2014 Volume 78 Issue 8 Pages 1782-1790
2014 Volume 78 Issue 8 Pages 1782-1790
Recent advances in structural heart intervention have produced increasing demand for transseptal access, which was first introduced as a diagnostic tool to directly measure left atrial pressure. Transseptal access allows safe and adequate approach to the left atrium and surrounding structures. Percutaneous transcatheter mitral valve repair using the MitraClip device is a safe and less invasive treatment for selected patients with significant mitral regurgitation, who are at high risk for surgery. This is an echocardiographic- and fluoroscopic-guided procedure requiring accurate transseptal access of the left atrium and clipping of the mitral leaflets at the precise location of their malcoaptation. Percutaneous transcatheter closure of the left atrial appendage is another novel procedure that requires transseptal access of the left atrium, followed by closure or ligation of the left atrial appendage. This catheter-based therapy has been shown to be a safe and effective alternative to long-term anticoagulant therapy for the prevention of stroke in patients with atrial fibrillation. In this article, we systematically review these novel structural heart interventions. (Circ J 2014; 78: 1782–1790)
Transseptal access of the left atrium (LA) was first used in the 1950 s as a means to directly measure LA pressure.1 Subsequently, this approach has been used extensively for catheter-based procedures in the LA, pulmonary vein, and mitral valve (MV).2–6 Percutaneous balloon mitral valvuloplasty is a conventional transcatheter intervention using the transseptal approach,2,3 and considered to be a standard treatment option for severe mitral stenosis.7,8 In the electrophysiological field, the transseptal approach is used in pulmonary vein isolation for atrial fibrillation (AF).5,6 Initially, transseptal puncture was performed using only fluoroscopic guidance, which often could be challenging in patients with enlarged atria and distorted septum. The use of transesophageal and intracardiac echocardiography in addition to fluoroscopy has significantly improved the safety and accuracy of a transseptal puncture.9,10
Recently, percutaneous MV repair and left atrial appendage (LAA) occlusion have emerged as novel transcatheter interventions utilizing the transseptal approach. In the present article, we systematically review these structural heart interventions, with an emphasis on procedural technique and current perspectives.
Percutaneous transcatheter MV repair with the MitraClip system (Abbott Vascular, Menlo Park, CA, USA) is a novel approach to the treatment of selected patients with degenerative and functional mitral regurgitation (MR). The MitraClip therapy has demonstrated superior safety and compatible clinical efficacy in comparison with conventional MV surgery.11,12 The safety and efficacy of the MitraClip therapy was further confirmed in a high surgical risk population,13 and in a real-world setting.14 Currently, this catheter-based therapy is considered a standard treatment option for severe MR in patients who are at significant risk for open heart surgery.6,7
The MitraClip SystemThe concept of MitraClip therapy is based on the surgical edge-to-edge repair that was pioneered by Dr Alfieri and his colleagues in the early 1990s. In this therapy, one or more clips are delivered through a transvenous transseptal route. The clips are used to clip together the most malcoapted regions of the mitral leaflets, resulting in a competent double orifice MV. The MitraClip system consists of a 24Fr steerable guiding catheter (GC) and clip delivery system (CDS) (Figures 1A,B). The clip is attached to the distal end of CDS, and delivered via a transseptal approach (Figures 1C–F). The MitraClip system has important features. During the procedure, the grasped leaflets can be released, followed by repositioning or removal of the clip without causing significant injury to the leaflets. Also, during the procedure more than 1 clip can be used to achieve optimal results. These features add to the safety and efficacy of the procedure.
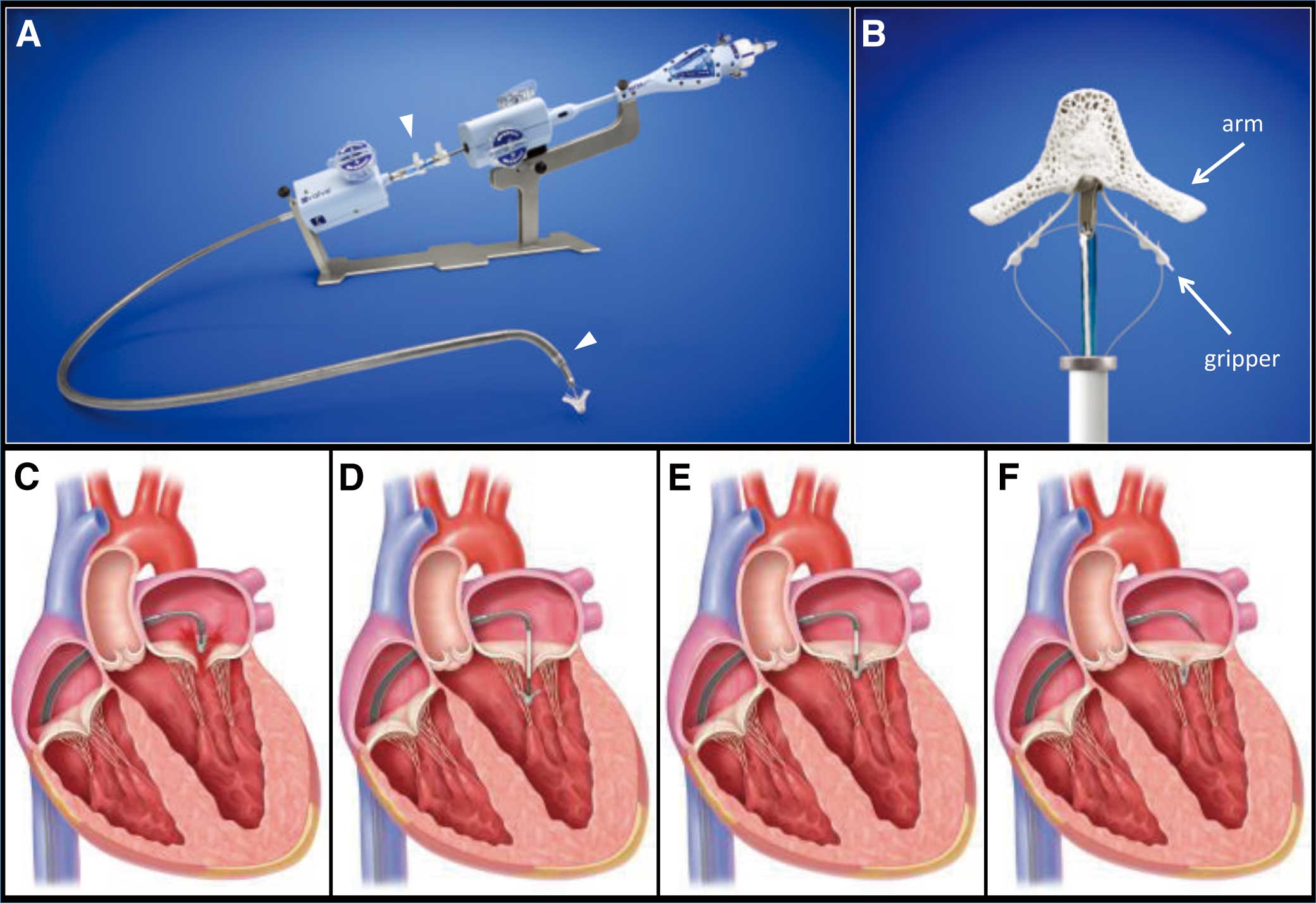
The MitraClip system and an illustration of the procedure (courtesy of Abbott Vascular. ©2014 Abbott. All rights reserved). (A) The 24Fr steerable guide catheter (between white arrowheads) and the clip delivery system. (B) The clip has 2 arms and grippers. (C) The clip is positioned above the origin of the regurgitant jet. (D) The clip is advanced into the left ventricle. (E) The clip is slowly pulled back toward the mitral valve, and grasps the leaflets. (F) The clip is released from the clip delivery system.
The MitraClip procedure is performed under general anesthesia with transesophageal echocardiographic (TEE) and fluoroscopic guidance. TEE is the main guide for the MitraClip procedure, so the interventionalist needs sufficient knowledge of echocardiography. The MitraClip procedure is composed of transseptal puncture, clip positioning in the LA, leaflet grasping, and hemostasis.
Transseptal Puncture and Insertion of the Guide Catheter Transseptal puncture is a key step in the MitraClip procedure. Because the CDS has a limited range of motion in the LA, a specific location in the atrial septum needs to be punctured (Figure 2). First, the tenting point of the transseptal puncture system is visualized on mid-esophageal bicaval and short-axis views. The tenting point is then positioned to the middle-superior aspect on the bicaval view, and the middle-posterior aspect on the short-axis view. The next step is to visualize the tenting point on the 4-chamber view, and measure the distance between the level of the tenting point and MV coaptation. Because the working length of the CDS is approximately 5 cm, the optimal distance needs to be between 4.0 and 4.5 cm. Once access is achieved in the LA, a stiff wire is advanced into the body of the LA or left upper pulmonary vein. The transseptal catheter is removed over the wire and exchanged for the 24Fr specialized tip deflected GC.

Transseptal puncture. (A) The tenting point for transseptal puncture (white arrows) is positioned in the middle-superior aspect on the bicaval view, and the middle-posterior aspect on the short-axis view. (B) In the 4-chamber view, the distance between the level of the tenting point and mitral valve coaptation (yellow arrow) needs to be within 4.0 to 4.5 cm.
Clip Positioning in the LA Following successful transseptal puncture, the CDS is introduced into the LA through the GC. Using echocardiographic guidance, the clip is positioned above the origin of the MR jet (Figure 3A). The clip is opened and the arms are positioned perpendicular to the line of coaptation using TEE (Figure 3B).
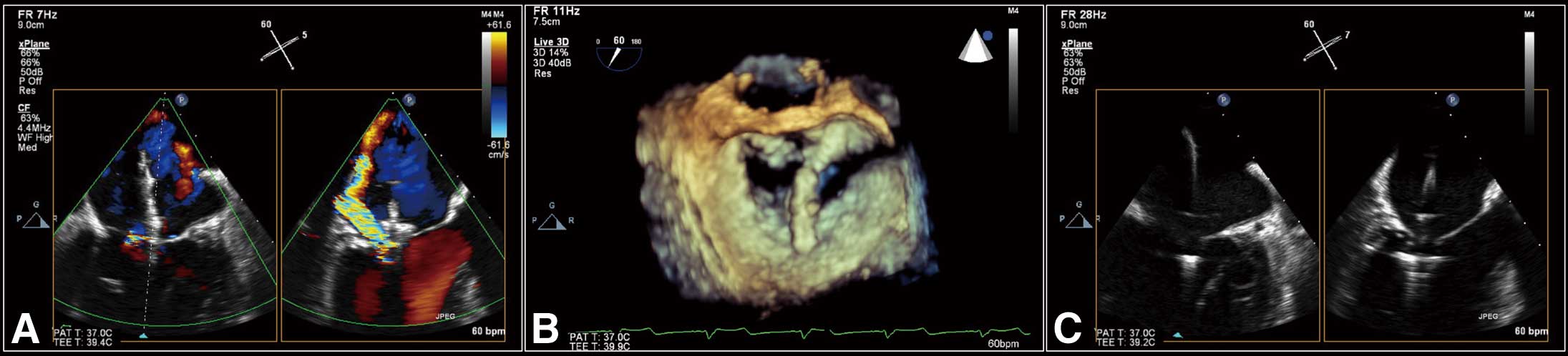
Maneuvering the clip delivery system. (A) Bicommissural and LVOT views showing the clip above the origin of the regurgitant jet. (B) 3D en face view showing the clip arm, which is perpendicular to the line of mitral valve coaptation. (C) Bicommissural and LVOT views showing the clip advanced into the left ventricle. LVOT, left ventricular outflow tract.
Leaflet Grasping The clip is advanced into the LV until just below the MV (Figure 3C), then slowly pulled back toward the MV with a clip arm angle of 120 degrees. Once both leaflets are resting on the clip arms, grippers are lowered and the clip is closed. After confirmation of leaflet insertion, MR reduction, and absence of significant MV stenosis, the clip is deployed (Figure 4). If there is residual MR, additional clips can be deployed in a similar fashion.
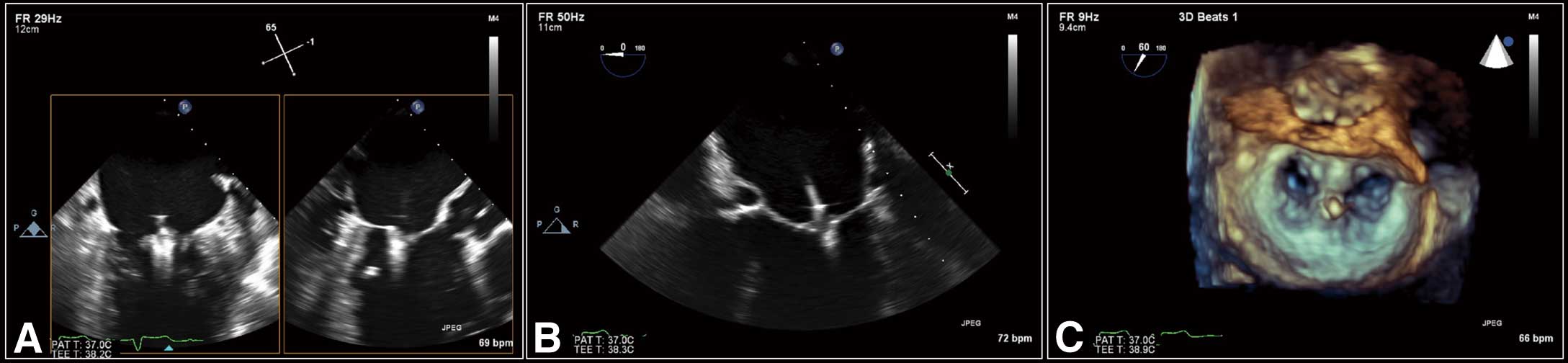
Assessment of leaflet insertion. (A) LVOT view (right side) showing satisfactory grasping of both leaflets. (B) 4-chamber view showing satisfactory grasping of both leaflets. (C) 3D en face view showing double orifice mitral valve. LVOT, left ventricular outflow tract.
Hemostasis In our institution, preclosure technique with Perclose suture-mediated closure system (Abbott Vascular, Redwood City, CA, USA) is used for hemostasis of the femoral vein access site. If hemostasis is suboptimal with this technique, a Figure-of-Eight technique is also used.
Clinical Experience With the MitraClipOver 14,000 patients have been treated with the MitraClip device and approximately 2,000 patients have been enrolled in prospective clinical trials. The device is approved for clinical usage in Europe, some countries in Asia, and recently in the USA. At present, the device is not available in Japan. The initial clinical studies were conducted in North America,11–13 followed by post-market registries in Europe.14
The EVEREST II trial was the first prospective randomized study conducted in North America to compare the MitraClip therapy with traditional surgery for standard risk patients with significant MR.11 In this study, 184 and 95 patients were randomized to the MitraClip therapy and conventional surgery, respectively. The rates of major adverse events at 30 days, which was the primary safety endpoint, were significantly lower in the MitraClip group than in the surgery group (15% vs. 48%, P<0.001). The primary efficacy endpoint defined as 12 months freedom from death, MV surgery, and MR grade ≥3+ was significantly lower in the MitraClip group (55% vs. 73%, P=0.007). This was primarily driven by a higher incidence of requirement of MV surgery because of recurrence and inadequate reduction in MR in the MitraClip group (20% vs. 2%, P<0.001). However, both groups achieved improvement in clinical symptoms as assessed by New York Heart Association (NYHA) functional class, LV reverse remodeling, and quality of life measures at 12 months.
The 4-year outcomes of the EVEREST II randomized control trial were reported in 2013.12 The rates of the composite endpoint of freedom from death, surgery, and MR grade ≥3+ were still lower in the MitraClip group than in the surgery group (40% vs. 53%, P=0.070). Of importance, between 1 and 4 years, only 3 and 2 patients in the MitraClip and surgery groups, respectively, required surgery. These data confirm that once a successful reduction of MR is achieved by the MitraClip, there is evidence of durability for up to 4 years. This emphasizes the importance of case selection and meticulous reduction of MR during the index clip procedure.
The EVEREST II High Surgical Risk Cohort included 351 high-risk patients pooled from the EVEREST II High Risk Study and the EVEREST II REALISM Continued Access Study.13 The data from this cohort were presented to the US Food and Drug Administration (FDA) panel. The mean Society of Thoracic Surgeons mortality score was 11±8%. Approximately 70% of patients had functional MR, and 60% of patients had previous cardiac surgery. The actual 30-day mortality was only 4.8%. At 12 months, there was significant improvement of NYHA functional class, reduction in the number of hospitalizations for heart failure, and improvement of MR, and LV reverse remodeling in comparison with baseline measurements. The data for this large high-risk cohort, together with the randomized trial data, were used in the approval process by the US FDA panel.
The ACCESS-EU registry, which was a prospective, multicenter, nonrandomized post-approval study, enrolled a total of 567 patients with significant MR who underwent the MitraClip therapy at 14 European sites.14 This study is unique because it is the largest cohort of the use of the MitraClip device in a real-world setting. At baseline, the mean age was 74 years. The prevalence of functional MR and LV ejection fraction ≤40% was 77% and 53%, respectively. The mean logistic EuroSCORE at baseline was 23.0±18.3. MitraClip devices were successfully implanted in 99.6%. At 30-day follow-up, there had been 19 deaths (3.4%). The Kaplan-Meier analysis demonstrated that mortality at 1 year was 18.2%. At 1-year follow-up, 78.9% (258/327) and 71.4% (245/343) of patients had MR severity ≤2+ and NYHA functional class I/II, respectively. Significant improvements in the 6-min walk test and quality of life measures were also observed. These results confirmed the safety and efficacy of the MitraClip in the real-world setting.
Current PerspectiveThere is an unmet need in the management of patients with MR. MV surgery has demonstrated excellent long-term outcomes with acceptable operative mortality,15 and is considered the treatment of choice for patients with severe degenerative MR.7,8 However, studies have shown that a significant number of patients with severe MR are not considered for surgery because of their age, LV dysfunction and other comorbidities.16 There is therefore a need for a safe and effective treatment of option for this large group of patients. The MitraClip therapy is clearly one such option.17 In patients with primary MR who are at high risk for surgery, there is no controversy that the MitraClip should be considered if the patient has a suitable anatomy. On the other hand, in patients with functional MR, which is a ventricular disease, the role of mechanical correction of MR is still controversial.18,19 Medical therapy, including cardiac resynchronization therapy, is the mainstay of treatment, but the MitraClip therapy may have added benefits. Having said so, in the commercial setting outside the USA, the majority of patients treated are those with functional MR who are high risk for surgery.14
Based on clinical usage and data, the European Society of Cardiology therefore recommends MitraClip therapy for patients with both primary and functional MR who are at high risk for surgery (Class IIb, Level of Evidence C).7 On the other hand, the US FDA has approved the MitraClip device for high surgical risk patients with symptomatic MR caused by a primary disorder (degenerative) of the MV apparatus. This indication is also part of the American College of Cardiology (ACC)/American Heart Association (AHA) guidelines (Class IIb, Level of Evidence B).8 In the USA, patients with functional MR for whom surgery is not indicated are encouraged to participate in a clinical trial evaluating the role of the MitraClip. The COAPT trial is a randomized study in North America, in which patients with functional MR are randomized to MitraClip plus best medical treatment or best medical treatment alone. A similar study named the RESHAPE trial is being conducted in Europe.
Summary of the MitraClip TherapyThe MitraClip therapy is a tour-de-force technology for the management of MR. This catheter-based therapy is a safe and effective treatment option for selected patients with degenerative and functional MR. Currently, the MitraClip therapy is indicated as a standard treatment option for high surgical risk patients with severe MR. In the future, the MitraClip alone or in conjunction with other transcatheter procedures could be used in patients who are at low to moderate risk for open heart surgery.
AF is the most common sustained rhythm disorder and its prevalence increases with age. Approximately 3.8% of populations aged 60 years or older and 9.0% of populations aged 80 years or older have AF.20 Cardiogenic embolic stroke is the most important and life-threatening complication of AF, which is associated with higher mortality and morbidity as compared with non-cardiogenic stroke.21 Therefore, prevention of stroke is the main component in the management of patients with AF. Long-term oral anticoagulant therapy is the standard of care for stroke prophylaxis in patients with AF.22–25 However, despite its proven efficacy there are definite limitations, including the risk of major and minor bleeding, drug and diet interactions, issues of compliance, and finally a small incidence of drug failure. Echocardiographic and autopsy studies have demonstrated that the LAA is a source of thromboembolism in more than 90% of patients with nonvalvular AF.26–28 Based on these findings, percutaneous transcatheter LAA occlusion/ligation, which excludes the LAA from systemic circulation, has emerged as a novel approach to stroke prophylaxis. In this intervention, the transseptal access is used to approach the LAA. Here, we review the most widely-used percutaneous LAA occlusion systems: the Watchman LAA closure device (Atritech, a subsidiary of Boston Scientific, Plymouth, MN, USA), the AMPLATZERTM Cardiac Plug (ACP) device (St. Jude Medical, Minneapolis, MN, USA), and the LARIAT Suture Delivery Device (SentreHEART, Inc, Palo Alto, CA, USA).
The Watchman LAA Closure DeviceSystem and Procedure The Watchman LAA closure system consists of a 14Fr transseptal access sheath and a delivery system with an implantable device (Figures 5A–C). The device has a self-expanding nitinol frame with fixation barbs and a permeable polyester fabric cover. The device has a diameter range of 21, 24, 27, 30, and 33 mm to accommodate the wide range of size of the ostium of the LAA.

The Watchman left atrial appendage (LAA) closure system and cine angiogram of the procedure. (©2014 Boston Scientific Corporation or its affiliates. All rights reserved. Used with permission of Boston Scientific Corporation.) (A) The Watchman device. (B) The 14Fr single-curve transseptal access sheath. (C) The 14Fr double-curve transseptal access sheath. (D) Baseline angiogram shows chicken wing-type LAA. (E) The delivery system (white arrow) with the device (between white arrowheads) is introduced. (F) The device is deployed. (G) Final angiogram shows well-seated device without significant residual leak.
The procedure is performed under general anesthesia with TEE guidance. Figures 5D–G shows cine angiograms of the Watchman procedure. Similar to the MitraClip procedure, transseptal puncture is a key step in the procedure. Because the LAA is located in the anterolateral and superior aspect of the LA, the posterior and inferior aspect of the atrial septum needs to be punctured to manipulate the sheath coaxially into the LAA. Following transseptal puncture, a 14Fr single- or double-curve sheath is advanced into the LA in the direction of the left superior pulmonary vein. A pigtail catheter is then advanced into the access sheath, and directed into the LAA, and the sheath is then advanced over the pigtail catheter into the LAA. The use of a pigtail catheter is important to prevent perforation of the LAA and subsequent pericardial effusion. The device size is determined to be 10–20% larger than the maximum diameter of the LAA ostium. The delivery catheter with the device is advanced through the sheath into the LAA, and then the device is deployed and released after confirmation of adequate device compression (8–20% of its original size) and sufficient sealing of the LAA.
Results of Clinical Trials The PROTECT-AF (WATCHMAN Left Atrial Appendage System for Embolic Protection in Patients with Atrial Fibrillation) study was a multicenter, prospective, randomized controlled trial undergone at 59 sites in the USA and Europe.29 In this study, 707 patients with nonvalvular AF and a CHADS2 risk score ≥1 were randomized to Watchman device (n=463) or Coumadin (n=244) group in a 2:1 ratio. In the intervention group, the Watchman device was successfully implanted in 88% (408/463) of patients. At 45 days follow-up, 86% (349/408) of implanted patients discontinued warfarin therapy, and switched to dual antiplatelet therapy of aspirin and clopidogrel for 6 months, followed by lifelong use of aspirin only. At 1,065 patient-years of follow-up, the primary efficacy event rate (ischemic or hemorrhagic stroke, cardiovascular or unexplained death, or systemic embolism) in the intervention group (3.0 per 100 patient-years, 95% credible interval [CrI] 1.9–4.5) was non-inferior to the control group (4.9 per 100 patient-years, 95% CrI 2.8–7.1). The primary safety event rate (excessive bleeding or procedure-related complications) was higher in the intervention group (7.4 per 100 patient-years, 95% CrI 5.5–9.7) than in the control group (4.4 per 100 patient-years, 95% CrI 2.5–6.7). In the intervention group, 55% (27/49) of the primary safety events occurred on the day of the procedure. Conversely, 50% (8/16) of the events occurred between 45 days and 1 year in the control group.
Recently, the long-term results of the PROTECT-AF study were presented at the Heart Rhythm Society 2013.30 At a mean follow-up period of 45 months (2,621 patients-years), the primary efficacy event rate in the intervention group (2.3 per 100 patient-years, 95% CrI 1.7–3.2) was superior to the control group (3.8 per 100 patient-years, 95% CrI 2.5–4.9). Notably, the intervention group, as compared with the control group, demonstrated significant reduction of all-cause mortality (hazard ratio, 0.66; 95% confidence interval, 0.45–0.98), cardiovascular mortality (rate ratio 0.40; 95% CrI, 0.23–0.82), and hemorrhagic stroke (rate ratio 0.15; 95% CrI, 0.03–0.49).
The PROTECT-AF study reported the therapeutic efficacy of percutaneous LAA closure as an alternative to long-term warfarin therapy; however, one concern was the high incidence of safety events associated with LAA closure. The Continued Access Protocol (CAP), a nonrandomized registry that began subsequent to the completion of the PROTECT-AF study, demonstrated a significant improvement in the safety events with increased experience.31 The safety endpoint (bleeding- and procedure-related complications) of the CAP registry was significantly lower than those of the PROTECT-AF study (3.7% vs. 7.7%, P=0.007). A similar experience-related improvement was also observed for serious pericardial effusion (2.2% vs. 5.0%, P=0.019) and procedure-related stroke (0% vs. 0.9%, P=0.039).
Current Perspective Percutaneous LAA occlusion with the Watchman system is a first proof-of-concept that a “local” therapy is equivalent to systemic therapy in the prevention of stroke, death, or systemic embolization in patients with AF. The initial concern about the safety of the procedure has been tempered by the subsequent CAP registry, which showed that the learning curve has reduced the periprocedural complication rate.31 In addition, long-term data from PROTECT-AF has shown no late safety issues.30 The long-term data have clearly demonstrated meaningful reductions of stroke (ischemic and hemorrhagic), cardiovascular and all-cause mortality. Based on the results of PROTECT-AF study and CAP registry, the ESC updated their guideline to recommend percutaneous LAA occlusion as Class IIb indication for AF patients with high stroke risk and contraindication for long-term oral anticoagulation therapy.32 In the USA, the Watchman LAA closure system is still an investigational device, but the results of an FDA panel meeting in 2013 reflected the demand for this catheter-based stroke prophylaxis. The panel voted favorably for the Watchman LAA closure with regard to safety (13:1), effectiveness (13:1), and benefit over the risk (13:1).
The AMPLATZERTM Cardiac PlugSystem and Procedure The ACP device is a nitinol-made self-expanding device with a proximal disk and distal lobe (Figure 6A). The new-generation ACP device, the AMPLATZERTM Cardiac Plug 2 (AmuletTM), has the same base design as the first-generation ACP device, but there are several upgraded modifications to optimize the sealing of the LAA and minimize procedure-related complications. Major modifications of the second generation are: the device is preloaded inside the delivery system; the length of the distal lobe is 2–3 mm longer than in the first generation; the diameter of the proximal disk is 6–7 mm larger than the distal lobe (4–6 mm in the first generation); the waist between the proximal disk and distal lobe is 5.5–8 mm (4 mm in the first generation); the end-screw on the proximal disk is recessed inside the device; a 31- to 34-mm device is available.
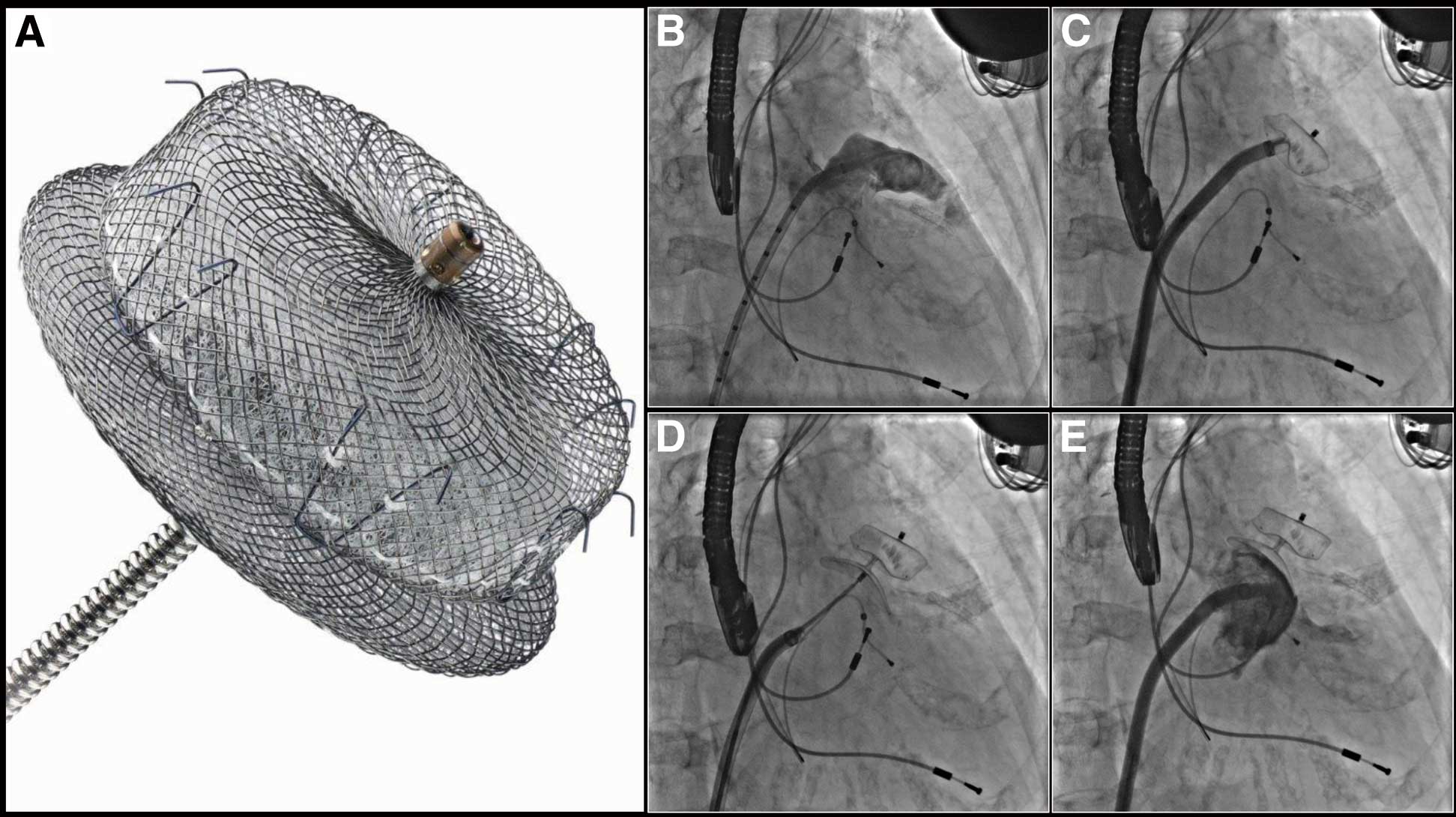
The AMPLATZERTM Cardiac Plug and cine angiograms of the procedure. (AMPLATZER and St. Jude Medical are trademarks of St. Jude Medical, Inc or its related companies. Reprinted with permission of St. Jude Medical, ©2014. All rights reserved.) (A) The AMPLATZERTM Cardiac Plug. (B) Baseline angiogram shows chicken wing-type left atrial appendage. (C) The distal lobe is deployed. (D) The proximal disk is deployed. (E) Final angiogram shows well-seated device without significant residual leak.
The procedure is performed under general anesthesia and TEE guidance. Figures 6B–E shows cine angiograms of LAA occlusion using the ACP device. The procedural steps are similar to the Watchman procedure. Following transseptal puncture (posterior and inferior aspect), the delivery catheter with the device is advanced into the LAA, the device is deployed and then released after confirmation of adequate sealing of the LAA on angiogram and TEE. The size of the device is based on the TEE measurement, and needs to be 2–4 mm larger than the maximum diameter of the landing zone, which is 1–2 cm distal from the LAA ostium.
Clinical Trials Despite the absence of randomized clinical trials of ACP LAA closure, previous studies demonstrated its feasibility and safety.
An investigator-initiated multicenter retrospective study was conducted to evaluate the initial experience of ACP LAA closure in Europe.33 The procedure was successfully performed in 132 of 137 patients (96%). Serious procedural complications were observed in 10 patients (7%): ischemic stroke (n=3), embolization of the device (n=2), and pericardial effusion requiring pericardiocentesis (n=5).
More recently, ACP LAA closure was evaluated in nonvalvular AF patients with a contraindication for anticoagulation therapy.34 The device was successfully implanted in 51 of 52 patients (98%). Device embolization was observed in 1 patient (1.9%). There were no cases of in-hospital stroke or death. During a mean follow-up of 20±5 months, there were 3 deaths (5.8%), 1 stroke (1.9%), no systemic embolism (0%), 1 pericardial effusion (1.9%), and 1 major bleeding (1.9%). Follow-up TEE at 6 months (n=37) revealed mild peri-device leak in 5 patients (16%). There were no cases of device thrombosis.
Current Perspective The ACP device received CE mark approval in 2008. The therapeutic concept of the ACP system is similar to that of the Watchman system. Therefore, despite the lack of data, ACP LAA closure may have similar clinical performance as Watchman LAA closure. Further clinical trials of ACP LAA closure are warranted to evaluate its efficacy and safety against Watchman LAA closure and also long-term oral anticoagulation therapy.
The LARIAT Suture Delivery DeviceSystem and Procedure The LARIAT Suture Delivery Device is a unique suture-based ligation system. Percutaneous LAA closure with the LARIAT Suture Delivery Device is performed using the EndoCATH Large Occlusion Balloon and the FindrWIRZ Magnetic Guide Wire System (SentreHEART, Inc) under general anesthesia with TEE guidance (Figure 7). Figure 8 shows cine angiograms of percutaneous LAA ligation. First, pericardial puncture is performed and a 17 G epidural needle is slowly advanced with a test injection of contrast using anterior-posterior and lateral fluoroscopic views. Once pericardial staining is confirmed, the needle is exchanged for a 14 Fr soft-tipped epicardial cannula over a 0.035-inch wire and then transseptal puncture is performed using a standard technique. Through the transseptal sheath, a magnet-tipped 0.025-inch endocardial wire with a balloon occlusion catheter is advanced into the LAA apex. Through the epicardial cannula, a magnet-tipped 0.035-inch epicardial wire is advanced toward the LAA apex, and creates end-to-end magnetic union with the endocardial wire. Using TEE and fluoroscopic guidance, the occlusion balloon is positioned at the LAA ostium. The LARIAT device is advanced over the epicardial wire, and the snare is closed. After removal of the endocardial wire and the balloon occlusion catheter from the LAA, the snare is further closed and the preloaded suture is released. LA angiography and color Doppler TEE are performed to confirm optimal ligation of the LAA.
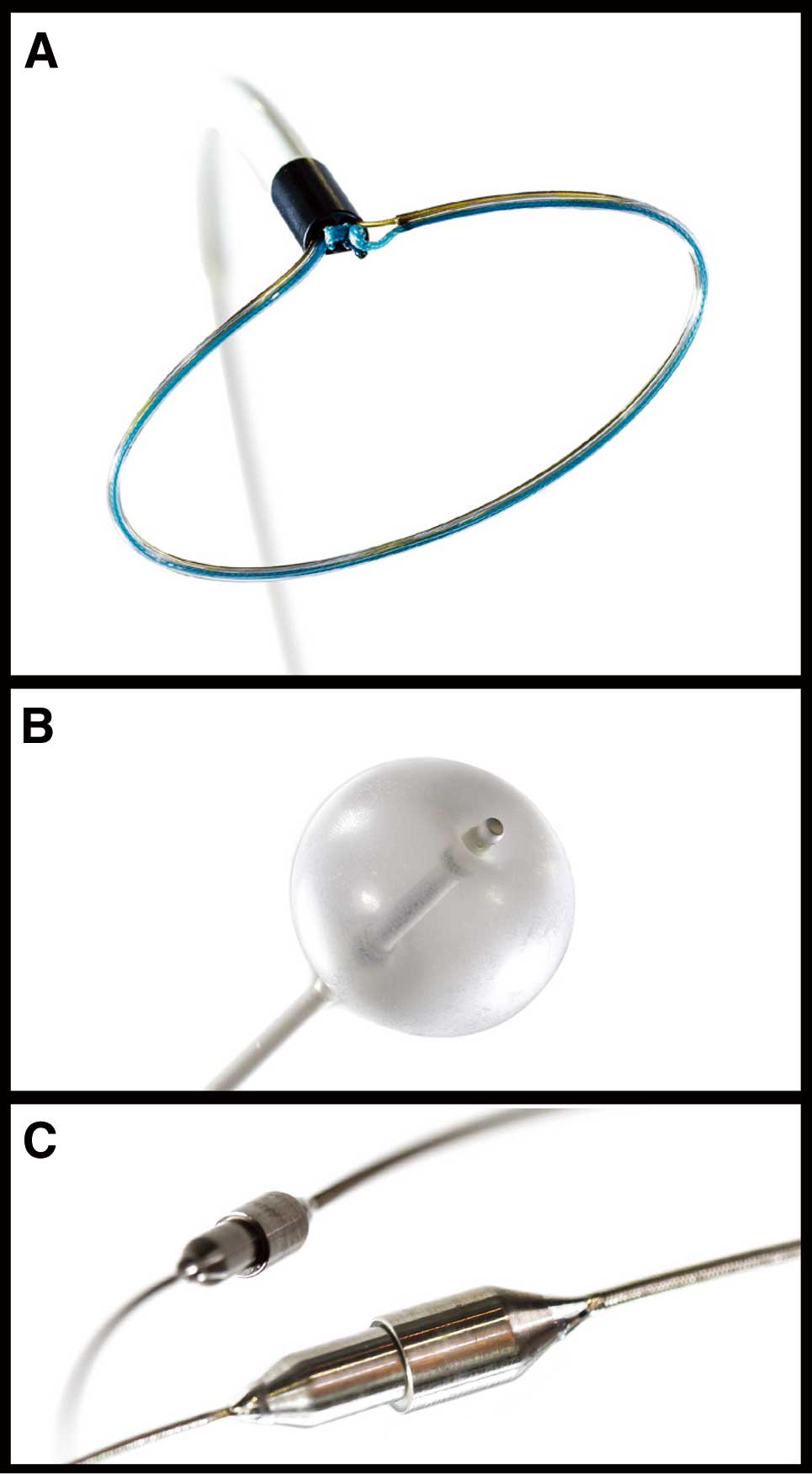
Components of percutaneous left atrial appendage ligation (courtesy of SentreHEART, Inc). (A) The LARIAT Suture Delivery Device. (B) The EndoCATH Large Occlusion Balloon. (C) The FindrWIRZ Magnetic Guide Wire System.

Cine angiograms of percutaneous left atrial appendage (LAA) ligation. (A) A 17G epidural needle is slowly advanced with a test injection of contrast (lateral fluoroscopic view). (B) A 0.035-inch wire is advanced into the pericardial space (lateral fluoroscopic view). (C) Baseline angiography is performed, which shows a cactus-type (LAA). (D) The epicardial wire is advanced toward the LAA apex through the epicardial cannula, and creates an end-to-end magnetic union with the endocardial wire. (E) The LARIAT Suture Delivery Device is advanced over the epicardial wire. (F) The snare is closed. (G) After removal of the endocardial wire and the balloon occlusion catheter from the LAA, the snare is further closed. (H) Final angiogram shows ligated LAA without residual communication.
Clinical Trials A previous prospective single-arm study reported procedural and 1-year follow-up results of the LARIAT procedure for nonvalvular AF with poor candidate or ineligibility for oral anticoagulation therapy.35 The procedure was successfully performed in 85 of 89 patients (96%). There were 3 periprocedural complications (3.3%); a right ventricular puncture and a laceration of a superficial epigastric vessel during pericardial puncture, and a pericardial effusion during transseptal puncture. Following the procedure, 20 of 85 patients developed chest pain, and 2 of 20 patients were diagnosed as having pericarditis requiring a nonsteroidal anti-inflammatory drug. During 1-year follow-up, there were 2 unexplained sudden deaths, 2 strokes (1 hemorrhagic, 1 lacunar), and 1 late pericardial effusion. TEE at 1-year follow-up (n=65) revealed complete closure in 64 patients (98%).
Current Perspective The LARIAT Suture Delivery Device received 510(k) approval from the US FDA for use as soft tissue ligation device in 2009. The device has been used off-label in the USA to ligate the LAA. There have been no trials conducted in the USA to confirm the safety and efficacy of this method. Despite the favorable results of previous studies, important technical limitations should be noted. First, this suture ligation system requires an intact pericardium to access the LAA; therefore, patients with a history of open heart surgery and pericarditis are contraindicated for this procedure. Second, the LARIAT system has anatomical exclusion criteria of the LAA; an LAA width >40 mm, LAA tip oriented behind the pulmonary artery trunk, LAA with multilobes that direct different planes exceeding 40 mm, and a posteriorly rotated heart. Indeed, in the previous study, 13% of patients were excluded because of the anatomical criteria.35 Despite these limitations, a potential advantage of this technique is that there is no foreign body left inside the cardiac chambers, which may have long-term advantages of endocardial closure of the LAA with a device.
Summary of Percutaneous LAA OcclusionIschemic stroke is a life-threatening complication of patients with AF. Although oral anticoagulation therapy effectively reduces the incidence of ischemic stroke, bleeding events still remain a major concern. Percutaneous LAA closure has emerged as a novel stroke prophylaxis for patients with nonvalvular AF. Previous studies demonstrated promising clinical outcomes of LAA closure. We believe that catheter-based stoke prophylaxis benefits patients with nonvalvular AF as an alternative to long-term anticoagulation therapy.
We reviewed percutaneous MV repair and percutaneous LAA occlusion, in which accurate and safe transseptal puncture is highly attributed to optimal procedural results. Although transseptal access was first utilized as a diagnostic tool, it is now recognized as an established safe and appropriate approach for structural heart interventions. The future is very bright, and this is just the beginning of a new era of transseptal interventions for structural heart disease.
We thank Asma Hussaini, PA, Mane Arabyan, Harrison Dinh, and Marilyn Grossi for their support in the preparation of this manuscript.
Saibal Kar received research grants and consulting fees from Abbott Vascular, Boston Scientific, and St. Jude Medical. No other author has relationships relevant to the contents of this manuscript to disclose.