 25.
deGoma EM, deGoma RL, Rader DJ. Beyond high-density lipoprotein cholesterol levels evaluating high-density lipoprotein function as influenced by novel therapeutic approaches. J Am Coll Cardiol 2008; 51: 2199–2211.
- 26.
Valente AJ, Yoshida T, Clark RA, Delafontaine P, Siebenlist U, Chandrasekar B. Advanced oxidation protein products induce cardiomyocyte death via Nox2/Rac1/superoxide-dependent TRAF3IP2/JNK signaling. Free Radic Biol Med 2013; 60: 125–135.
- 27.
Kalousova M, Zima T, Tesar V, Dusilova-Sulkova S, Skrha J. Advanced glycoxidation end products in chronic diseases-clinical chemistry and genetic background. Mutat Res 2005; 579: 37–46.
- 28.
Alagozlu H, Gorgul A, Bilgihan A, Tuncer C, Unal S. Increased plasma levels of advanced oxidation protein products (AOPP) as a marker for oxidative stress in patients with active ulcerative colitis. Clin Res Hepatol Gastroenterol 2013; 37: 80–85.
- 29.
Krzystek-Korpacka M, Neubauer K, Berdowska I, Boehm D, Zielinski B, Petryszyn P, et al. Enhanced formation of advanced oxidation protein products in IBD. Inflamm Bowel Dis 2008; 14: 794–802.
- 30.
Piwowar A, Knapik-Kordecka M, Warwas M. AOPP and its relations with selected markers of oxidative/antioxidative system in type 2 diabetes mellitus. Diabetes Res Clin Pract 2007; 77: 188–192.
- 31.
Nicholls SJ, Tuzcu EM, Sipahi I, Grasso AW, Schoenhagen P, Hu T, et al. Statins, high-density lipoprotein cholesterol, and regression of coronary atherosclerosis. JAMA 2007; 297: 499–508.
- 32.
Tarling EJ, Edwards PA. Dancing with the sterols: Critical roles for ABCG1, ABCA1, miRNAs, and nuclear and cell surface receptors in controlling cellular sterol homeostasis. Biochim Biophys Acta 2012; 1821: 386–395.
- 33.
Joyce CW, Amar MJ, Lambert G, Vaisman BL, Paigen B, Najib-Fruchart J, et al. The ATP binding cassette transporter A1 (ABCA1) modulates the development of aortic atherosclerosis in C57BL/6 and apoE-knockout mice. Proc Natl Acad Sci USA 2002; 99: 407–412.
- 34.
Ross R. The pathogenesis of atherosclerosis: A perspective for the 1990s. Nature 1993; 362: 801–809.
- 35.
Jun HJ, Hoang MH, Lee JW, Yaoyao J, Lee JH, Lee DH, et al. Iristectorigenin B isolated from Belamcanda chinensis is a liver X receptor modulator that increases ABCA1 and ABCG1 expression in macrophage RAW 264.7 cells. Biotechnol Lett 2012; 34: 2213–2221.
- 36.
Deguchi JO, Aikawa M, Tung CH, Aikawa E, Kim DE, Ntziachristos V, et al. Inflammation in atherosclerosis: Visualizing matrix metalloproteinase action in macrophages in vivo. Circulation 2006; 114: 55–62.
- 37.
Cherepanova OA, Pidkovka NA, Sarmento OF, Yoshida T, Gan Q, Adiguzel E, et al. Oxidized phospholipids induce type VIII collagen expression and vascular smooth muscle cell migration. Circ Res 2009; 104: 609–618.
- 38.
Cucuianu M, Coca M, Hancu N. Reverse cholesterol transport and atherosclerosis: A mini review. Rom J Intern Med 2007; 45: 17–27.
- 39.
Sato M, Kawata Y, Erami K, Ikeda I, Imaizumi K. LXR agonist increases the lymph HDL transport in rats by promoting reciprocally intestinal ABCA1 and apo A-I mRNA levels. Lipids 2008; 43: 125–131.
- 40.
Grote K, Luchtefeld M, Schieffer B. JANUS under stress: Role of JAK/STAT signaling pathway in vascular diseases. Vascul Pharmacol 2005; 43: 357–363.