2014 Volume 78 Issue 8 Pages 1960-1966
2014 Volume 78 Issue 8 Pages 1960-1966
Background: The aim of the present study was to assess the impact of pulmonary regurgitation (PR) and right ventricle (RV) volume on left ventricle (LV) longitudinal strain (LS) in patients with surgically repaired tetralogy of Fallot (r-TOF).
Methods and Results: The study subjects were 32 adolescent asymptomatic r-TOF subjects and 30 adolescent healthy controls. Two-dimensional (2-D) and 3-dimensional (3-D) echocardiography were performed in all subjects using Vivid E9 (GE Healthcare). On 3-D analysis, LV and RV volumes were measured using 4D auto LVEF and TomTec RV analysis. PR fraction (PRF) was calculated by the volumetric method (PRF=[RV stroke volume–LV stroke volume]/RV stroke volume). LV-LS was assessed on 2-D speckle tracking echocardiography, and “free wall LS” represented the average of 3 lateral segments. Although RVEF and LVEF were not different between the 2 groups, free wall LS (–17.1±3.2%) was reduced significantly in the r-TOF group compared to the control (–20.5±4.9%, P=0.008), and correlated with the RV variables RVEDVI, RV/LV EDVI ratio and PRF. On multivariate stepwise linear regression analysis, RVEDVI was the only risk factor for the reduction of free wall LS (P=0.004).
Conclusions: Free wall LS was significantly reduced despite preserved LVEF. The severity of LS reduction was correlated with RV dilation even in asymptomatic r-TOF patients. (Circ J 2014; 78: 1960–1966)
The prognosis of tetralogy of Fallot (TOF) has improved markedly in recent years thanks to advances in surgical techniques; the current 30-year survival rate is estimated to be >90%.1 Late survival is excellent in Japan,2 partially because the prevalence of serious arrhythmias is low in Japanese surgically repaired TOF (r-TOF) patients as compared with Western countries.3,4 The majority of patients, however, develop a variable degree of chronic pulmonary regurgitation (PR), which sometimes leads to right ventricular (RV) volume overload and dysfunction, with potential development of ventricular arrhythmias.5–8 Left ventricular (LV) ejection fraction (EF) is another potential risk factor for morbidity and mortality.9 Furthermore, recent studies have shown that reduced LV longitudinal strain (LV-LS), determined on 2-D speckle tracking strain measurements, is another serious risk factor for mortality.10 In this regard, the aforementioned technique is not angle dependent, and not affected by tethering effects. In subjects with r-TOF and impaired RV function, LV-LS is also associated with greater risk of cardiac death/life-threatening ventricular arrhythmias.11 In addition, assessment of LV and RV dysfunction indicated a close relationship between cardiac magnetic resonance imaging (cMRI) and speckle tracking, but only a few studies have assessed RV and LV function on 2-D and 3-D transthoracic echocardiography (TTE) in r-TOF subjects.12 The aim of this study was therefore to evaluate the impact of PR and RV remodeling on LV-LS using TTE in adolescent subjects.
This single-center cross-sectional study included 46 asymptomatic Japanese r-TOF patients (r-TOF group) who were seen at the outpatient clinic of the University of Tsukuba, between November 2011 and June 2013. The following exclusion criteria were applied: (1) history of coronary artery disease; (2) history of persistent tachycardia; (3) history of medication for the cardiovascular system; (4) significant structural cardiac anomalies (excluding pulmonary valve abnormalities); (5) inadequate imaging for offline volume analysis; and (6) presence of chromosomal abnormality (Figure 1). All of the present patients were classified into New York Heart Association (NYHA) class 1. The study also included control subjects. Thirty healthy controls were recruited. These control subjects were followed at the outpatient clinic for non-specific chest pain and palpitations for which no organic causes were identifiable. All subjects underwent echocardiographic assessment and 12-lead electrocardiogram.
Patient flow. r-TOF, surgically repaired tetralogy of Fallot; 3-D, 3-dimensional.
The study protocol was approved by Tsukuba University Institutional Review Board for human subjects and informed consent was obtained from the patient or guardian. We determined that at least 30 was needed in each group, because this is the minimum number to prove the statistical significance based on the Faul et al methods.13,14
TTEComprehensive echocardiography, including M-mode, Doppler, 2-D and 3-D echocardiography, was done in all subjects (GE Vivid E9; GE Healthcare, Tokyo, Japan). First, we recorded the peak flow velocity through the pulmonary valve, on continuous wave Doppler, to determine the severity of pulmonary stenosis (PS). Pressure gradient was calculated from the velocity using the simplified version of Bernoulli’s equation. Second, the LV long-axis function on M-mode echocardiography (MAPSE) was measured, as described in detail previously.11 Tissue Doppler echocardiography was then used to assess the lateral longitudinal myocardial velocity at the mitral annular level,15 which is described as LV lateral S’. Each variable was measured in apical 4-chamber view. The cine loops for assessment of 2D LV-LS were also recorded in apical 4-chamber view by changing the transducer scan width to achieve a frame rate of at least 40/s. LV-LS was assessed on 2-D speckle tracking echocardiography (Echo Pac; GE Healthcare). The endocardial borders were traced in the end-systolic frame of the 2-D images, and speckles were tracked frame by frame throughout the LV wall during the cardiac cycle. The values were measured at 6 segments: base lateral; mid lateral; apical lateral; base septal; mid-septal; apical septal (Figure 2). In addition, we used the global longitudinal strain (GLS), which represented the average of all 6 segments; and free-wall LS as the average of the lateral 3 segments: apical lateral, mid-lateral, base lateral.
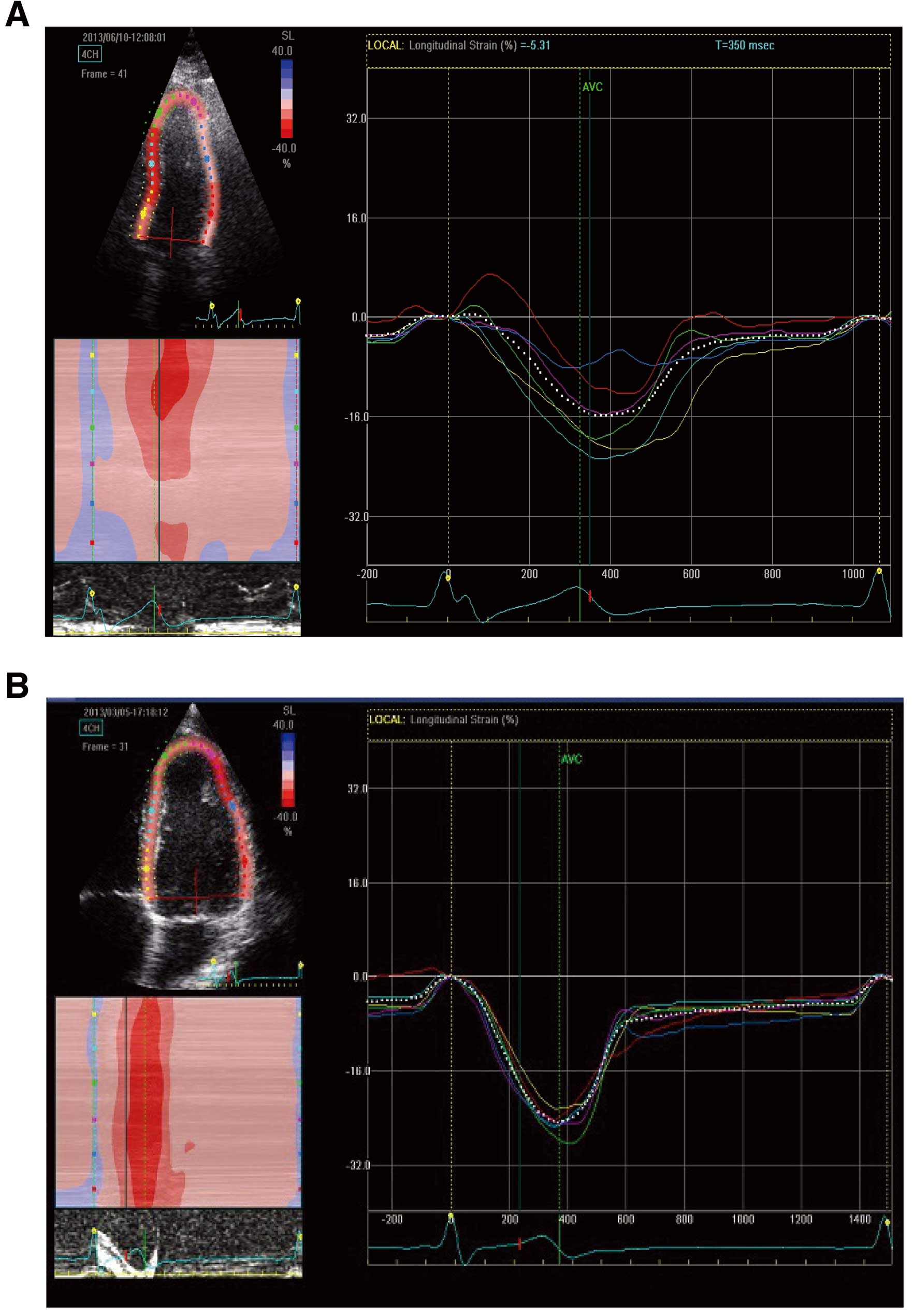
Longitudinal changes in parameters of 2-dimensional speckle tracking on apical 4-chamber view. Representative images of regional strain-time curves in (A) a surgical repair of tetralogy of Fallot patient, and (B) a control subject.
For the assessment of LV diastolic function, left atrial pressure was estimated using the algorithm recommended by the American Society of Echocardiography and European Society of Echocardiography,16 which is based on mitral inflow E- and A-wave peak velocities, early mitral annular velocities (e’), and left atrial area index. The left atrial area was measured from the apical 4-chamber view at end-diastolic phase, with manual tracing.
All 3-D echocardiographic images were acquired with a standard 4.0-MHz multi-plane transducer and ultrasound scanner adapted for 3-D image acquisition. 3-D LV and RV full-volume sweeps were acquired from apical views during end-expiratory breath-hold, when available. Especially for the RV, the probe was tilted interiorly in order to include the RV free wall and outflow in the same angle of view. The acquired images were stored in DICOM format and transferred to a separate workstation for offline data analysis. LV/RV volumes and function were analyzed using dedicated software. The 3-D data sets were analyzed as follows: each echocardiographic data set was opened with 4D auto LVEF (Echo Pac; GE) and TomTec RV. All images were viewed in sagittal, 4-chamber, and coronal views. The best possible views, containing a full-volume set, were chosen for the analysis. Each volume data set was imported into the application and manipulated by rotating, angulating, and slicing in any of the 3 displayed orthogonal planes. The software used an automated border detection algorithm with manual correction options. Measurements were made on the original full-volume echocardiographic images. The end-diastolic and end-systolic frames were chosen by visual inspection of the cine loops. The endocardial borders of the LV and RV were outlined at end-diastole and at end-systole at all levels. LV and RV trabeculations and LV papillary muscles were included within volume analysis, whereas the inter-ventricular septum was excluded from each volume assessment. At the end of the analysis, a final spatiotemporal reconstruction of the LV and RV model was created. Especially, 3-D construction images of the RV were also obtained in projections of the RV free wall, inter-ventricular septum, and outflow tract.
The software-derived functional parameters LV end-diastolic volume index (LVEDVI), LV end-systolic volume index, LV stroke volume index (LVSVI) and LVEF were measured using 4D auto LVEF. In the same way, RV end-diastolic volume index (RVEDVI), RV end-systolic volume index (RVESVI), RV stroke volume index (RVSVI) and RVEF were measured using TomTec RV analysis. Cardiac index was measured using the following formula: LVSVI×heart rate at the examination.
Calculation of PR Fraction (PRF)The PRF was calculated using the following formula: (RVSVI–LVSVI)/RVSVI, as described previously.17 This method could not be applied to subjects with other structural heart diseases, because RVSVI and LVSVI are influenced by coexistent valvular disease or residual shunts. For this reason, we excluded from this study subjects with structural cardiac anomalies, apart from those with pulmonary valve disease.
Statistical AnalysisAll data are presented as mean±SD. Comparisons between groups were made using Student’s t-test or non-parametric tests depending on data distribution. Similarly, associations among variables were explored using the Wilcoxon rank-sum test or Spearman rank correlation coefficient, as appropriate. RV variables and QRS duration with P<0.05 were entered into multivariate stepwise linear regression analysis to determine the risk factor for impairing LV free wall LS. Data were analyzed using SPSS ver. 21 (SPSS, Chicago, IL, USA). Two-tailed P<0.05 was considered statistically significant.
Table 1 lists the subject characteristics. Age, gender, height, weight, body surface area and heart rate at rest were not different between the 2 groups. The age at surgery of the 32 r-TOF patients ranged from 0.5 to 7 years (2.6±1.5 years). Among them, 30 underwent surgical repair that included pulmonary transannular patch, while an external conduit was used in the remaining 2 patients. QRS duration was significantly longer in the r-TOF group (116±30.2 ms) than the control group (86.1±8.0 ms, P=0.001), but did not exceed 180 ms in any of the patients.
| r-TOF (n=32) | Control (n=30) | P-value | |
|---|---|---|---|
| Age (years) | 18.6±5.8 | 16.9±7.4 | 0.11† |
| M/F (n) | 16/16 | 16/14 | 0.81§ |
| Age at TOF repair (years) | 2.6±1.5 | – | – |
| Surgical procedure | |||
| Pulmonary transannular patch | 30 | – | – |
| External conduit repair | 2 | – | – |
| Height (cm) | 157±9.8 | 155±17.6 | 0.53‡ |
| Weight (kg) | 51.7±11.4 | 44.5±16.6 | 0.07‡ |
| Body surface area (m2) | 1.49±0.20 | 1.45±0.29 | 0.57‡ |
| Heart rate (beats/min) | 80.9±15.0 | 75.0±15.5 | 0.13‡ |
| QRS duration (ms) | 116±30.2 | 86.1±8.0 | 0.001† |
Data given as mean±SD. †Mann-Whitney U-test; ‡Student’s t-test; §χ2 test.
r-TOF, surgically repaired tetralogy of Fallot; TOF, tetralogy of Fallot.
Table 2 lists the results of 3D-TTE analysis of the RV and LV volumes, and the PS pressure gradient. RVEDVI, RVESVI, RVSVI and PS were significantly larger in the r-TOF group than in the control. In contrast, LVEDVI and LVSVI were significantly smaller in the r-TOF group than in the control. In addition, the PRF and RV/LV EDVI ratio were larger in the r-TOF group compared with the control. There was a positive correlation between PRF and RVEDVI (r=0.542, P<0.001), but cardiac index, RVEF and LVEF were not different between the 2 groups. In the control group, there was no significant difference between RVEDVI and LVEDVI, or between RVSVI and LVSVI (Table 2).
| r-TOF (n=32) | Control (n=30) | P-value | |
|---|---|---|---|
| RVEDVI (ml/m2) | 82.3±22.3 | 60.3±13.8 | <0.001† |
| RVESVI (ml/m2) | 35.0±13.3 | 26.7±7.7 | 0.004‡ |
| RVSVI (ml/m2) | 47.3±11.9 | 34.3±8.0 | <0.001† |
| RVEF (%) | 59.2±7.5 | 56.6±7.0 | 0.16‡ |
| LVEDVI (ml/m2) | 53.7±9.8 | 61.6±13.4 | 0.01‡ |
| LVESVI (ml/m2) | 23.3±5.1 | 26.8±6.3 | 0.21† |
| LVSVI (ml/m2) | 30.7±7.2 | 34.7±8.3 | 0.03† |
| LVEF (%) | 56.9±6.9 | 56.1±4.7 | 0.59‡ |
| Cardiac index (L·beats–1·min–1·m–2) | 2.25±0.99 | 2.44±0.71 | 0.31† |
| PRF | 0.33±0.17 | –0.01±0.08 | <0.001‡ |
| PS (mmHg) | 25.7±21.2 | 3.3±1.0 | <0.001† |
| RV/LV EDVI ratio | 1.55±0.56 | 0.98±0.13 | <0.001† |
Data given as mean±SD. †Mann-Whitney U-test; ‡Student’s t-test.
EF, ejection fraction; EDVI, end-diastolic volume index; ESVI, end-systolic volume index; LV, left ventricle; PRF, pulmonary regurgitation fraction; PS, pulmonary stenosis; r-TOF, surgically repaired tetralogy of Fallot; RV, right ventricle; SVI, stroke volume index.
Table 3 lists the results of 2D-TTE analysis of parameters of LV longitudinal function and diastolic function. LV free wall LS was significantly reduced in the r-TOF group than the control (Figure 3). In addition, each apical LS was reduced compared to mid- and base LS at both the lateral and septal side in the r-TOF group, unlike in the control group. In contrast, MAPSE and LV lateral S’ were not significantly different between the 2 groups. None of the parameters of LV diastolic function was significantly different between the 2 groups.
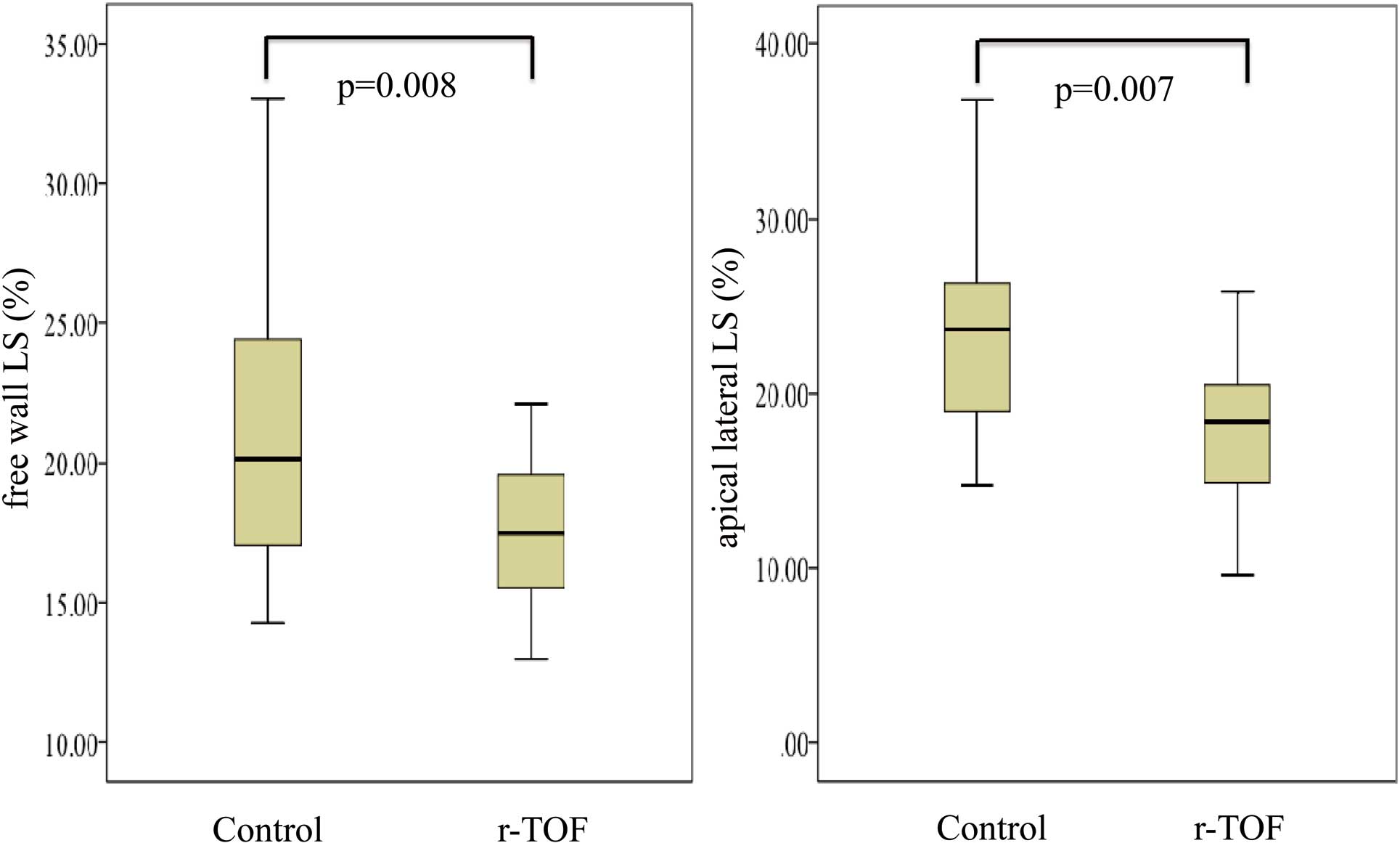
Comparison of free wall longitudinal strain (LS) and apical lateral LS. Free wall LS, especially apical lateral LS, was significantly reduced in the surgically repaired tetralogy of Fallot (r-TOF) group compared with the control. LS is given as an absolute value.
| r-TOF (n=32) | Control (n=30) | P-value | |
|---|---|---|---|
| GLS (%) | –17.7±2.9 | –21.8±3.7 | <0.001‡ |
| Free wall LS (%) | –17.1±3.2 | –20.5±4.9 | 0.008† |
| Base lateral LS (%) | –14.6±4.4 | –16.7±5.5 | 0.10‡ |
| Mid-lateral LS (%) | –18.6±5.0 | –21.9±6.0 | 0.03‡ |
| Apical lateral LS (%) | –18.1±4.6 | –21.6±10.4 | 0.007† |
| Base septal LS (%) | –19.1±4.8 | –21.2±5.1 | 0.16† |
| Mid-septal LS (%) | –19.9±3.0 | –23.3±3.0 | <0.001† |
| Apical septal LS (%) | –17.2±5.0 | –25.7±5.0 | <0.001† |
| MAPSE (mm) | 14.8±2.8 | 13.9±2.4 | 0.18‡ |
| LV lateral S’ velocity (cm/s) | 8.5±1.3 | 9.4±2.2 | 0.06‡ |
| E/A | 2.5±0.83 | 2.2±0.58 | 0.09‡ |
| E/e’ | 6.6±1.9 | 6.0±1.2 | 0.17‡ |
| LA area index (cm2/mm2) | 6.4±1.7 | 6.3±2.0 | 0.83† |
Data given as mean±SD. †Mann-Whitney U-test; ‡Student’s t-test.
See Table 2 for abbreviations. GLS, global longitudinal strain; LA, left atrium; LS, longitudinal strain; MAPSE, mitral annular plane systolic excursion.
Table 4 lists the correlations between RV-related factors and LV regional wall strain. LV free wall LS mildly correlated with the RV variables: RVEDVI, RV/LV EDVI ratio, PRF and PS.Table 5 presents the effect of RV variables and QRS duration on the reduction of LV free wall LS on multivariate stepwise linear regression analysis. RVEDVI was the only risk factor for impairment of LV free wall LS.
| RVEDVI | RV/LV EDVI ratio | PRF | PS | |||||
|---|---|---|---|---|---|---|---|---|
| r | P-value | r | P-value | r | P-value | r | P-value | |
| GLS (%) | 0.45 | <0.001‡ | 0.46 | <0.001‡ | 0.43 | 0.001‡ | 0.38 | 0.004‡ |
| Free wall LS (%) | 0.38 | 0.003‡ | 0.36 | 0.005‡ | 0.29 | 0.03‡ | 0.30 | 0.02‡ |
| Base lateral LS (%) | 0.22 | 0.10‡ | 0.18 | 0.17‡ | 0.04 | 0.76‡ | 0.23 | 0.10‡ |
| Mid-lateral LS (%) | 0.30 | 0.03‡ | 0.27 | 0.04‡ | 0.23 | 0.09‡ | 0.32 | 0.02‡ |
| Apical lateral LS (%) | 0.29 | 0.03‡ | 0.41 | 0.002‡ | 0.43 | 0.001‡ | 0.19 | 0.18‡ |
| Base septal LS (%) | 0.43 | 0.001‡ | 0.19 | 0.16‡ | 0.15 | 0.25‡ | 0.14 | 0.33‡ |
| Mid-septal LS (%) | 0.45 | <0.001‡ | 0.41 | 0.001‡ | 0.31 | 0.02‡ | 0.33 | 0.02‡ |
| Apical septal LS (%) | 0.39 | 0.003‡ | 0.57 | <0.001‡ | 0.58 | <0.001‡ | 0.48 | <0.001‡ |
‡Spearman correlation t-test. See Tables 2,3 for abbreviations.
| Multivariate β | P-value | |
|---|---|---|
| RVEDVI (ml/m2) | 0.38 | 0.003 |
| RV/LV EDVI ratio | NS | |
| PRF | NS | |
| PS (mmHg) | NS | |
| QRS duration (ms) | NS |
See Tables 2,3 for abbreviations.
In the present study it was found that LV free wall LS was reduced in r-TOF subjects, despite the absence of cardiac failure signs. The severity of LS reduction was correlated with RV dilation. Previous studies showed that RV dilation correlated with impaired LV systolic performance,14 but no study has demonstrated the relation between RV volume and LV free wall LS using TTE.
RVEDVI/SVI and LVEDVI/SVI determined on 3D-TTE were almost the same in the control group. This result was consistent with the fact that RVEDVI and LVEDVI were equivalent.18 The assessment of RV volume and function on 3D-TTE, however, remains a challenge in congenital heart disease.19–21 Therefore, we applied 3D-TTE to both RV and LV volume analysis. A good correlation was found between the SVI of the LV and RV, and a reasonable RV/LV EDVI ratio in the control group (Table 2). As for the r-TOF group, the RV/LV EDVI ratio (1.55±0.56) in the present study was similar to those in previous reports.22 Zhang et al reported that the correlations between 3-D echocardiography and cMRI measurements were acceptable.23 In our next step, we will compare RV volume obtained on 3D-TTE with that on cMRI to validate the accuracy and reproducibility.
LV-LS is available from routine TTE assessment, and GLS has been confirmed previously to be reliable, independent of the observer experience.24 In addition, LV-LS and MAPSE were reported to be the most sensitive parameters for the prediction of mortality in adults with r-TOF.25 The major finding of the present study is that LV free wall LS assessed using this method was significantly reduced, and associated with RVEDVI in adolescents with r-TOF (Tables 3, 5). Especially, each apical LS at both the lateral and septal side was reduced compared to the mid- and base LS in the r-TOF group, unlike in the control group. In contrast, MAPSE, LV lateral S’ velocity and the parameters of diastolic function were not significantly different between the 2 groups, and correlated with RV variables. Therefore, this indicates that the reduction of LV-LS would be going on gradually from apical lesion, even if the r-TOF subjects had no heart failure signs. We should follow up not only RV variables, but also LV-LS over time.
The strain at the septal wall was significantly reduced rather than that at the lateral wall in the present study (Table 3). In previous reports, it was also found that LV-LS was reduced at both the septal wall and free wall. The former was more greatly reduced than the latter, similar to the present study.26 As for RV, the RV free wall as well as the septal wall were impaired in the r-TOF subjects.27 RV remodeling due to PR, the patch used in the radical operation, and abnormal muscle fiber architecture could affect RV strain in the subjects with r-TOF.26 Additionally, impairment of LV free wall LS might be induced by hypoxemia.28
The present study, however, did not investigate the mechanisms of impaired LV dysfunction in r-TOF subjects. Previous studies have suggested that myocardial ischemia due to hypoxemia before corrective surgery might be related to LV dysfunction.28 In the present study, all the r-TOF subjects were <30 years of age, without a history of coronary events, hypertension or diabetes based on the medical records. Alternatively, other studies have suggested that myocardial fibers are shared by the 2 ventricles and that the adverse impact of septal shift was responsible for LV dysfunction, although one cannot also rule out a simple geometrical interaction between the 2 ventricles in the limited space of pericardial sac and through septal shift.29 In the present study, LVEDVI and LVSVI were significantly reduced in r-TOF, although cardiac index was preserved. To clarify the mechanism of LV dysfunction, we should take into account the impact of septal shift, radial strain (RS) and circumferential strain (CS). We must assess, however, the interaction among septal shift, LV-RS and LV-CS before assessing LV function, because the through-plane phenomenon at the short axis would cause incorrect measurement of LV-RS and CS.30
The present study focused on the impact of RV volume on LV free wall LS. Other factors, however, such as age and duration of cyanosis, were not associated with LV free wall LS in the present study. These factors could influence survival. Therefore, we need take a long-term view of the relationship between RV volume and free wall LS. Further studies are needed to identify the mechanisms of reduced LV function based on RV variables throughout the whole life of r-TOF patients.
Study LimitationsThe present study had 3 major limitations. First, we did not use the gold standard method for validation of volume analysis, such as cMRI. Second, not all images obtained on 3D-TTE were appropriate for analysis. Ingenuity is needed in order to record clear 3-D echocardiography images, especially for RV analysis (eg, changes in body position, a wide angle of view and breath-holding). In the present study, we excluded 3 r-TOF patients and 2 control subjects from analysis due to poor imaging. Third, we did not apply the volumetric method for calculation of PRF in r-TOF subjects with other structural anomalies in addition to PR. In r-TOF, significant tricuspid regurgitation or aortic regurgitation could affect the outcome. We should take into account these coexisting lesions for more effective analysis of prognosis, and compare PRF obtained on 3D-TTE with that on cMRI to validate the accuracy.
Using 2-D and 3-D TTE, the presence of reduced LV free wall LS in asymptomatic r-TOF subjects with well-preserved LVEF was demonstrated. Longitudinal LV myocardial dysfunction will develop in association with RV remodeling.
The authors declare no conflict of interest.