2014 Volume 78 Issue 8 Pages 1808-1815
2014 Volume 78 Issue 8 Pages 1808-1815
Left ventricular outflow tract obstruction (LVOTO) has important prognostic implications in patients with hypertrophic cardiomyopathy (HCM). Echocardiography provides critical information to establish LVOTO as a unique feature of HCM by demonstrating heterogeneity of hypertrophy patterns and the systolic anterior motion of mitral leaflets, resulting in mitral-septal contact. Currently, 2 treatment strategies are available for reduction of muscle mass to relieve LVOTO: surgical myectomy and percutaneous alcohol septal ablation. Both focus on mechanical removal of the hypertrophied septum. However, this alone is not the best approach to abolishing LVOTO, because recurrence is common and requires additional septal reduction. Recent 3-dimensional in vivo measurements and other noninvasive cardiac imaging modalities have confirmed primary alterations of the mitral valvular apparatus, including leaflet elongation with increased surface area and abnormal displacement of papillary muscles. More importantly, these extra-myocardial changes appear to be independent factors associated with the development of LVOTO. Other important anatomical changes include anomalous papillary muscle insertion into the anterior mitral leaflet and midventricular obstruction because of apposition of the hypertrophied mid-septum and the papillary muscle. Thus, the myocardium is not the only tissue affected in patients with HCM. A tailored approach to correcting primary changes of the mitral valvular apparatus and hypertrophy pattern based on a comprehensive evaluation using noninvasive imaging modalities is necessary to improve long-term outcomes. (Circ J 2014; 78: 1808–1815)
Over the past several decades, we have witnessed a dramatic evolution of hypertrophic cardiomyopathy (HCM) from a relatively common disease with a notable paucity of treatment options to a contemporary treatable disease.1 Prevention of sudden death, the most visible and devastating consequence of HCM, is now a realistic option for young patients with HCM.2 Left ventricular outflow tract obstruction (LVOTO) is another characteristic adverse clinical manifestation of HCM, and the issues surrounding it have evoked the most discussion.3 As well as the cumulative genetic data in patients with HCM,4 echocardiography and other noninvasive imaging modalities have provided important information to establish effective treatment options, especially for patients with LVOTO.
LVOTO used to be a pathognomonic and integral finding indispensable for a diagnosis of HCM.5,6 However, this phenomenon has been the source of periodic and often intense controversy regarding its clinical and pathophysiologic significance. A vigorously contracting left ventricle (LV) may result in excessively rapid ejection and artifacts produced by an entrapped catheter within the recesses of hypertrophied LV trabeculations during vigorous isometric contraction are other skeptical explanations for LVOTO.7,8 This debate could be ended by echocardiographic documentation of marked heterogeneity in hypertrophy patterns in patients with HCM. Moreover, the demonstration of systolic anterior motion (SAM) of the mitral valve leaflets resulting in systolic contact with an hypertrophied septum as a main mechanism of LVOTO (Figure 1) provides a solid platform for fundamental understanding of HCM with a wide clinical spectrum.9–12 This finding has provided a scientific rationale and background to the surgical removal of the hypertrophied septum to relieve LVOTO.13,14 In addition to an initial operative mortality between 5% and 8%, the failure rate between 10% and 20% to relieve symptoms after myectomy15 tempered initial enthusiasm for this surgical approach. Only during the past 10–15 years has the frequency and importance of LVOTO within the clinical spectrum of HCM been fully recognized.16,17 Echocardiography has contributed significantly to this. The morphologic spectrum of HCM is characterized by marked heterogeneity, including septal, basal, midventricular, and apical hypertrophy. Although the myocardium is the primary affected site in patients with HCM, abnormalities in other anatomical structures, especially the mitral valvular apparatus, have been appropriately evaluated. This information is critical for innovative modifications in surgical techniques, which have shown a strikingly low operative mortality, ≤1%, because of contemporary myocardial preservation techniques and increased surgical experience.18,19

Representative frame-by-frame echocardiographic images showing systolic anterior motion (SAM) of the mitral leaflet in a patient with asymmetrical septal hypertrophy. At the beginning of systole (Left), the coaptation point of the mitral valve (arrow) lies in the middle of the left ventricle (LV) and, with LV contraction, it moves toward the hypertrophied interventricular septum (IVS) and eventually SAM-septal contact develops (Middle and Right). Ao, aorta; LA, left atrium; LVOT, left ventricular outflow tract.
Diverse structural alterations of the mitral valve were first reported in pathologic specimens of human hearts more than 20 years ago. These included elongation of the leaflets with increased leaflet area and anomalous papillary muscle insertion directly into the anterior mitral leaflets.20 Those authors could not conclude whether the elongated leaflets were responsible for LVOTO, as the leaflet area was larger in patients without LVOTO than in those with LVOTO. Moreover, no evidence was provided on whether these changes in the mitral valve apparatus were primary or secondary. However, the observations were very important as they provided a sound scientific background for presenting an evolution of the precept that HCM is structurally confined to cardiac muscle. Detailed 2-dimensional echocardiographic studies have shown abnormalities in the morphology and motion of the mitral leaflets in patients with HCM. Mitral leaflets were longer in 10 patients with obstructive HCM, compared with 10 patients with either LV hypertrophy (nonobstructive type) or normal controls.21 This finding was confirmed by another group using transesophageal echocardiography with better resolution, delineating the leaflet tips from the chordae tendinae.22 Recent technical advancements in imaging methods enable vivo measurements of the leaflet area, which confirmed that increased mitral leaflet area is an independent factor associated with LVOTO (Figure 2).23 In a clinical study using 3-dimensional echocardiographic data, the leaflet area was larger in patients with LVOTO than in those without.23 Another study using magnetic resonance imaging also confirmed mitral leaflet elongation in patients with HCM, and provided evidence supporting a primary change of the mitral leaflet by showing definite discrepancies between the degree of LV hypertrophy and mitral leaflet length (Figure 3).24 Some patients with massive hypertrophy had normal leaflet length, whereas other preclinical patients with genetic abnormalities responsible for HCM (gene-positive/phenotype-negative) showed normal septal wall thickness and markedly elongated mitral leaflets. Demonstration of mitral leaflet elongation in a large study of patients with both overt and preclinical HCM supports the idea that a comprehensive evaluation of abnormalities of the mitral leaflets is necessary for the selection of optimal treatment strategies.

In vivo measurements of the open mitral leaflet area using real-time 3-dimensional echocardiography. Representative leaflet area measurements are shown in green and purple for the anterior and posterior leaflet viewed from the side. The leaflet area was larger in patients with asymmetrical septal hypertrophy (ASH) and those with ASH and left ventricular outflow tract obstruction (LVOTO) showed the largest leaflet area. (Reproduced with permission from Kim DH, et al.23) Ao, aorta; LA, left atrium; LV, left ventricle.

Representative cardiac magnetic resonance images showing the broad spectrum of mitral leaflet abnormalities in patients with hypertrophic cardiomyopathy (HCM). Arrows represent either elongated anterior (A), posterior (B), or both leaflets (C). There was no association between septal thickness and leaflet length (D,E). In a patient with preclinical HCM, a markedly elongated mitral leaflet was observed, despite normal wall thickness (F). (Reproduced with permission from Maron MS, et al.24) Ao, aorta; LA, left atrium; LV, left ventricle.
The papillary muscle is another structure associated with relatively frequent anatomical changes in patients with HCM.21,23,25–27 The most common and important abnormality is anterior displacement of the thickened papillary muscle (Figure 4), which can contribute to slackness in the mitral leaflet. Anterior displacement of the papillary muscle by itself brings about early systolic flow posterior to the mitral leaflets; this is a critical condition for the development of SAM, which sweeps the mitral leaflets into the septum, just as a spinnaker is easily pushed by the wind when the wind strikes its undersurface. Several investigators reported a strong association between anterior displacement of the papillary muscle and an increased prevalence of SAM and LVOTO, which was independent of septal thickness.23,28 This finding can explain the development of SAM and LVOTO in patients without significant septal hypertrophy. Abnormal attachments or connections between the papillary muscles and the anterior wall of the LV have been frequently found in patients with anteriorly displaced papillary muscles, which became plausible surgical targets. Anomalous papillary muscle insertion into the anterior mitral leaflet is another well-recognized phenomenon,20,29 which requires special attention during the initial echocardiography to avoid preoperative misdiagnosis (Figure 5).

Representative echocardiographic images (Upper) and averaged data obtained from each group by 3-dimensional echocardiography showing positional changes of papillary muscles and the effect on the pathogenesis of SAM and LVOT obstruction. In ASH+LVOTO patients, besides significant hypertrophy, medial displacement toward the center and anterior displacement of both papillary muscles resulted in a short systolic inter-papillary muscle distance. (Modified with permission from Kim DH, et al.23) Ao, aorta; ASH, asymmetrical septal hypertrophy; LVOTO, left ventricular outflow tract obstruction; PM, papillary muscle; SAM, systolic anterior motion.

Representative echocardiographic images of anomalous insertion of the PM into the anterior mitral leaflet in a patient with HCM and LVOTO. This patient showed ASH and a typical dagger-shaped Doppler signal, suggesting LVOTO (Left upper). Transthoracic (Right upper) and transesophageal echocardiography (Left lower) showed no evidence of SAM and the thick muscular connection between the PM and the anterior mitral leaflet was characteristic. Surgical removal of this muscular connection (Right lower) resolved LVOTO. Ao, aorta; ASH, asymmetrical septal hypertrophy; IVS, interventricular septum; LA, left atrium; LV, left ventricle; LVOTO, left ventricular outflow tract obstruction; PM, papillary muscle; SAM, systolic anterior motion
Beta-receptor blocking agents are the mainstay of pharmacologic therapy and the first-line agents for symptomatic patients with HCM and significant LVOTO.30 Their negative inotropic effects also improve the myocardial oxygen supply-demand relationship and hence reduce myocardial ischemia. In those patients unable to tolerate β-blockers or those with symptoms unresponsive to β-blockers, calcium-channel blockers may provide effective symptomatic relief. In patients with obstructive HCM who remain symptomatic despite the use of β-blockers and calcium-channel blockers, alone or in combination, class 1A antiarrhythmic drugs blocking the sodium channel, such as disopyramide31 or cibenzoline,32 may be effective. Implantation of a dual-chamber pacemaker was proposed as an alternative treatment for patients with severe symptomatic obstructive HCM. Although there was an initial enthusiasm for dual-chamber pacing, the exact mechanism of improvement with pacing remains unknown and a subsequent randomized comparison trial demonstrated long-lasting beneficial results in only a small minority of patients, whereas most of the perceived improvement was judged to be a placebo effect.33–35
Mechanical removal of a hypertrophied septum remains the main treatment option for patients with HCM and LVOTO with intractable symptoms, and clinical information obtained by critical evaluation of imaging data are important for decision-making. Surgical myectomy and percutaneous alcohol septal ablation (ASA) are 2 available strategies for reduction of muscle mass to relieve LVOTO, and the debate regarding the clinical efficacy of both techniques has evoked turmoil. The classic treatment strategy for patients with HCM and documented LVOTO was surgical removal of the hypertrophied septum to create a wider LVOT.13,14 In addition to a significant rate of surgical mortality, probably associated with poor techniques for myocardial preservation, the failure rate was reported to be higher than 10%.15 To improve these outcomes, “extended transaortic myectomy” with a wider initial septal incision including both subaortic and the midventricular septum was introduced and considered to be the standard treatment for symptomatic patients with LVOTO.36 However, echocardiographic data shows that, besides the hypertrophied septum, alterations of the mitral valvular apparatus, especially elongated mitral leaflets, contribute significantly to the development of LVOTO; therefore, modifications in the surgical techniques have been inevitable.37 According to the largest contemporary single-center study,38 more than 25% of patients (181/299) needed mitral valve procedures. Moreover, an LVOT gradient >50 mmHg was observed in 16% of cases after uneventful surgery and, during a mean follow-up of 6.2±3 years, 24 patients (3.4%) required redo cardiac surgeries to relieve residual symptomatic LVOTO: 5 repeat myectomies, 16 mitral valve replacements, and 3 myectomies+mitral valve replacements. These findings reinforce the clinical importance of a comprehensive preoperative evaluation of the mitral valve apparatus.
As an alternative to surgical myectomy for treating LVOTO, ASA has been adopted in an increasing number of cardiac centers worldwide.39–44 Early clinical efficacy with septal ablation has been demonstrated in short-term studies and the benefits of ASA compared with myectomy include a shorter hospital stay, less pain, and avoidance of complications associated with surgery and cardiopulmonary bypass.45 However, long-term outcome data are quite contradictory. According to a European study with clinical follow-up of 5.4±2.5 years, approximately 1 in 3 patients with HCM who underwent ASA had major cardiovascular complications during the procedure and follow-up, including cardiac death or resuscitated sudden cardiac death (SCD) in approximately 1 in 5 patients.46 Compared with myectomy patients, ASA patients had a 5-fold increase in the estimated annual primary endpoint rate (4.4% vs. 0.9%). A logical inference of the risk factor data is that ASA itself is a risk factor for SCD, regardless of the amount of ethanol, patient’s age or comorbidities. As the septal morphological appearance post-ASA is myocardial infarction,47 arrhythmogenicity from the ASA-induced scar has become a serious concern, affecting long-term outcomes. Thus, the authors concluded that choosing ASA as a therapeutic approach is limited by the lack of long-term safety data compared to myectomy.46 A recent report from the United States showed that survival free of all-cause mortality was similar to that of age- and sex-matched patients who underwent isolated surgical myectomy with the same similar 8-year survival estimate of 79%.48 Late survival was similar to that observed in an age- and sex-matched general US population, without an increased risk of SCD after ASA. However, among 147 patients surviving ablation with clinical follow-up, 99 patients were asymptomatic, but persistent or recurrent severe symptoms occurred in 20 patients (14%), leading to the need for additional septal reduction therapy (myectomy or repeat ASA) in 15 patients (10%). Thus, the 8-year survival rate free of the combined end point of death and need for additional septal reduction therapy was 69.8% (60.9–78.8%). This was lower than that observed in patients undergoing myectomy (78.9%; 71.2–86.6%; P=0.04), owing to the greater frequency of myectomy in follow-up for patients who had ASA. A more important finding was that the residual LVOT gradient was higher in ablation patients than in those who underwent surgical myectomy; the post-ASA LVOT gradient was an independent significant predictor of long-term survival. As long-term survival was favorable and similar to that of an age- and sex-matched general population, and also to patients undergoing surgical myectomy, the authors concluded that their data supported the role of septal ablation in the management of patients with drug-refractory symptoms from obstructive HCM, when performed by experienced surgeons. It is quite surprising to observe such a big difference in the clinical efficacy of ASA, as reported by 2 established institutions. However, clinical data from ablation patients shared 1 important pathophysiologic implication with data from surgical myectomy patients. Despite favorable long-term outcomes after ASA, ASA by itself could not relieve LVOTO effectively in a certain proportion of patients, who eventually needed repeat intervention (Figure 6) and the LVOT gradient after ASA was associated with long-term survival.46,48 This finding supports the possibility that targeting the hypertrophied septum alone is not ideal for abolishing LVOTO and achieving better long-term outcomes. The recent ACCF/AHA guideline recommends that ASA should be performed only by experienced operators in the context of a comprehensive HCM clinical program and only for the treatment of eligible patients with severe drug-refractory symptoms and LVOTO.30
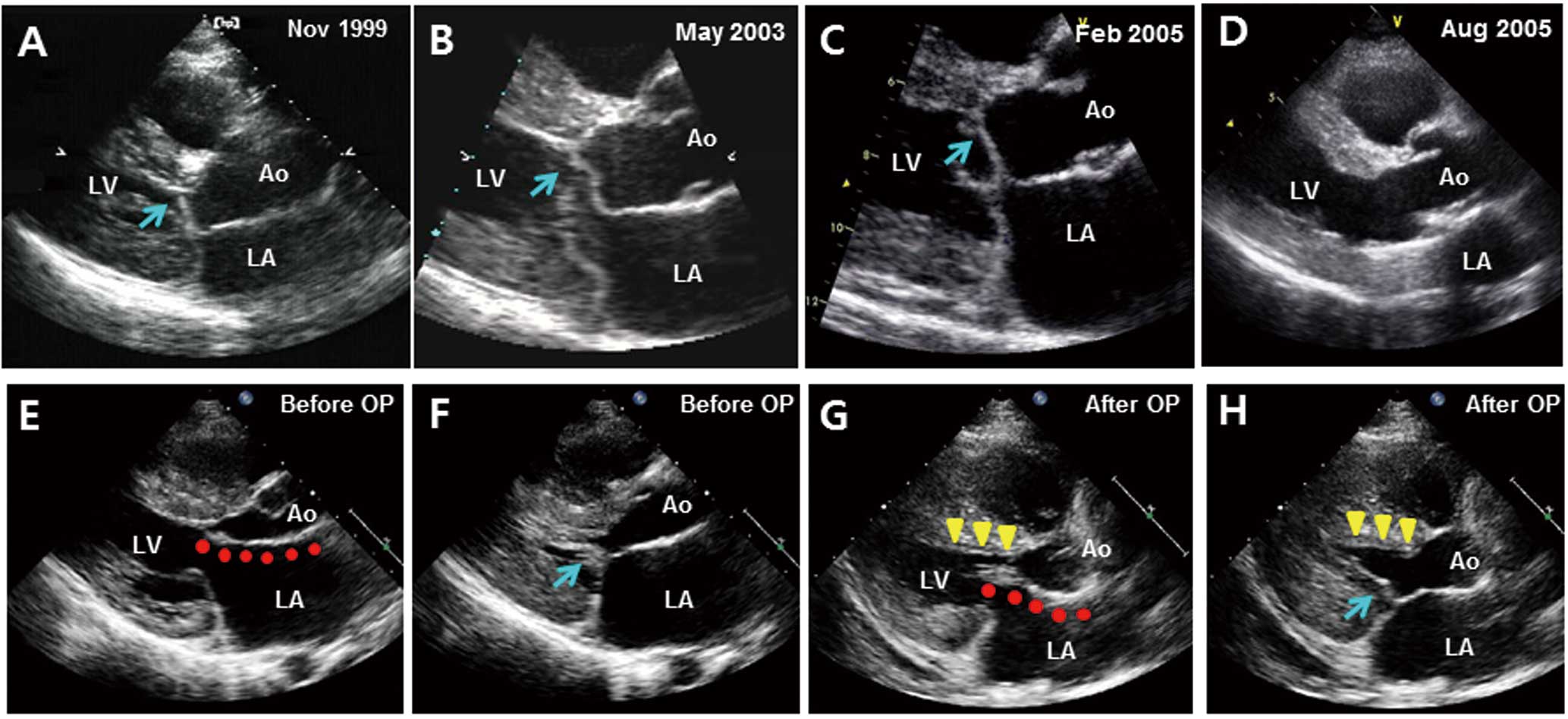
Representative echocardiographic images after alcohol septal reduction (ASA) and myectomy with mitral valve plication. (A–D) Serial echocardiographic images in a patient who needed additional septal reduction therapy after ASA. This patient underwent the first ASA on in November 1999, to control left ventricular outflow tract obstruction (LVOTO). He needed repeat ASA to control persistent systolic anterior motion (SAM) of the mitral leaflet (arrow in B) and recurrent LVOTO, which was performed in May 2003. However, final mitral valve replacement was needed to abolish SAM caused by an elongated leaflet (arrow in C), which was performed in March 2005. (E–H) Diastolic and systolic echocardiographic images of a patient before (E,F) and after myectomy and mitral valve plication (G,H). After plication, in addition to myectomy (triangles in G,H), reduced leaflet length and decreased mobility because of increased leaflet stiffness was characteristic (arrow in H), and contributed to complete resolution of LVOTO. Ao, aorta; LA, left atrium; LV, left ventricle.
Surgical procedures for mitral leaflet changes associated with HCM have evolved significantly. Mitral valve replacement was once the main technique to eliminate SAM,49 but now is selectively considered for the few patients with intrinsic mitral valve disease or elongated mitral leaflets despite normal thickness of the LV.50 Other modifications include repair and plication.51–53 Horizontal plication of elongated leaflets leaves the coaptation zone untouched and stiffens the midportion of the leaflet, resulting in excellent relief of LVOTO (Figure 6). As this procedure is technically simple and easy to duplicate and this portion of the leaflet is easy to access from the aortotomy used for the myectomy, horizontal plication has attracted the interest of many surgeons. In rare cases, patch extension of the anterior leaflet can be applied to increase its stiffness and cause lateral displacement of the secondary chords, perhaps moving the leaflet posteriorly out of the LVOT. However, heavy manipulation of the leaflets, especially in young adults, may cause fibrosis or calcification, resulting in dysfunctional leaflets. Thus, serious consideration of the potential advantages and disadvantages of valve repair over valve replacement is necessary. Abnormal papillary muscle geometry is another target of surgical modifications: division of any abnormal attachments that the papillary muscles may have to the lateral wall of the LV with thinning of the papillary muscle is critical for the treatment of SAM. The term “RPR (Resection, Plication, and Release) procedure” is being used to represent the comprehensive process of surgical modification: resection refers to the extended myectomy, plication is the treatment of the anterior leaflet itself where it is extremely redundant, and release refers to dividing any abnormal attachments of the papillary muscle.37
SAM with septal contact resulting in significant LVOTO is not the only cause of heart failure in patients with HCM. A rare variant of significant obstruction within the LV is midventricular obstruction,54 in which apposition between the septum and the papillary muscle occurs at the midventricular level (Figure 7). As this level is difficult to address through the traditional transaortic approach for surgical myectomy, prognosis is reported to be worse than for other variants of HCM. Recently, the apical approach was introduced successfully and short-term results seem to be very promising.55 Transapical myectomy is also reported to be an effective way of removing hypertrophied septum confined to the LV apical area in order to augment the LV cavity size.56

Representative echocardiographic images of a patient with midventricular obstruction. Neither the diastolic (A,B) nor systolic (C,D) images show evidence of systolic anterior motion (SAM). However, systolic apposition of the interventricular septum with the papillary muscle (arrow head in C,D) results in flow acceleration from the midventricle (arrow, E) with a markedly elevated pressure gradient within the left ventricle (F). Ao, aorta; LA, left atrium; LV, left ventricle; RV, right ventricle.
A deeper understanding of the marked heterogeneity of hypertrophy patterns and associated anatomical changes beyond the hypertrophied septum allows targeted therapeutic approaches to relieving LVOTO. As LVOTO is an important determinant of long-term survival in patients with HCM and, regardless of the treatment options, a post-procedural LVOT gradient has been proved to be strongly associated with prognosis, a comprehensive evaluation is mandatory to delineate the mechanism of the LVOTO. An exciting development is that recent technological advances now enable 3D analysis of the LV and mitral valvular apparatus, which definitely contributes to appropriate clinical decision-making. Although the randomized clinical trial is used to provide answers to the debate on better treatment options, a prospective multicenter registry that provides detailed baseline characteristics and descriptions of the procedure, as well as careful and systematic follow-up, has not been attempted, and does not seem viable in the near future. Moreover, surgical myectomy and ASA may have different optimal candidates based on the morphologic variation of both the hypertrophied septum and the mitral valvular apparatus, including leaflets and the papillary muscle. Thus, communication and a team approach among interventional cardiologists, surgeons, and imaging specialists to achieve the best individualized treatment option, is the wisest approach currently available (Table). The clinical importance of information based on noninvasive cardiac imaging modalities cannot be over-emphasized.
| Structural abnormality | Treatment options |
|---|---|
| LV hypertrophy | |
| Basal and asymmetrical septal | Transaortic myectomy |
| Alcohol septal ablation | |
| Midventricular | Transapical myectomy |
| Apical | Transapical myectomy |
| Mitral leaflet elongation | Mitral valve replacement |
| Plication | |
| Other repair including Alfieri-type stitch | |
| Papillary muscle adhesion, displacement and abnormal attachments |
Division of papillary muscle |
| Release of abnormal attachments | |
LV, left ventricular.