2014 Volume 78 Issue 11 Pages 2719-2726
2014 Volume 78 Issue 11 Pages 2719-2726
Background: In hypertensive patients, left ventricular hypertrophy (LVH) may persist despite satisfactory blood pressure (BP) control. The efficacy of thiazide diuretics in Western countries has been reported, but whether this applies to hypertensive Japanese patients is uncertain.
Methods and Results: We randomly assigned 94 patients whose BP was poorly controlled with usual doses of angiotensin-II receptor blockers (ARB), to losartan/hydrochlorothiazide (HCTZ) fixed-dose combination vs. maximum doses of ARB. After 6 months follow-up, decrease in BP, regression of electrocardiographic LVH, and changes in laboratory measurements were examined. Although a similar decrease in BP was observed in both groups, the decrease in LV Sokolow-Lyon voltage, from 34.4±9.2 to 29.4±8.8 mm in the losartan/HCTZ vs. from 29.9±10.2 to 29.1±8.4 mm in the ARB group (P=0.0003), and the decrease in serum B-type natriuretic peptide (BNP) level, from 30.1±28.5 to 26.8±28.0 pg/ml vs. from 23.7±14.8 to 29.8±29.3 pg/ml (P=0.045) were greater in the losartan/HCTZ group. By single variable logistic regression analysis, ∆BNP (P=0.012) and treatment with losartan/HCTZ (P<0.0001) correlated with the regression of LVH. By multiple variable logistic regression analysis, both ∆BNP (P=0.035) and treatment with losartan/HCTZ (P=0.0003) remained significant. No major adverse effects were observed.
Conclusions: Greater regression of LVH was safely achieved with losartan/HCTZ in patients whose BP was poorly controlled with an ARB. (Circ J 2014; 78: 2719–2726)
Because of the high rate of adverse cardio- and cerebrovascular events associated with hypertension, strict control of systemic blood pressure (BP) is recommended.1,2 However, adverse clinical events may, in some hypertensive patients, develop despite tight control of BP, suggesting that latent injury to the target organs may be developing despite administration of appropriate treatment. Of the many trials that have addressed the management of BP, few have focused attention on the detection and prevention of latent hypertension-induced organ injury.
Editorial p 2633
Renin-angiotensin system (RAS) inhibitors and thiazide diuretics are the most prescribed drugs for their antihypertensive and cardioprotective effects.3 The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure recommended the use of thiazide diuretics for uncomplicated hypertension,4 and the eighth report recommended thiazide diuretics, calcium-channel blockers, angiotensin-converting enzyme (ACE) inhibitors and angiotensin-II receptor blockers (ARB) as first-line therapy.5 However, in Japan, thiazide diuretics are not prescribed as often as RAS inhibitors, despite being listed as first-line drugs in the guidelines issued by the Japanese Society of Hypertension,2 in part because of their adverse effects, such as hyponatremia, hypokalemia and hyperuricemia, and adverse effects on the glucose and lipid profiles.6 These adverse effects can be mitigated, however, by administration in low doses.7
The therapeutic effects of thiazide diuretics on the cardiovascular system have been described.8,9 For example, when combined with an ARB in a substudy of the LIFE trial, thiazide diuretics induced regression of left ventricular hypertrophy (LVH).10 On the basis of these observations, we conducted a randomized multicenter trial to examine the effectiveness and safety of losartan combined with a hydrochlorothiazide (HCTZ) in hypertensive Japanese patients whose BP was not controlled with usual doses of ARB.
Between October 2007 and April 2012, this open-label, randomized trial enrolled, at 6 medical institutions, 94 ambulatory, hypertensive patients between the ages of 20 and 80 years, whose BP, according to the 2004 guidelines of the Japanese Society of Hypertension,11 was not controlled with losartan 50 mg, candesartan 8 mg, valsartan 80 mg, telmisartan 40 mg or olmesartan, 20 mg daily. Patients were excluded if they presented with a history of malignant or secondary hypertension, gout, myocardial infarction, cerebral infarction or hemorrhage, percutaneous coronary intervention or coronary artery bypass graft surgery within the past 6 months; or a current diastolic BP ≥110 mmHg, angina pectoris, heart failure or LV dysfunction (LV ejection fraction <40% on echocardiography within 6 months before entry), active inflammation (suffering from rheumatoid arthritis, necrosis by arteriosclerosis obliterans, any infections, etc), hemoglobin A1c ≥8.0%, serum uric acid level ≥8.0 mg/dl or creatinine ≥2.0 mg/dl, severe liver disease (aspartate aminotransferase or alanine aminotransferase >3-fold the normal limit), pregnancy, or an allergy or intolerance to a pharmaceutical used in this trial.
A 12-lead electrocardiogram was performed at baseline and at 6 months, and both the Sokolow-Lyon voltage (RV5+SV1) and Cornell voltage (RAVL+SV3) were calculated manually in each institution.
This trial was approved by the ethics committees of all participating institutions and written informed consent was given by all patients before their enrolment.
Study DesignAfter a 2-month run-in period during which they were treated with usual doses of ARB, the patients were randomly assigned to treatment with losartan 50 mg and HCTZ 12.5 mg daily, vs. ARB only at maximum recommended daily doses in Japan, including losartan 100 mg, candesartan 12 mg, valsartan 160 mg, telmisartan 80 mg, or olmesartan 40 mg. A telephone method provided by Metem Corporation (Tokyo, Japan) was used for the randomization scheme. The patients were followed for 6 months thereafter, and BP and heart rate were recorded at each visit. At 3 months, an antihypertensive drug other than an ARB or an ACE inhibitor could be added, or the medication burden could be alleviated depending on the patient’s BP and clinical status. A 12-lead ECG, complete blood count and screening biochemical tests were obtained at baseline and at 6 months (Figure) to compare the efficacy and safety of losartan/HCTZ with the maximal doses of other ARBs. The study protocol was posted on the homepage of the Japanese Heart Foundation on April 30, 2008.
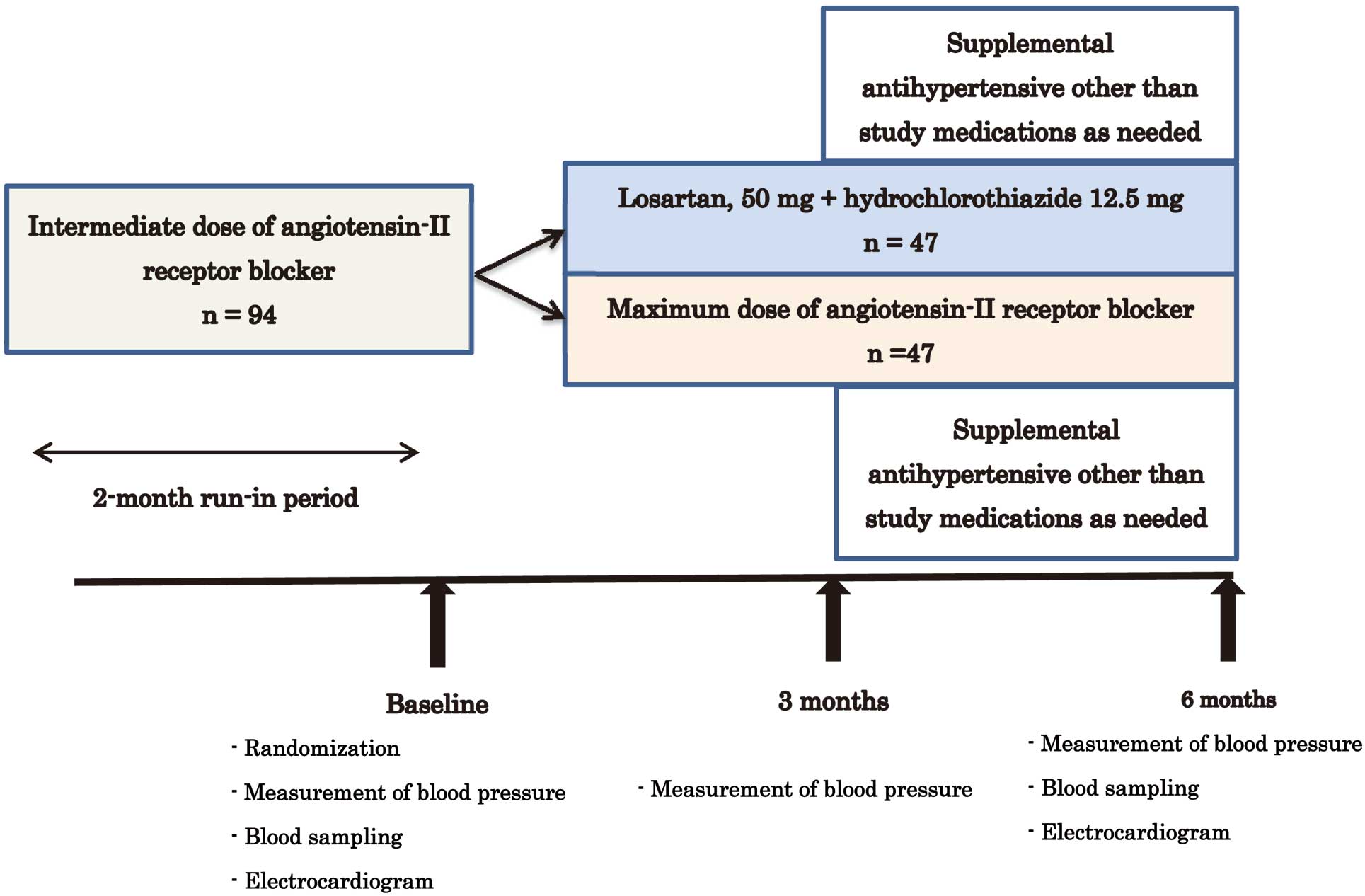
Summary of study protocol. Intermediate doses of angiotensin-II receptor blocker=losartan 50 mg, candesartan 8 mg, valsartan 80 mg, telmisartan 40 mg or olmesartan 20 mg daily. Maximum doses of angiotensin-II receptor blocker=losartan 100 mg, candesartan 12 mg, valsartan 160 mg, telmisartan 80 mg or olmesartan 40 mg daily.
A complete blood count and levels of total cholesterol, uric acid, hemoglobin A1c (HbA1c), human fatty acid-binding protein, B-type natriuretic peptide (BNP) and high-sensitivity C-reactive protein were obtained at both baseline and 6 months and analyzed by a core laboratory (SRL Inc, Tokyo, Japan). The estimated glomerular filtration rate (eGFR) was calculated, using the formula of the Japanese Society of Nephrology:
eGFR (ml·min−1·1.73 m−2)=194×(serum creatinine)−1.094×age−0.287(×0.739 if female).12
Statistical AnalysisThe data are expressed as mean±SD, or as count and percentage. Differences in categorical values between the study groups were examined by Pearson’s chi-square or Fisher’s exact tests. Between-treatment group differences were analyzed by unpaired t-test or analysis of covariance (ANCOVA). Single and multiple variable logistic regression analyses were used to identify the correlates of LVH regression. P value <0.05 was considered statistically significant. All analyses were performed using Stat View-J 5.0 (Hulinks Inc, Tokyo, Japan).
The study assigned 94 patients randomly and evenly to treatment with losartan/HCTZ vs. a maximum recommended dose of an ARB. The baseline characteristics of the study groups were similar, except for age, serum uric acid level, and the Sokolow-Lyon voltage (Table 1).
| ARB (n=47) |
Losartan+HCTZ (n=47) |
P value | |
|---|---|---|---|
| Age, years | 65.3±9.3 | 61.1±10.7 | 0.043 |
| Men | 26 (55.3) | 17 (36.2) | 0.40 |
| Arterial BP, mmHg | |||
| Systolic | 154.3±13.9 | 154.4±14.7 | 0.98 |
| Diastolic | 87.2±11.2 | 87.2±11.8 | 0.98 |
| Heart rate, beats/min | 70.4±11.6 | 73.7±12.7 | 0.19 |
| Sokolow-Lyon voltage, mm | 29.9±10.2 | 34.4±9.2 | 0.03 |
| Cornell voltage, mm | 18.9±6.4 | 18.6±6.8 | 0.87 |
| C-reactive protein, mg/L | 754±1,059 | 1,474±2,865 | 0.11 |
| BNP, pg/ml | 23.7±14.8 | 30.1±28.5 | 0.17 |
| Human fatty acid-binding protein, ng/ml | 3.1±1.4 | 2.9±1.5 | 0.55 |
| eGFR, ml·min–1·1.73 m–2 | 76.5±12.7 | 73.3±17.8 | 0.32 |
| Uric acid, mg/dl | 4.9±1.4 | 5.5±1.2 | 0.016 |
| Hemoglobin, g/dl | 13.7±1.1 | 13.8±1.5 | 0.75 |
| Hemoglobin A1c, % | 5.4±0.5 | 5.3±0.5 | 0.36 |
| Total cholesterol, mg/dl | 199±35 | 202±29 | 0.69 |
| Serum sodium, mEq/L | 141±2.3 | 142±2.6 | 0.59 |
| Serum potassium, mEq/L | 4.2±0.3 | 4.2±0.4 | 0.44 |
| Diabetes mellitus | 5 (10.6) | 4 (8.5) | 0.73 |
| Dyslipidemia | 12 (25.5) | 14 (29.8) | 0.65 |
| Target BP reached | 31 (66.0) | 26 (55.3) | 0.29 |
| Drug therapy | |||
| β-adrenergic blocker | 7 (14.9) | 6 (12.8) | 0.77 |
| Calcium-channel blocker | 25 (53.2) | 26 (55.3) | 0.84 |
| Supplemental antihypertensive drug | 4 (8.5) | 2 (4.3) | 0.40 |
Data are mean±SD, or n (%) of observations.
ARB, angiotensin-II receptor blocker; BNP, B-type natriuretic peptide; BP, blood pressure; eGFR, estimated glomerular filtration rate; HCTZ, hydrochlorothiazide.
No significant differences in previous treatments were observed for either study group (Table 2). The baseline systolic and diastolic BP values were similar in both groups and, in the entire patient population, decreased from 154.4±14.2 and 87.2±11.5 mmHg at baseline to 134.5±15.9 and 77.8±11.1 mmHg (P<0.0001) at 6 months. Systolic BP decreased similarly, from 154.4±14.7 to 134.7±15.3 mmHg in the losartan/HCTZ group and from 154.3±13.9 to 134.4±16.6 mmHg (P=0.08) in the ARB group (Table 3). After the 3-month visit, supplemental antihypertensive drugs were prescribed for 2 patients in the losartan/HCTZ, and 4 patients in the ARB group (Table 4).
| ARB (n=47) | Losartan+HCTZ (n=47) | P value | |
|---|---|---|---|
| Valsartan | 12 (25.5) | 3 (6.4) | |
| Candesartan | 4 (8.5) | 6 (12.8) | |
| Losartan | 16 (34.0) | 13 (27.7) | |
| Olmesartan | 8 (17.0) | 15 (31.9) | |
| Telmisartan | 7 (14.9) | 10 (21.3) | |
| 0.067 |
Data are n (%) of observations. Abbreviations as in Table 1.
| ARB (n=47) | Losartan+HCTZ (n=47) | P value* | |||||
|---|---|---|---|---|---|---|---|
| Baseline | 6-month | P value | Baseline | 6-month | P value | ||
| BP, mmHg | |||||||
| Systolic | 154.3±13.9 | 134.4±16.6 | <0.0001 | 154.4±14.7 | 134.7±15.3 | <0.0001 | 0.08 |
| Diastolic | 87.2±11.2 | 76.5±11.5 | <0.0001 | 87.2±11.8 | 79.0±10.7 | <0.0001 | 0.21 |
| Heart rate, beats/min | 70.4±11.6 | 71.6±11.6 | 0.27 | 73.7±12.7 | 72.7±11.3 | 0.60 | 0.18 |
| Voltage, mm | |||||||
| Sokolow-Lyon | 29.9±10.2 | 29.1±8.4 | 0.25 | 34.4±9.2 | 29.4±8.8 | <0.0001 | 0.0003 |
| Cornell | 18.9±6.4 | 17.9±6.7 | 0.049 | 18.6±6.8 | 16.9±6.0 | 0.003 | 0.33 |
| BNP, pg/ml | 23.7±14.8 | 29.8±29.3 | 0.052 | 30.1±28.5 | 26.8±28.0 | 0.35 | 0.045 |
| Human fatty acid-binding protein, ng/ml | 3.1±1.4 | 3.2±1.5 | 0.33 | 2.9±1.5 | 3.6±2.3 | 0.0003 | 0.02 |
| eGFR, ml·min–1·1.73 m–2 | 76.5±12.7 | 75.4±11.3 | 0.38 | 73.3±17.8 | 69.2±19.5 | 0.005 | 0.09 |
| Uric acid, mg/dl | 4.9±1.4 | 5.1±1.4 | 0.08 | 5.5±1.2 | 5.7±1.4 | 0.16 | 0.96 |
| C-reactive protein, mg/L | 754±1,059 | 612±758 | 0.40 | 1,474±2,865 | 842±841 | 0.15 | 0.30 |
| Hemoglobin, g/dl | 13.7±1.1 | 13.5±1.2 | 0.07 | 13.8±1.5 | 13.7±1.5 | 0.35 | 0.50 |
| Hemoglobin A1c, % | 5.4±0.5 | 5.4±0.7 | 0.35 | 5.3±0.5 | 5.4±0.5 | 0.01 | 0.68 |
| Total cholesterol (mg/dl) | 199±35 | 197±30 | 0.46 | 202±29 | 199±30 | 0.29 | 0.88 |
| Serum level, mEq/L | |||||||
| Sodium | 141.3±2.3 | 141.8±2.0 | 0.19 | 141.6±2.6 | 140.8±3.8 | 0.019 | 0.01 |
| Potassium | 4.2±0.3 | 4.3±0.3 | 0.40 | 4.2±0.4 | 4.0±0.4 | 0.006 | 0.007 |
Data are mean±SD. *Between-group difference of the change between baseline and 6-month follow-up. Abbreviations as in Table 1.
| Study group | Supplemental medication | Dose, mg/day |
|---|---|---|
| Losartan+HCTZ | Celiprolol | 100 |
| ARB | Indapamide | 1 |
| ARB | Amlodipine | 5 |
| Losartan+HCTZ | Amlodipine | 5 |
| ARB | Amlodipine | 2.5 |
| ARB | Bisoprolol | 2.5 |
Abbreviations as in Table 1.
The baseline laboratory measurements were similar in both study groups, except for the serum uric acid level (Table 1). In the between-group analysis, serum sodium (141.6±2.6 to 140.8±3.8 mEq/L in the losartan/HCTZ vs. 141.3±2.3 to 141.8±2.0 mEq/L in the ARB group; P=0.01) and potassium (4.2±0.4 to 4.0±0.4 mEq/L in the losartan/HCTZ vs. 4.2±0.3 to 4.3±0.3 mEq/L in the ARB group; P=0.007) levels decreased significantly in the losartan/HCTZ group, while human fatty acid-binding protein increased significantly in the losartan/HCTZ group (Table 3), compared with the ARB group. The serum BNP level did not change significantly in the entire study sample (Table 3). It is, however, noteworthy, that the serum BNP level increased in the ARB group, whereas it decreased in the losartan/HCTZ group (Table 3).
Regression of Electrocardiographic Signs of LVHAt baseline, the Cornell voltage was similar in both groups, whereas the Sokolow-Lyon voltage was significantly higher in the losartan/HCTZ group (Table 1). At the 6-month follow-up, a significant decrease in both voltages was observed in the entire study sample (from 32.1±9.9 to 29.3±8.6 mm in Sokolow-Lyon voltage, P<0.0001 and from 18.7±6.6 to 17.4±6.3 mm in Cornell voltage, P=0.0004), though a considerably greater decrease in the Sokolow-Lyon voltage (P=0.0003) was measured in the losartan/HCTZ than in the ARB group, despite a similar degree of BP lowering in both groups (Table 3).
Determinants of LVH RegressionThe median decrease in Sokolow-Lyon voltage in the entire study sample was 2.0 mm. To identify the factors correlated with regression of LVH, we subdivided the study population (Table 5) into a group of 42 patients whose Sokolow-Lyon voltage decreased by <2.0 mm (no regression) and a group of 52 patients whose Sokolow-Lyon voltage decreased by ≥2.0 mm (regression). At baseline, the 2 groups were similar, except for the significantly younger mean age and higher mean Sokolow-Lyon voltage of the regression vs. the non-regression group (Table 5). By single variable logistic regression analysis, ∆BNP and losartan/HCTZ correlated with regression of LVH (Table 6). By multiple variable analysis, both ∆BNP and losartan/HCTZ therapy correlated with regression of LVH, though, after adjustment for age, sex, systolic BP and baseline Sokolow-Lyon voltage, only losartan/HCTZ therapy remained correlated (Table 6).
| Regression (n=52) | No regression (n=42) | P value | |
|---|---|---|---|
| Age, years | 60.9±10.7 | 66.0±8.8 | 0.014 |
| Men | 31 (59.6) | 25 (59.5) | 0.99 |
| BP, mmHg | |||
| Systolic | 154.0±14.6 | 154.8±13.9 | 0.78 |
| Diastolic | 87.7±12.5 | 86.6±10.1 | 0.66 |
| Heart rate, beats/min | 73.2±11.4 | 70.7±13.0 | 0.33 |
| Voltage, mm | |||
| Sokolow-Lyon | 35.3±9.2 | 28.2±9.4 | 0.0004 |
| Cornell | 19.8±6.5 | 17.4±6.5 | 0.07 |
| C-reactive protein, mg/L | 1,286±2,663 | 900±1,362 | 0.40 |
| BNP, pg/ml | 28.1±27.2 | 25.3±15.9 | 0.56 |
| Human fatty acid-binding protein, ng/ml | 2.9±1.4 | 3.1±1.4 | 0.39 |
| eGFR, ml·min–1·1.73 m–2 | 77.1±18.2 | 72.1±10.8 | 0.12 |
| Uric acid, mg/dl | 5.4±1.2 | 5.0±1.4 | 0.08 |
| Hemoglobin, g/dl | 13.8±1.4 | 13.8±1.2 | 0.90 |
| Hemoglobin A1c, % | 5.4±0.5 | 5.3±0.5 | 0.58 |
| Total cholesterol, mg/dl | 200±32 | 202±32 | 0.77 |
| Serum level, mEq/L | |||
| Sodium | 142±2.6 | 141±2.4 | 0.18 |
| Potassium | 4.2±0.4 | 4.2±0.3 | 0.61 |
| Diabetes, % | 6 (11.5) | 3 (7.1) | 0.47 |
| Dyslipidaemia, % | 11 (21.2) | 15 (35.7) | 0.12 |
| Ischemic heart disease, % | 3 (5.8) | 6 (14.3) | 0.16 |
| Target BP reached, % | 33 (63.5) | 24 (57.1) | 0.53 |
| β-adrenergic blockade, % | 8 (15.4) | 5 (11.9) | 0.63 |
| Calcium-channel blockade, % | 26 (50.0) | 25 (59.9) | 0.36 |
| Supplemental antihypertensive therapy, % | 2 (3.8) | 4 (9.5) | 0.26 |
Data are mean±SD, or n (%) of observations. Abbreviations as in Table 1.
| Analysis | ||
|---|---|---|
| Single variable | Multiple variable | |
| ΔBP, mmHg | ||
| Systolic | 0.30; 1.013 (0.988–1.039) | |
| Diastolic | 0.99; 1.000 (0.961–1.040) | |
| ΔHeart rate, beats/min | 0.46; 0.986 (0.951–1.023) | |
| ΔBNP, pg/ml | 0.012; 1.035 (1.008–1.064) | 0.035; 1.027 (1.002–1.054) |
| ΔHuman fatty acid-binding protein, ng/ml | 0.26; 0.808 (0.559–1.168) | |
| ΔeGFR, ml·min–1·1.73 m–2 | 0.19; 1.032 (0.984–1.083) | |
| ΔUric acid, mg/dl | 0.21; 0.741 (0.464–1.182) | |
| ΔHemoglobin, g/dl | 0.08; 0.639 (0.386–1.056) | |
| Losartan+HCTZ therapy | <0.0001; 0.158 (0.064–0.390) | 0.0003; 0.179 (0.070–0.456) |
| Adjusted* | Adjusted** | |
| ΔBNP, pg/ml | 0.07; 1.023 (0.998–1.049) | 0.07; 1.025 (0.998–1.052) |
| Losartan+HCTZ therapy | 0.0005; 0.18 (0.068–0.474) | 0.0022; 0.208 (0.076–0.568) |
Data are: P value; odd ratio (95% confidence interval). Abbreviations as in Table 1.
Δ=change between baseline and 6-month follow-up; *for age, sex and systolic BP; **for age, sex, systolic BP and baseline Sokolow-Lyon voltage.
The prognosis of arterial hypertension is aggravated by the development of LVH,13,14 whereas regression of LVH is associated with a decrease in the risk of adverse cardiovascular events.9,15 The Sokolow-Lyon16 and the Cornell17 voltages are major electrocardiographic criteria used for the detection and evaluation of LVH. Although the Sokolow-Lyon voltage is the gold standard, the Cornell voltage has gained in status in recent years, especially since the publication of the LIFE study.9 In LIFE, the degree of LVH assessed by these 2 criteria correlated with the rate of adverse cardiovascular events and with mortality, independently of the decrease in BP and treatment regimen.9 Although the Cornell voltage has been found to be superior to the Sokolow-Lyon voltage in some studies,18 this might not be applicable to Japanese hypertensive patients, as Japanese are slimmer and tend to present with higher voltage than Western patients.
Adding HCTZ to losartan further decreased LVH in the LIFE substudy.10 On the other hand, the Treatment of Mild Hypertension Study found that chlorthalidone was as effective as ACE inhibitors and calcium-channel blockers for regression of LVH.19 Finally, in the LIVE study, the regression of LVH induced by indapamide was greater than that induced by enalapril.20
Despite these observations, and a diet richer in salt than in Western country, the administration of diuretics in Japan remains limited. According to the Ministry of Health, Labour and Welfare, Japanese adults consume on average 10.6 g of salt daily, in contrast with 7 g and 5 g daily by adult American men and women, respectively, according to the National Center for Health Statistics.21 Furthermore, between 1996 and 2003, diuretics were included in <10% of antihypertensive drug regimens in Japan, compared with nearly 30% in the United States.22
The intake of salt inhibits the activity of the RAS, which decreases the therapeutic effects of ARBs.23,24 Consequently, because of their high salt consumption, hypertensive Japanese are likely to be ARB resistant. However, by promoting the excretion of sodium, diuretics, as with dietary reduction in salt intake, restore the effects of ARBs and can counteract nocturnal hypertension.25 Accordingly, an increase in the prescription of diuretics for hypertensive Japanese seems appropriate.
In the LIFE study, a 30-mmHg decrease in the systolic BP was associated with a 15.3% and 9.0% regression in the Sokolow-Lyon voltage criterion of LVH in the losartan and atenolol treatment groups, respectively.10 In our study, assignment to the losartan/HCTZ treatment group lowered the systolic BP by approximately 20 mmHg and caused a 14.5% regression in the Sokolow-Lyon voltage criterion of LVH. By contrast, an increase in the ARB regimen was associated with nearly the same decrease in systolic BP, though only a 2.7% regression of LVH. This suggests a prominent effect contributed by losartan/HCTZ in the regression of the Sokolow-Lyon voltage criterion, as the participants in our study had already been treated with an ARB.
When classified according to the effect of treatment on LVH, the group who benefited from regression was significantly younger, perhaps because the younger patients had been exposed to hypertension for a shorter amount of time, and less myocardial fibrosis had developed. This also suggests that early introduction of antihypertensive therapy in patients who have developed LVH is beneficial.
A significantly greater decrease in the serum level of BNP was observed in the group whose LVH regressed, compared with the group in which it had not regressed. In hypertensive patients, BNP and N-terminal proBNP have been correlated with LVH.26 Furthermore, the serum levels of BNP and N-terminal proBNP are considerably lower in hypertensive patients than in patients suffering from heart failure; higher BNP and N-terminal proBNP levels in hypertensive patients are reported to be associated with worse outcome, even in such lower degree.27–29 Consequently, the present study’s results confirm the unequivocal BP-lowering and cardioprotective effects of diuretics in hypertensive Japanese.
By logistic regression analysis, prescription of losartan/HCTZ and a decrease in the serum level of BNP strongly correlated with regression of LVH, as was observed in the LIFE study. Although office systolic and diastolic BP values decreased similarly in both groups, we did not recruit 24-h ambulatory BP monitoring. Because diuretics are reported to improve nocturnal hypertension, regression of LVH can be attributed to changes in nighttime and morning BP by HCTZ administration.30,31 On the other hand, a statistically significant decrease was observed in the serum sodium and potassium levels in the losartan/HCTZ group, an observation reported previously and considered clinically insignificant.8,32 It is clear, however, that diuretics should be avoided in the presence of hyponatremia or hypokalemia,33 though these disorders are rare among patients who eat a balanced diet. In addition, the blood level of human fatty acid-binding protein increased significantly in the losartan/HCTZ group, presumably because of a subtle decrease in renal function, but further investigation is needed to exclude true myocardial injury.
A total of 32 of the 94 patients fulfilled the criterion of Sokolow-Lyon voltage ≥35 mm (20 patients in the losartan/HCTZ group, 12 patients in ARB group). In this subgroup, Sokolow-Lyon voltage (from 43.0±6.8 to 36.0±8.5 in the losartan/HCTZ group and from 43.4±6.0 to 38.8±7.4 in the ARB group, P=0.35) and systolic BP decreased similarly (from 161.0±16.4 to 135.7±16.3 in the losartan/HCTZ group and from 160.2±20.3 to 133.4±19.9 in the ARB group, P=0.14). Systolic BP tended to decrease more in the ARB group, and LVH tended to regress more in the losartan/HCTZ group, but both changes were not significant. This was partly because of the small sample size and different numbers of patients in each group.
Despite potential concerns with respect to the possible adverse effects of losartan combined with HCTZ, no significant difference was observed between the 2 study groups in the evolution of lipids, glucose and uric acid in response to treatment. Although a slight deterioration of glucose tolerance was observed in the losartan/HCTZ group, variation of HbA1c was very small (from 5.3±0.5 to 5.4±0.5), and seems not to be clinically important. As was found in the Antihypertensive and Lipid-Lowering treatment to prevent Heart Attack Trial,8 low doses of diuretics do not cause adverse metabolic changes, including in Japanese patients. On the other hand, compared with low doses, diuretics administered in high doses have little additional antihypertensive properties, and are associated with a greater risk of adverse effects such as hypokalemia.34–37 In this respect, the recently introduced ARB/diuretic combination is desirable, as it includes only 6.25 or 12.5 mg of HCTZ. Although these drug combinations are reported to have no positive effect on patient’s adherence,38 practically they are cost-effective.
Study LimitationsThe first major study limitation is the higher baseline Sokolow-Lyon voltage in the losartan/HCTZ than in the ARB group. Although the baseline Cornell voltage was similar in both groups, the losartan/HCTZ group could be a LVH progressive group. Because the baseline LV mass index is reported to be the determinant of LVH regression, the losartan/HCTZ group was thought to respond better to the treatment in the first place.39 The second major study limitation was the recruitment of patients based on the electrocardiographic evidence of LVH. The use of echocardiographic entry criteria would have been desirable to more accurately measure the changes in LV dimensions. The third major limitation is the treatment regimen before entry. Although we recruited the intermediate dose of ARB as previous treatment, a considerable number of patients are administered the maximum dose of ARB in clinical practice. Switching to losartan/HCTZ from a maximum dose of an ARB could impair the efficacy mentioned in this study.
Losartan/HCTZ induced regression of LVH and a decrease in serum BNP level in hypertensive patients in this randomized multicenter trial. Because these measurements are used as surrogate markers of the efficacy of antihypertensive treatment, the administration of losartan/HCTZ seems more beneficial than the uptitration of an ARB, though the latter is unequivocally an effective organ protector. Therefore, additional studies using direct endpoints are needed to further compare these therapeutic regimens.
This study was supported by grants from the Japan Heart Foundation.
The authors have no potential conflict of interest to disclose.
Principal Investigator
Hisayoshi Fujiwara, MD, PhD, Hyogo Prefectural Amagasaki Hospital.
Collaborating Investigators and Participating Institutions
Yukihito Sato MD, PhD, Yoshiki Takatsu, MD, PhD, Hyogo Prefectural Amagasaki Hospital; Mitsuo Matsuda, MD, PhD, Takashi Uegaito, MD, PhD, Kishiwada City Hospital; Masaru Tanaka, MD, PhD, Tsukasa Inada, MD, PhD, Osaka Red Cross Hospital; Shunichi Miyazaki, MD, PhD, Mitsugu Taniguchi, MD, PhD, Kinki University; Yutaka Furukawa, MD, PhD, Natsuhiko Ehara, MD, PhD, Kobe City Medical Center General Hospital; Yoshio Kita, MD, PhD, Hyogo Prefectural Tsukaguchi Hospital.