2014 Volume 78 Issue 7 Pages 1646-1653
2014 Volume 78 Issue 7 Pages 1646-1653
Background: The safety of exercise-based cardiac rehabilitation (CR) has not been investigated in Japan, so a nationwide survey was conducted to investigate the incidence of adverse events (AEs) associated with CR and exercise testing.
Methods and Results: In total, 136 hospitals reported operating recovery-phase CR programs, amounting to 383,096 patient-hours of exercise training. The incidence rates of all AEs and life-threatening AEs (LAE: death, cardiac arrest, acute myocardial infarction, cardiac rupture) during exercise sessions were 12 and 1 event/383,096 patient-hours (3.13 and 0.26 events/100,000patient-hours), respectively. When CR programs were categorized as “Formal” in which an exercise prescription based on exercise testing was issued to individual patients or “Non-formal” without exercise prescription, the incidence of AEs during and within the 24 h after an exercise session was significantly lower in the Formal than the Non-formal CR programs (P<0.001), despite similar hospital size and coronary intervention volumes between the 2 category hospitals. Moreover, LAEs did not occur in 277,721 patient-hours in Formal CR, whereas 2 LAEs occurred in 105,375 patient-hours in Non-formal CR (P<0.05). During 469,215 exercise testing sessions, 3 LAEs (0.64 event/100,000tests) and 31 non-LAEs (6.61 events/100,000tests) occurred.
Conclusions: This first nationwide survey in Japan revealed that both exercise-based CR and exercise testing are generally safe, and that Formal CR, in which an individual exercise prescription is determined by exercise testing, is particularly safe. (Circ J 2014; 78: 1646–1653)
Exercise-based cardiac rehabilitation (CR) has been shown to be beneficial in improving exercise capacity and quality of life and/or prolonging survival in patients with coronary artery disease including acute myocardial infarction (AMI).1–5 In parallel with efficacy studies, a number of studies have been published on the safety of exercise-based CR,6–10 but most of them (except for a French registry11 ) were published before the year of 2000 (ie, before the widespread use of primary percutaneous coronary intervention [PCI]), and therefore are not directly applicable to current cardiology practice.
Editorial p1569
In Japan, recovery-phase CR has been reported as markedly underused, partly because of the rigorous regulatory standards for CR facilities and the paucity of evidence for CR in Japan.12 Indeed, there has not been a multicenter study or nationwide survey on the efficacy and/or safety of CR in Japan.
As for the safety of exercise stress testing, most previous studies, again, have been published before the era of widespread use of coronary reperfusion and revascularization.13,14 In Japan, a multicenter survey on the safety of exercise testing has been reported,15 but it was conducted approximately 20 years ago and not necessarily related to CR.
Accordingly, the purpose of the present study was to present large-scale data on the safety of both exercise-based CR and exercise stress testing in Japan. To this end, we analyzed data on adverse events (AEs) related to CR and exercise testing that were extracted from the previously conducted nationwide survey on CR in Japan.12
This study was conducted by the research group of “Study on the current status and promotion of CR in Japan (Japanese Cardiac Rehabilitation Survey)” supported by a Research Grant for Cardiovascular Diseases (15A-2) from the Ministry of Health, Labor and Welfare, Japan. The rationale and complete design of the questionnaires have been published elsewhere.12 Questionnaires were sent to a total of 1,875 hospitals including all of 859 “cardiology training hospitals” authorized by the Japanese Circulation Society (JCS), 311 “JCS associated training hospitals” and 705 randomly sampled non-training hospitals. Effective replies were obtained from 1,059 hospitals (56%) including 526 training hospitals (61% of 859 training hospitals), 194 associated hospitals (62% of 311 associated hospitals), and 339 non-training hospitals (48% of 705 non-training hospitals).
The investigation items included hospital data (eg, number of beds), cardiology practice data (eg, annual volumes of AMI and PCI), CR practice data (eg, annual volumes of recovery-phase CR and exercise testing), and data for AEs related to CR and exercise testing. The data sheets were collected and analyzed at the Department of Cardiovascular Medicine, National Cerebral and Cardiovascular Center.
Definition of CR ProgramIn Japan, because the regulatory standards require that a CR facility should be a medical institution with a cardiology/cardiac surgery section that has at least 1 cardiologist/cardiac surgeon and an experienced CR physician as full-time employees, CR facilities are in fact limited to hospitals that are running cardiology practice. Thus, in the case of patients with AMI, “acute-phase CR” usually starts at bedside in a cardiology ward a few days after admission (unless hemodynamic instability continues), proceeds to “in-hospital recovery-phase CR” in a rehabilitation room for 1–2 weeks, and shifts to “outpatient recovery-phase CR” in the same cardiology hospital. All exercise sessions are supervised by medical staff (at least 1 nurse or physical therapist and 1 cardiologist) with ECG monitoring. The length of the recovery-phase CR program is usually 3 months (maximally 5 months). In the present study, we defined recovery-phase CR as exercise training in a rehabilitation room (not in a coronary care unit or ward), including both the in-hospital and outpatient setting.
CR programs were categorized as “Formal” or “Non-formal”, according to whether or not an exercise prescription for each patient was determined according to individual exercise testing. Because the present survey was conducted on an institute-basis, not a patient-basis, we considered all patients in an institute with a Formal (or Non-formal) CR program to be the participants in the Formal (or Non-formal) CR program, regardless of the actual status of the individual exercise prescription. In addition, the total volume of recovery-phase CR was expressed as the product of the total number of patients and the duration of an exercise session (=patient-hours), considering that 1 exercise session in recovery-phase CR lasts for 1 h in routine practice in Japan.
Definition of AEsAEs related to CR were defined as unexpected events that occurred during or within 24 h after a CR exercise session and required emergency treatment or hospitalization. Specifically, AEs included occurrence of AMI, cardiac arrest, death, cardiac rupture, unstable angina, worsening of heart failure requiring hospitalization, ventricular tachycardia, cerebrovascular event, and severe orthopedic injury. Of these AEs, AMI, cardiac arrest, death, and cardiac rupture were referred to as life-threatening (LAEs), and the remaining events as non-life-threatening (non-LAEs). AEs were also classified according to the time of occurrence: during an exercise session or within 24 h after the end of an exercise session. AEs related to exercise testing were defined in a similar manner, but limited to the time period during and within 1 h after an exercise testing session.
Statistical AnalysisData on AEs were analyzed according to the type of CR program (Formal or Non-formal), severity (LAE or non-LAE), and time of occurrence (during or within 24 h after an exercise session). Numerical data are presented as mean ± standard deviation. To determine the statistical significance, numerical data were compared by unpaired t-test and categorical data were compared by chi-square test. P<0.05 was considered to be statistically significant.
Ethical ConsiderationsThis study did not deal with data from individual patients, and conformed to the 2007 revised version of the Ethical Guidelines of Epidemiological Study by the Ministry of Education, Culture, Sports, Science and Technology and the Ministry of Health, Labor, and Welfare of Japan.
Among the 1,059 hospitals responding with effective replies, 136 were providing recovery-phase CR. The rate of implementation of recovery-phase CR in each hospital category was 21.5% (113/526) in JCS-training hospitals, 9.3% (18/194) in JCS-associated training hospitals, and 1.5% (5/339) in non-training hospitals. The total volume of CR amounted to 383,096 patient-hours in 3 years, of which 277,721 patient-hours were performed in Formal CR and 105,375 patient-hours in Non-formal CR. Table 1 summarizes hospital profiles and cardiology practice data according to implementation of recovery-phase CR and the type of CR program (Formal vs. Non-formal). As anticipated, both hospital size and cardiology practice procedure volumes such as coronary arteriography and PCI were significantly greater in hospitals with than without CR. However, it should be noted that there were no differences in hospital size or procedure volume between hospitals with Formal and Non-formal CR programs, except for the numbers of cardiologists and approval for CR facility standards.
| Recovery-phase CR (–) | Recovery-phase CR (+) | P value | Formal | Non-formal | P value | |
|---|---|---|---|---|---|---|
| No. of hospitals | 923 | 136 | 75 | 61 | ||
| No. of exercise sessions (per 3 years) | 0 | 383,096 | 277,721 | 105,375 | ||
| Hospital data | ||||||
| No. of hospital beds | 304±233 | 463±241 | <0.001 | 470±243 | 455±238 | NS |
| No. of cardiology beds | 24±21 | 47±17 | <0.001 | 50±18 | 43±14 | NS |
| No. of cardiologists (full-time+part-time) | 3.9±6.1 | 11±8.3 | <0.001 | 13±10 | 8.4±5.8 | <0.05 |
| Coronary care unit | 324 (35%) | 99 (73%) | <0.001 | 56 (75%) | 43 (70%) | NS |
| Cardiac surgery section | 242 (26%) | 84 (62%) | <0.001 | 47 (63%) | 37 (61%) | NS |
| Approved for specific intensive care facility standards | 189 (20%) | 85 (63%) | <0.001 | 50 (67%) | 35 (57%) | NS |
| Approved for CR facility standards | 9 (1%) | 60 (44%) | <0.001 | 43 (57%) | 17 (28%) | <0.001 |
| Status of cardiology care | ||||||
| No. of hospitals treating AMI | 739 (80%) | 130 (96%) | <0.001 | 72 (96%) | 58 (95%) | NS |
| No. of patients with AMI (per year) | 33±41 | 74±49 | <0.001 | 80±54 | 67±43 | NS |
| No. of hospitals implementing coronary arteriography | 521 (56%) | 130 (96%) | <0.001 | 72 (96%) | 58 (95%) | NS |
| No. of coronary arteriography (procedures/year) | 314±449 | 885±616 | <0.001 | 933±639 | 825±583 | NS |
| No. of hospitals implementing PCI | 484 (52%) | 127 (93%) | <0.001 | 71 (95%) | 56 (92%) | NS |
| No. of PCI (procedures/year) | 96±148 | 272±192 | <0.001 | 271±189 | 273±196 | NS |
| No. of hospitals implementing emergency PCI | 461 (50%) | 121 (89%) | <0.001 | 69 (92%) | 52 (85%) | NS |
| No. of emergency PCI (procedures/year) | 30±44 | 80±54 | <0.001 | 83±58 | 77±49 | NS |
| No. of CABG (procedures/year) | 15±32 | 67±43 | <0.001 | 77±53 | 53±29 | NS |
AMI, acute myocardial infarction; CABG, coronary artery bypass grafting; CR, cardiac rehabilitation; PCI, percutaneous coronary intervention.
Numbers of AEs related to CR exercise sessions are summarized in Table 2. In a total of 383,096 patient-hours of exercise sessions, 50 AEs were reported, of which 12 (3.13 events/100,000patient-hours) occurred during exercise sessions and 38 within 24 h after exercise sessions. The 12 AEs during exercise sessions consisted of 1 LAE and 11 Non-LAEs, and the 38 AEs within 24 h after exercise sessions consisted of 1 LAE and 37 Non-LAEs. Thus, the incidence rate of LAEs during exercise sessions was 1/383,096 patient-hours (0.26 events/100,000patient-hours) and there were no fatal events during the exercise sessions.
| During exercise | Within 24 h after exercise | During exercise and within 24 h after exercise | Total | ||||
|---|---|---|---|---|---|---|---|
| Formal CR | Non-Formal CR | Formal CR | Non-formal CR | Formal CR | Non-Formal CR | ||
| Exercise sessions (patient-hours) | 277,721 | 105,375 | 277,721 | 105,375 | 277,721 | 105,375 | 383,096 |
| No. of LAEs | |||||||
| Death | 0 | 0 | 0 | 1 | 0 | 1 | 1 |
| Cardiac arrest | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| AMI | 0 | 1 | 0 | 0 | 0 | 1 | 1 |
| Cardiac rupture | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| LAEs subtotal | 0 | 1 | 0 | 1 | 0 | 2 | 2 |
| No. of Non-LAEs | |||||||
| Unstable angina | 1 | 0 | 0 | 21 | 1 | 21 | 22 |
| Heart failure | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| VT | 3 | 0 | 2 | 5 | 5 | 5 | 10 |
| Cerebrovascular events | 4 | 1 | 4 | 5 | 8 | 6 | 14 |
| Severe orthopedic injury | 0 | 2 | 0 | 0 | 0 | 2 | 2 |
| Non-LAEs subtotal | 8 | 3 | 6 | 31 | 14 | 34 | 48 |
| No. of all AEs | 8 | 4 | 6 | 32 | 14 | 36 | 50 |
| Incidence rate | |||||||
| Incidence rate of LAEs (/100,000 patient-hours) | 0 | 0.95 | 0 | 0.95 | 0 | 1.90* | |
| LAEs in all hospitals (/100,000 patient-hours) | 0.26 | 0.26 | 0.52 | ||||
| Incidence rate of all AEs (/100,000 patient-hours) | 2.88 | 3.80 | 2.16 | 30.37† | 5.04 | 34.16† | |
| AEs in all hospitals (/100,000 patient-hours) | 3.13 | 9.92 | 13.05 | ||||
*P<0.05 and †P<0.001 compared with Formal.
AE, adverse event; LAEs, life-threatening adverse events; Non-LAEs, non-life-threatening adverse events; VT, ventricular tachycardia. Other abbreviations as in Table 1.
When the Formal and Non-formal CR programs were compared, 14 AEs occurred during and within 24 h after exercise sessions in 277,721 patient-hours of Formal CR (5.04 events/100,000patient-hours), and 36 occurred in 105,375 patient-hours of Non-formal CR (34.16 events/100,000patient-hours, P<0.001 compared with Formal CR) (Table 2). No LAEs occurred in Formal CR, whereas 2 LAEs occurred in Non-formal CR programs. Those 2 LAEs were 1 fatal cardiac rupture, which occurred 3 h after a 15-min walking exercise 14 days after the onset of AMI, and 1 AMI event, which occurred during treadmill exercise at 2.5 km/h 15 days after the onset of AMI. Although the incidence rates of LAEs (Formal 0 vs. Non-formal 0.95 per 100,000 patient-hours, NS) and Non-LAEs (2.88 vs. 2.85 per 100,000 patient-hours, NS) during exercise were not significantly different between Formal and Non-formal programs (Figure 1), the combined incidence rates during and within 24 h after exercise were significantly lower in the Formal than in the Non-formal programs for both LAEs (0 vs. 1.90 per 100,000 patient-hours, P<0.05) and Non-LAEs (5.04 vs. 32.27 per 100,000 patient-hours, P<0.001). It is of note that 21 unstable angina events occurred in Non-formal CR programs, whereas only 1 case occurred in the Formal CR programs (P<0.001) (Table 2).
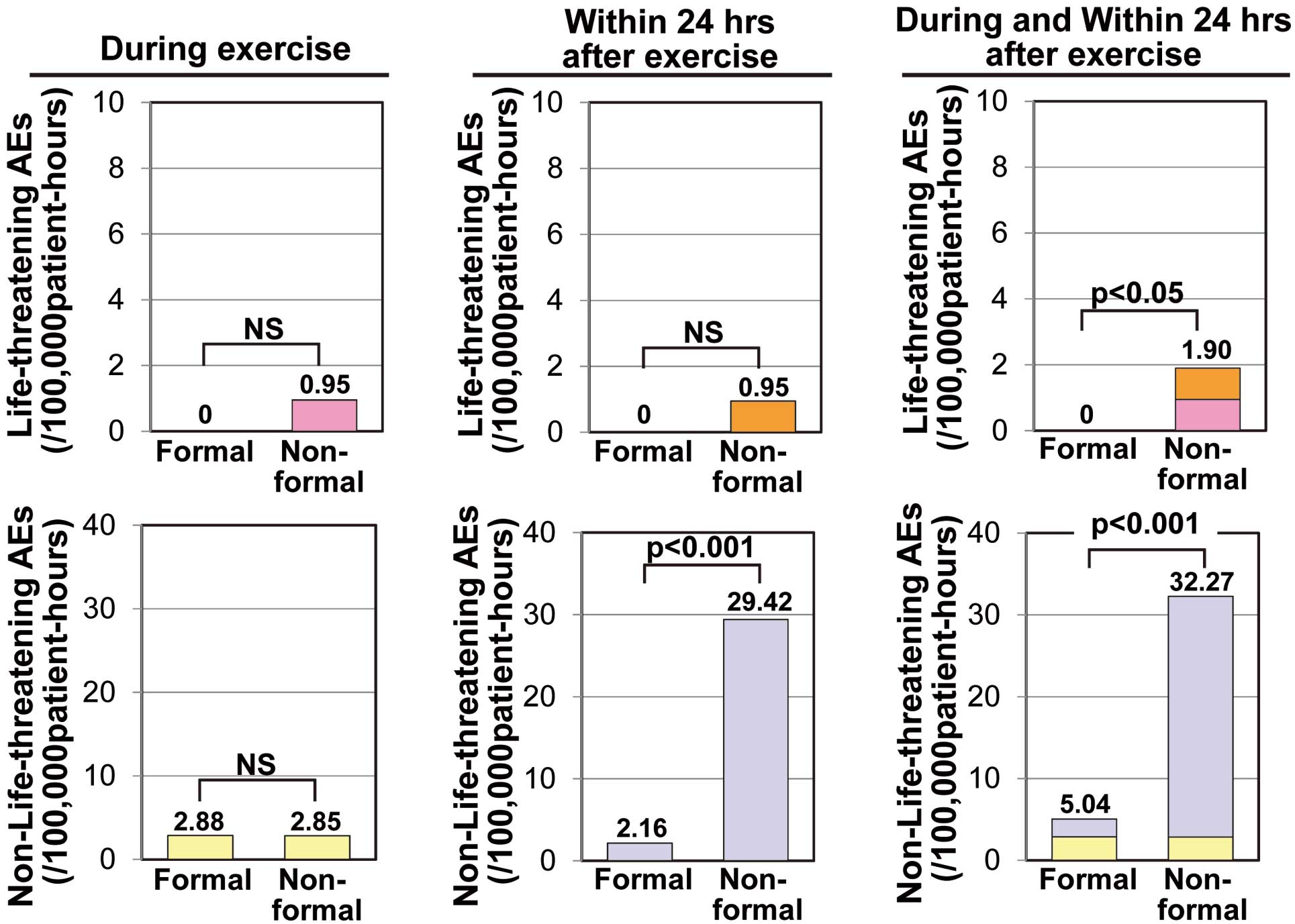
Comparison of the incidence rates of life-threatening and non-life-threatening adverse events (AEs) between Formal and Non-formal cardiac rehabilitation programs. Life-threatening AEs included acute myocardial infarction, cardiac arrest, death, and cardiac rupture, and non-life-threatening AEs included unstable angina, worsening of heart failure, ventricular tachycardia, cerebrovascular event, and severe orthopedic injury. Incidence rates are expressed as the rate per 100,000 patient-hours of exercise training.
The total number of exercise tests was 469,215 in 3 years, consisting of 433,269 treadmill tests and 35,946 cardiopulmonary exercise tests (Table 3). Noticeably, 91% (32,859/35,946) of all cardiopulmonary exercise tests were performed in Formal CR, indicating that cardiopulmonary exercise testing in Japan has been utilized mainly for CR. As anticipated, the annual volume of exercise tests per hospital was significantly smaller in hospitals without recovery-phase CR (73±119 vs. 640±654 tests/year, P<0.001) (Figure 2A). However, it is surprising that the annual volume of exercise tests per hospital was markedly smaller in hospitals with Non-formal CR than in those with Formal CR (142±194 vs. 1,160±965 tests/year, P<0.001) (Figure 2A), despite the similar hospital size and PCI volumes (Table 1).
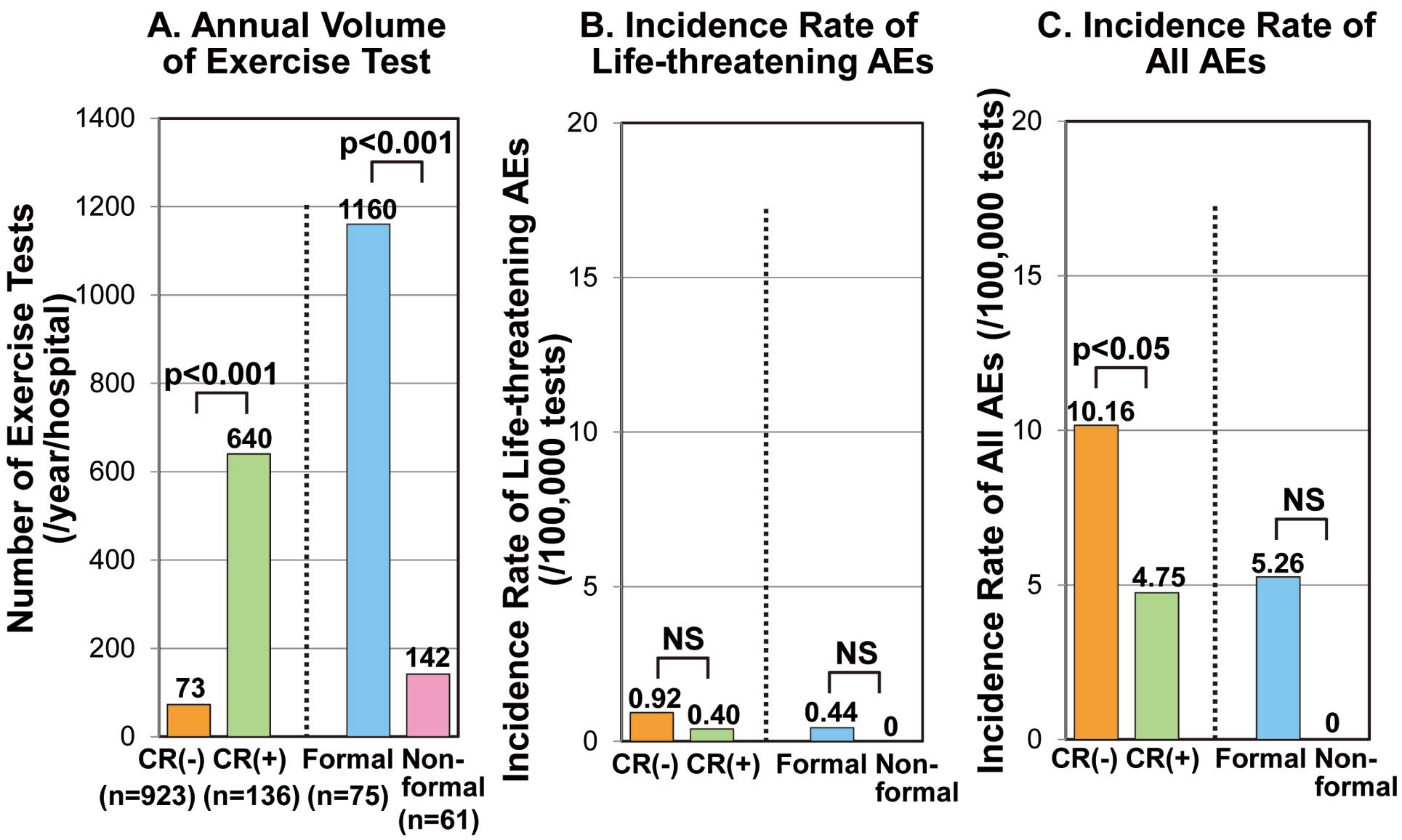
Comparison of (A) annual volumes of exercise tests and (B, C) incidence rates of adverse events (AEs) between hospitals with and without cardiac rehabilitation and with and without Formal programs. Incidence rates are expressed as the rate per 100,000 patient-hours of exercise training. CR, recovery-phase cardiac rehabilitation.
| Hospital category | |||||
|---|---|---|---|---|---|
| Total (n=1,059) | Recovery-phase CR (–) (n=923) | Recovery-phase CR (+) (n=136) | Formal CR (n=75) | Non-formal CR (n=61) | |
| Exercise tests | |||||
| Treadmill (/3 years) | 433,269 | 214,236 | 219,033 | 195,312 | 23,721 |
| Cardiopulmonary exercise test (/3 years) | 35,946 | 2,262 | 33,684 | 32,859 | 825 |
| Total exercise tests (/3 years) | 469,215 | 216,498 | 252,717 | 228,171 | 24,546 |
| Total exercise tests (procedures/year/hospital) | 142±220 | 73±119 | 640±654† | 1,160±965 | 142±194# |
| LAEs | |||||
| Death | 0 | 0 | 0 | 0 | 0 |
| Cardiac arrest | 0 | 0 | 0 | 0 | 0 |
| AMI | 3 | 2 | 1 | 1 | 0 |
| LAEs subtotal | 3 | 2 | 1 | 1 | 0 |
| Non-LAEs | |||||
| Unstable angina | 17 | 15 | 2 | 2 | 0 |
| Heart failure | 0 | 0 | 0 | 0 | 0 |
| VT | 13 | 5 | 8 | 8 | 0 |
| Cerebrovascular events | 0 | 0 | 0 | 0 | 0 |
| Severe orthopedic injury | 1 | 0 | 1 | 1 | 0 |
| Non-LAEs subtotal | 31 | 20 | 11 | 11 | 0 |
| All AEs | 34 | 22 | 12 | 12 | 0 |
| Incidence rate of AEs | |||||
| Incidence rate of LAEs (/100,000 tests) | 0.64 | 0.92 | 0.40 | 0.44 | 0 |
| Incidence rate of Non-LAEs (/100,000 tests) | 6.61 | 9.24 | 4.35* | 4.82 | 0 |
| Incidence rate of all AEs (/100,000 tests) | 7.25 | 10.16 | 4.75* | 5.26 | 0 |
*P<0.05 and †P<0.001 compared with Recovery-phase CR (–), #P<0.001 compared with Formal CR.
Abbreviations as in Tables 1,2.
There were 34 AEs (3 LAEs, 31 Non-LAEs) during and within 1 h after exercise testing (Table 3). All AEs occurred during treadmill testing, and none occurred during cardiopulmonary exercise testing (which is usually performed on a cycle ergometer in Japan). All of the 3 LAEs were AMIs, and no cases of death or cardiac arrest were observed. Of the 31 Non-LAEs, 17 unstable angina events (55%) and 13 ventricular tachycardia events (42%) comprised the majority (97%). The 1 cases of severe orthopedic injury requiring orthopedic surgery was an acromio-clavicular dislocation caused by a simple fall during treadmill exercise testing.
The incidence rates of LAEs related to exercise testing were not significantly different between hospitals with and without recovery-phase CR or between those with Formal and Non-formal CR programs (Figure 2B). However, the incidence rate of all AEs was significantly higher in hospitals without than in those with recovery-phase CR (Figure 2C), mainly because of the higher incidence of unstable angina in the hospitals without CR (Table 3). The incidence rate of all AEs was not significantly different between hospitals with Formal and Non-formal CR programs.
The major findings of the present study are: (1) the incidence rates of AEs and LAEs during exercise training in recovery phase CR in Japan were 12 and 1 event/383,096 patient-hours (3.13 and 0.26 events/100,000patient-hours, respectively); (2) the incidence rates of AEs during and within 24 h after exercise sessions were significantly lower in Formal than in Non-formal CR programs despite similar cardiology practice volumes; (3) no LAE occurred in Formal CR programs; and (4) there were 3 LAEs (0.64 event/100,000tests) and 31 non-LAEs (6.61 events/100,000tests) during 469,215 exercise testing sessions. These findings indicate that both recovery-phase CR and exercise testing in Japan are generally safe and that exercise training in Formal CR programs is particularly safe. The present study is the first nationwide survey of the safety of recovery-phase CR and exercise testing in Japan, and therefore the results enable us to compare data between Japan and Western countries and thereby contribute to the promotion of safe and effective CR and exercise testing in Japan.
AEs Related to CR Exercise SessionThe present study was a nationwide multicenter survey that covered most hospitals currently managing cardiology practice and recovery-phase CR programs in Japan. Although the response rate to the questionnaire (56%) was not very high, it was similar to that of the French registry6 in which 65 (57%) of 115 CR centers responded. Because many of the previous studies were single-center studies or were published before the widespread use of PCI, the French registry11 and the present study are the only 2 nationwide multicenter surveys on the safety of CR after the year of 2000.
Table 4 summarizes incidence rates of LAEs related to exercise-based CR in both previous reports and the present study. Studies before the PCI era6–10 reported approximately 1 cardiac arrest or nonfatal AMI per 35,000–120,000 patient-hours of exercise training. In contrast, after the year of 2000, both the French registry11 and the present study demonstrated that the incidence rate for cardiac arrest or nonfatal AMI during exercise sessions had markedly decreased to approximately 1 event per 380,000–740,000 patient-hours. Moreover, although 3 fatal events occurred during a cumulative 1,060,000 patient-hours of exercise training in the studies conducted before the year of 1990,6–8 no fatal event has been reported during a cumulative 1,680,000 patient-hours of exercise training in the studies since 1990,9–11 including the present study. These data indicate that exercise training in the medically supervised CR setting is generally safe in the present era of standardized medical therapy and coronary revascularization.
| Study | Year | Total exercise sessions
(patient-hours) |
Cardiac arrest
(event/patient-hours) |
Nonfatal AMI
(event/patient-hours) |
Cardiac arrest or nonfatal AMI
(event/patient-hours) |
Fatal events
(event/patient-hours) |
|---|---|---|---|---|---|---|
| Haskell6 | 1960–1974 | 1,629,634 | 1/38,801 | 1/325,927 | 1/34,673 | 1/116,402 |
| Van Camp and Peterson7 | 1980–1984 | 2,351,916 | 1/111,996 | 1/293,990 | 1/81,101 | 1/783,972 |
| Digenio et al8 | 1982–1988 | 480,000 | 1/120,000 | – | 1/120,000 | 1/160,000 |
| Vongvanich et al9 | 1986–1995 | 268,503 | 1/89,501 | 1/268,503 | 1/67,126 | 0/268,503 |
| Franklin et al10 | 1982–1998 | 292,254 | 1/146,127 | 1/97,418 | 1/58,451 | 0/292,254 |
| Pavy et al11 | 2003 | 743,471 | 1/743,471 | 0/743,471 | 1/743,471 | 0/743,471 |
| Present study (All CR programs) | 2001–2003 | 383,096 | 0/383,096 | 1/383,096 | 1/383,096 | 0/383,096 |
| Present study (Formal CR programs) | 2001–2003 | 277,721 | 0/277,721 | 0/277,721 | 0/277,721 | 0/277,721 |
Abbreviations as in Tables 1,2.
An important new finding in the present study is that Formal CR programs reported no LAE and also significantly lower incidence rates of LAEs, non-LAEs and all AEs than Non-formal CR programs for the combined time period of during and within 24 h after exercise sessions (Figure 1 , Table 2). This cannot be attributed to differences in coronary revascularization strategy between Formal and Non-formal CR hospitals, because the average annual volumes of coronary arteriography and PCI were similar in the 2 hospital categories (Table 1). Considering that the incidence of unstable angina was significantly less frequent in Formal than in Non-formal CR, the most likely explanation for the lower rate of all AEs in Formal CR programs is that either high-risk patients are screened out by the initial exercise testing or an appropriate individual exercise prescription based on exercise testing prevented the occurrence of AEs. Another possible explanation is that the significantly greater number of cardiologists in the Formal CR hospitals might have helped reduce AEs through more sufficient patient management or education. Unfortunately, because the diagnosis of each AE was left to each institute, the exact reasons for the different AE rates between the 2 hospital categories are unknown.
AEs Related to Exercise TestingThe present study demonstrated that the incidence rate of AEs related to exercise testing was generally low (7.25 events/100,000 tests), and that particularly, the rate of LAEs was very low (0.64 events/100,000 tests). In addition, the fact that the incidence rate of exercise testing-related AEs was significantly lower in hospitals with than without recovery-phase CR (Table 3) suggests that exercise testing related to recovery-phase CR is safe. Although the difference in the incidence rate of AEs between hospitals with and without recovery-phase CR might be related to differences in patient characteristics or skillfulness of medical staff for exercise testing, we do not have data to confirm this.
Table 5 summarizes incidence rates of LAEs related to exercise testing in previous reports11,13–15 and the present study. For cardiac arrest or nonfatal AMI, the previous studies reported approximately 1 cardiac arrest or nonfatal AMI per 1,100–42,000 tests, whereas the present study found a markedly lower incidence rate (approximately 1 event per 160,000 tests overall, and 1 event per 250,000 tests in CR hospitals). Additionally, it should be noted that both the French registry11 and the present study conducted after the year of 2000 did not report any fatal event related to exercise testing. These findings suggest that the risk of AEs in exercise testing has substantially decreased in modern cardiology practice.16
| Study | Year | No. of exercise
tests |
Cardiac arrest
(event/tests) |
Nonfatal AMI
(event/tests) |
Cardiac arrest or
nonfatal AMI (event/tests) |
Fatal events
(event/tests) |
|---|---|---|---|---|---|---|
| Stuart et al13 | 1976 | 518,448 | – | 1/2,857 | – | 1/20,000 |
| Hamm et al14 | 1975–1986 | 151,949 | 1/2,412 | 1/1,948 | 1/1,078 | 1/3,706 |
| Musha et al15 | 1983–1994 | 1,779,352 | 1/63,548 | 1/77,363 | 1/34,889 | 1/296,599 |
| Pavy et al11 | 2003 | 42,419 | 1/42,419 | 0/42,419 | 1/42,419 | 0/42,419 |
| Present study (overall) | 2001–2003 | 469,215 | 0/469,215 | 1/156,405 | 1/156,405 | 0/469,215 |
| Present study (CR hospitals) | 2001–2003 | 252,717 | 0/252,717 | 1/252,717 | 1/252,717 | 0/252,717 |
Abbreviations as in Tables 1,2.
An additional important finding in the present study is that recovery-phase CR and exercise testing remain markedly underused in Japan. In the present study, the implementation rate of recovery-phase CR was only 12.8% (136/1,059 hospitals) overall and 21.5% (113/526 hospitals) in the JCS training hospitals, and a Formal CR program was implemented in only 55% (75 hospitals) of the 136 hospitals providing recovery-phase CR. One reason for the marked underuse of CR in Japan has been attributed to physicians’ or hospitals’ ignorance of CR, because many large-sized hospitals that should have both sufficient space and human resources had not implemented CR.12 In addition, a recent review suggested that the relatively strict Japanese CR facility standards (ie, requirement of a cardiology/cardiac surgery section that has at least 1 cardiologist/cardiac surgeon and an experienced CR physician as full-time employees), which appear to not be necessary in providing safe and effective CR, are potentially limiting greater dissemination of CR delivery in Japan.17 Thus, enhancing the motivation toward providing CR of both physicians and hospitals and relaxation of regulatory facility standards are critically important for greater dissemination of CR in Japan.
The annual volume of exercise testing was markedly lower in hospitals without than with recovery-phase CR and in hospitals with Non-formal than with Formal CR programs (Figure 2A). The lower volume of exercise testing in non-CR hospitals than CR hospitals may be attributable to differences in the volume of cardiology practice between the 2 hospital categories. However, the extremely lower volume of exercise testing in Non-formal CR hospitals than in Formal CR hospitals is inexplicable, even after the volume of additional exercise tests for exercise prescription in the Formal CR programs is taken into account, because the volume of cardiology practice, such as coronary arteriography or PCI, was similar between the 2 hospital categories (Table 1). In fact, in Non-formal CR hospitals, the average volume of exercise testing (142±194 tests/year) appeared disproportionately low compared with the volumes of coronary arteriography (825±583 procedures/year) and PCI (273±196 procedures/year). Perhaps, the appropriateness of coronary arteriography and PCI should be questioned in these hospitals in the light of the appropriateness criteria and the recommendation of noninvasive diagnostic testing in the guidelines of the management of stable coronary artery disease.18–20 On the basis of safety data presented in both the present study and the French registry,11 exercise testing should be implemented more widely and appropriately in modern Japanese cardiology practice.21
Study LimitationsBecause the present study was a retrospective, hospital-based survey using questionnaires, the reliability of data depends on the accuracy of data collection in the surveyed hospitals. However, the total numbers of hospitalized AMI patients in Japan estimated from the present survey (71,201 patients in 2003)12 closely agreed with that in another nationwide survey (66,459 patients in 2000),22 suggesting that data in the present survey are reasonably accurate.
Our categorization of all patients in an institute with a Formal (or Non-formal) CR program as being participants in the Formal (or Non-formal) CR program regardless of the actual status of individual exercise prescriptions might have caused some bias. However, this bias, if any, should increase the numbers of patients who did not have individual exercise prescriptions in the Formal program and those who had exercise prescriptions in the Non-formal program, yielding a tendency toward an increase in AEs in Formal programs and a decrease in Non-formal programs (ie, a bias toward the opposite direction to the present results).
The response rate in the present study (56%) was relatively low, which might have yielded a potential bias in the results. However, when the hospitals that did and did not reply the present questionnaire were compared, there were no significant differences in the hospital size or regional distribution,12 suggesting that a statistical bias caused by the low reply rate, if any, should be negligible. In addition, the response rate of 56% was similar to that of the French registry (57%),11 and even higher than that in another nationwide survey in Japan (44%).23
The present study, which is the first nationwide survey of the safety of recovery-phase CR and exercise testing in Japan, has demonstrated that both are generally safe in modern cardiology practice, and that exercise training in Formal CR programs based on individual exercise prescription according to exercise testing is particularly safe.
This study was supported in part by a Research Grant for Cardiovascular Diseases (15A-2) from Ministry of Hearth, Labor and Welfare, Japan. We greatly thank the directors and staff of the 1,059 hospitals for their cooperation in the conducting of this survey.
The Japanese Cardiac Rehabilitation Survey Investigators
Yoichi Goto, MD (Chair, National Cerebral and Cardiovascular Center); Taiki Higo, MD (National Cerebral and Cardiovascular Center); Muneyasu Saito, MD, Nahoko Ikeda, MD (Jichi Medical School Ohmiya Medical Center); Toshiji Iwasaka, MD, Yutaka Kimura, MD (Kansai Medical University); Hiroyuki Daida, MD, Kazunori Shimada, MD, Miho Nishitani Yokoyama, MD (Juntendo University Graduate School of Medicine); Masahiro Kohzuki, MD, Nobuyoshi Mori, MD (Tohoku University); Kenji Ueshima, MD (Kyoto University); Masahiko Saito, MD (Morioka Red Cross Hospital); Shigeru Makita, MD (Saitama Medical University); Hitoshi Adachi, MD (Gunma Prefectural Cardiovascular Center); Hiroyoshi Yokoi, MD (Kokura Memorial Hospital); Kazuto Omiya, MD (St. Marianna University School of Medicine); Hiroshi Mikouchi, MD, Mika Yamamoto, MD (National Hospital Organization Okayama Medical Center); Hiroyuki Yokoyama, MD (National Cerebral and Cardiovascular Center); Jun Tanabe, MD (National Hospital Organization, Shizuoka Medical Center).