2014 Volume 78 Issue 9 Pages 2317-2324
2014 Volume 78 Issue 9 Pages 2317-2324
Background: Saving more limbs of patients with peripheral arterial disease (PAD) from amputation by accelerating angiogenesis in affected limbs has been anticipated for years. We hypothesized that an anti-Alzheimer drug, donepezil (DPZ), can activate angiomyogenic properties of satellite cells, myogenic progenitors, and thus be an additional pharmacological therapy against PAD.
Methods and Results: In a murine hindlimb ischemia model, we investigated the angiogenic effects of a clinical dose of DPZ (0.2 mg·kg–1·day–1) and its combination with cilostazol, a platelet aggregation inhibitor and a conventional therapeutic drug against PAD. The combination therapy most effectively improved skin coldness and most effectively upregulated vascular endothelial growth factor (VEGF)-producing satellite cells in ischemic hindlimbs. Computed tomography revealed that DPZ remarkably attenuated ischemic muscle atrophy and induced super-restoration in affected hindlimbs. The in vitro study with human aortic endothelial cells showed that DPZ or its combination with cilostazol effectively upregulated the expression of pAkt, hypoxia inducible factor-1α, and VEGF protein. Likewise, in primary cultured satellite cells, DPZ, alone or in combination, upregulated the expression of VEGF, interleukin-1β, and fibroblast growth factor 2 protein.
Conclusions: The present results suggest that a clinical dosage of DPZ accelerates angiomyogenesis by directly acting on both endothelial and satellite cells. Therefore, DPZ is a potential additional choice for conventional drug therapy against PAD. (Circ J 2014; 78: 2317–2324)
Peripheral arterial disease (PAD) is a recognized atherosclerotic disease that involves the arteries of the entire body, with the exception of the coronary and intracranial arteries. Intermittent claudication is the earliest and most common symptom observed in patients with PAD.1 In the advanced stages, patients often suffer from skin ulcers and necrosis of the toes, a pathological phenotype of PAD referred to as critical limb ischemia.2
There are several therapeutic strategies currently available for PAD, including pharmacological therapy using antiplatelet agents and vasodilators, endovascular treatment, and surgical bypass grafting.1,2 To accelerate neovascularization, autologous transplantation of bone marrow cells3 and intramuscular vascular endothelial growth factor (VEGF) gene transfer4,5 have been tried for years, but it remains to be proven whether these are appropriate for the treatment of vascular diseases.2 Recently, the induction of angiogenesis using thermal therapy or prostacyclin therapy has been reported.6,7 In addition to clinically available therapies, novel neovascularization-accelerating therapies have been anticipated.
Our previous study demonstrated that a large dosage (5 mg·kg–1·day–1) of donepezil (DPZ), an acetylcholinesterase inhibitor, upregulated a non-neuronal cholinergic system in the vasculature to efficiently accelerate angiogenesis, and that such angiogenic effects were not mediated by a cholinesterase-inhibitory mechanism.8 In that study, we focused on the effects of DPZ on vascular cells; however, our preliminary studies thereafter have suggested that these DPZ-induced angiogenic signals are derived from cells other than endothelial cells.
Satellite cells, candidate skeletal muscle stem cells, are located in a specific niche between the plasma membrane (sarcolemma) of the myofiber and the basal lamina,9 and are strikingly close to capillaries.10 These cells are normally quiescent in adult skeletal muscle cells, but will be activated after myotrauma, and then drive myogenesis (ie, proliferation and self-renewal) through VEGF, fibroblast growth factor (FGF), interleukin (IL)-1β, hepatocyte growth factor, and platelet-derived growth factor,11–13 finally differentiating into multinucleated myofibers.11,14 It has been reported that the endothelial cells enhance satellite cell growth through FGF and VEGF,10 and that satellite cells undergoing differentiation also accelerate angiogenesis by secreting VEGF.15 Therefore, it is considered that angiogenesis and myogenesis share VEGF as a co-regulatory factor.16,17 We hypothesized that DPZ-enhanced VEGF protein expression by satellite cells would promote not only angiogenesis but also myogenesis, and thus improve critical limb ischemia. Therefore, in the present study using a murine hindlimb ischemia model, we examined whether DPZ targeted skeletal muscle, in particular, myogenic satellite cells. Moreover, we evaluated whether or not DPZ exhibited a synergistic effect with cilostazol (CSZ), which is a type III phosphodiesterase inhibitor possessing antiplatelet potency and widely used for intermittent claudication caused by PAD.18,19 Whereas a large dosage of DPZ was used in our previous in vivo study,8 a clinical dosage of 0.2 mg·kg–1·day–1 was selected for the present in vivo studies.
All experimental animals received humane care in strict accordance with the guiding principles of the Physiological Society of Japan. All experimental designs and surgical procedures were approved by the Animal Research Committee of Kochi Medical School (Permit Number: F-00072). All surgeries were performed under sodium pentobarbital anesthesia or isoflurane, and all efforts were made to minimize suffering. Mice were killed quickly by cervical dislocation and organs were excised for further experiments.
Human Aortic Endothelial Cell (HAEC) Culture StudyAs reported previously,8 HAECs were cultured in EGM-2 culture medium (Lonza, Walkersville, MD, USA) according to the manufacturer’s instructions. Pharmacologic experiments were performed in 3 groups, and the final concentrations of each reagent were as follows: 10 μmol/L of CSZ (Otsuka Pharmaceutical, Tokushima, Japan), 1 μmol/L of DPZ (Eisai, Tokyo, Japan), and the combined treatments (CSZ+DPZ). CSZ was dissolved in dimethyl sulfoxide and DPZ was dissolved in distilled water. HAECs were treated with each reagent for 12–24 h.
Primary Satellite Cell Culture StudyPrimary satellite cells were isolated from the hindlimbs of male C57BL/6 mice (SLC, Hamamatsu, Japan) by enzymatic dissociation, according to a previously described technique.20 The cells were cultured in Dulbecco’s modified Eagle’s medium (Sigma-Aldrich, St. Louis, MO, USA) supplemented with 4.5 g/L of glucose, 20% horse serum, 4 mmol/L of L-glutamine, 10 mmol/L HEPES, antibiotics and 3% chick embryo extract prepared using 9-day-old eggs of a TOSA-JIRO chicken (Shimasaki Chicken Farm, Kochi, Japan) in our laboratory. We had been previously confirmed that the cells were positive for immunoreactivity of paired box 7 (Pax7), a marker specific to satellite cells, and that they underwent terminal differentiation into muscle fibers with a lower serum concentration. Similar to the HAEC study, the effects of CSZ and DPZ on satellite cells were investigated. In addition, to investigate the signal transduction of DPZ through PI3K/Akt, satellite cells were pretreated with a PI3K inhibitor, LY294002 (Cell Signaling, Beverly, MA, USA), followed by treatment with DPZ.
Unilateral Hindlimb Ischemia ModelMale C57BL/6 mice aged 9–10 weeks were administered pentobarbital sodium (30 mg/kg IP) and the left common femoral artery was ligated proximally to create a hindlimb ischemia model. The mice were randomly assigned to 3 treatment groups: no treatment (control), CSZ (30 mg·kg–1·day–1), and CSZ+DPZ (0.2 mg·kg–1·day–1). All drugs were orally administered for 6 weeks in the drinking water. CSZ was initially suspended in 0.5% carboxymethyl cellulose sodium salt and mixed with drinking water because it is not water soluble. In a preliminary experiment, the daily drinking volume was estimated to be approximately 3 ml. In an additional experiment with a VEGF receptor tyrosine kinase inhibitor, axitinib, hindlimb ischemia mice were divided into 3 groups: no treatment (control), DPZ (0.2 mg·kg–1·day–1), and DPZ+axitinib (10 mg/kg twice daily, IP, Abcam, Cambridge, MA, USA). The dosage of axitinib was selected according to in vivo experiments previously reported.21,22
Thermography StudyHeart rate and blood pressure were measured noninvasively in conscious mice by a tail cuff method (Softron, Tokyo, Japan). As detailed previously,8 the skin temperature of the hindlimbs was repeatedly measured at 1, 3, and 5 weeks after ligation. The laterality of skin temperature between the ischemic and nonischemic hindlimbs was calculated.
At 6 weeks after ligation, the mice were humanely killed and the femoral quadriceps muscles were excised for immunohistochemical, western blot, and PCR analysis.
Immunohistochemical AnalysisAs reported previously,8 samples of hindlimb muscles were prepared for immunohistochemistry. Pax7 and VEGF immunoreactivities were identified with a monoclonal anti-Pax7 antibody at 1:200 dilution (Abcam) and a polyclonal anti-VEGF antibody at 1:100 dilution (Santa Cruz, CA, USA), respectively.
Western BlottingHAECs were harvested with sample buffers after the 12- and 24-h treatments. Satellite cells were also harvested after a 24-h treatment. Cell lysates were fractionated by SDS-PAGE and transferred onto membranes. Muscle cell lysates were taken from the quadriceps of ischemic hindlimbs and similarly processed for western blot analysis. The blotted membranes were incubated with primary antibodies against pAkt (Cell Signaling, Beverly, MA, USA) at 1:2,000 dilution, hypoxia inducible factor (HIF)-1α (Novus, Littleton, CO, USA) at 1:300 dilution, VEGF (Santa Cruz) at 1:1,000 dilution, FGF2 (Santa Cruz) at 1:1,000 dilution, and α-tubulin (Lab Vision, Fremont, CA, USA) at 1:2,000 dilution. After the reaction, membranes were washed, followed by conjunction with IR Dye conjugated goat anti-mouse IgG or anti-rabbit IgG antibodies (Li-Cor, St. Lincoln, NE, USA). They were then scanned with an infrared imaging system (Odyssey, Li-Cor). As previously reported,23 the HIF-1α protein level was quantified by immunoblotting.
Reverse Transcriptase PCRAs described previously,8 total RNA (0.5 μg) was isolated from muscle cells and reverse-transcribed to obtain single-stranded cDNA. The synthesized cDNA was amplified with gene-specific primers for VEGF and GAPDH. The sense and antisense gene-specific primers were as follows:
VEGF (sense), 5’-CCAGCACATAGGAGAGATGAGCTTC-3’
VEGF (antisense), 5’-GGTGTGGTGGTGACATGGTTAATC-3’
GAPDH (sense), 5’-CGTATGGGCGGCTGGTCACCAG-3’
GAPDH (antisense), 5’-GACCTTGCCCACAGCCTTGGCAG-3’
The optimal annealing temperature and the number of cycles for each template were as follows: 68°C, 30 cycles for VEGF and 69°C, 30 cycles for GAPDH. The ratio of the RT-PCR product for VEGF was quantified and compared with that of GAPDH.
Computed Tomography (CT) StudyThe effect of treatment on hindlimb muscle mass was evaluated by CT (LCT-200, Hitachi Aloka, Tokyo, Japan) in 4 groups as follows: no treatment (control), CSZ (30 mg·kg–1·day–1), DPZ (0.2 mg·kg–1·day–1), and CSZ+DPZ. For each animal, the mass of the gastrocnemius-soleus muscles distal to the knee joint was repeatedly measured at 2, 3, 4, and 5 weeks after treatment.
Statistical AnalysisStatistical analysis was performed with JMP (SAS, Cary, NC, USA) and STATA (StataCorp, College Station, TX, USA) software packages. Differences among 3 or 4 treatment groups were analyzed by the Kruskal-Wallis H test and the Steel-Dwass test. Because the Shapiro-Wilk W test indicated that values of the muscle mass ratio were assumed to be normally distributed, we performed multiple regression analysis to reveal whether there were significant relationships for the muscle mass ratio among 4 treatment groups. Data are presented as mean±SE, and differences were considered statistically significant at P<0.05.
All 3 treatments significantly increased the expression level of pAkt in 12 and/or 24 h compared with vehicle (Figure 1A). A significant increase in HIF-1α protein expression was observed between DPZ and vehicle at 24 h after treatment (Figure 1B). VEGF protein expression levels were significantly increased in both the DPZ and CSZ+DPZ groups at 24 h after treatment (Figure 1C).
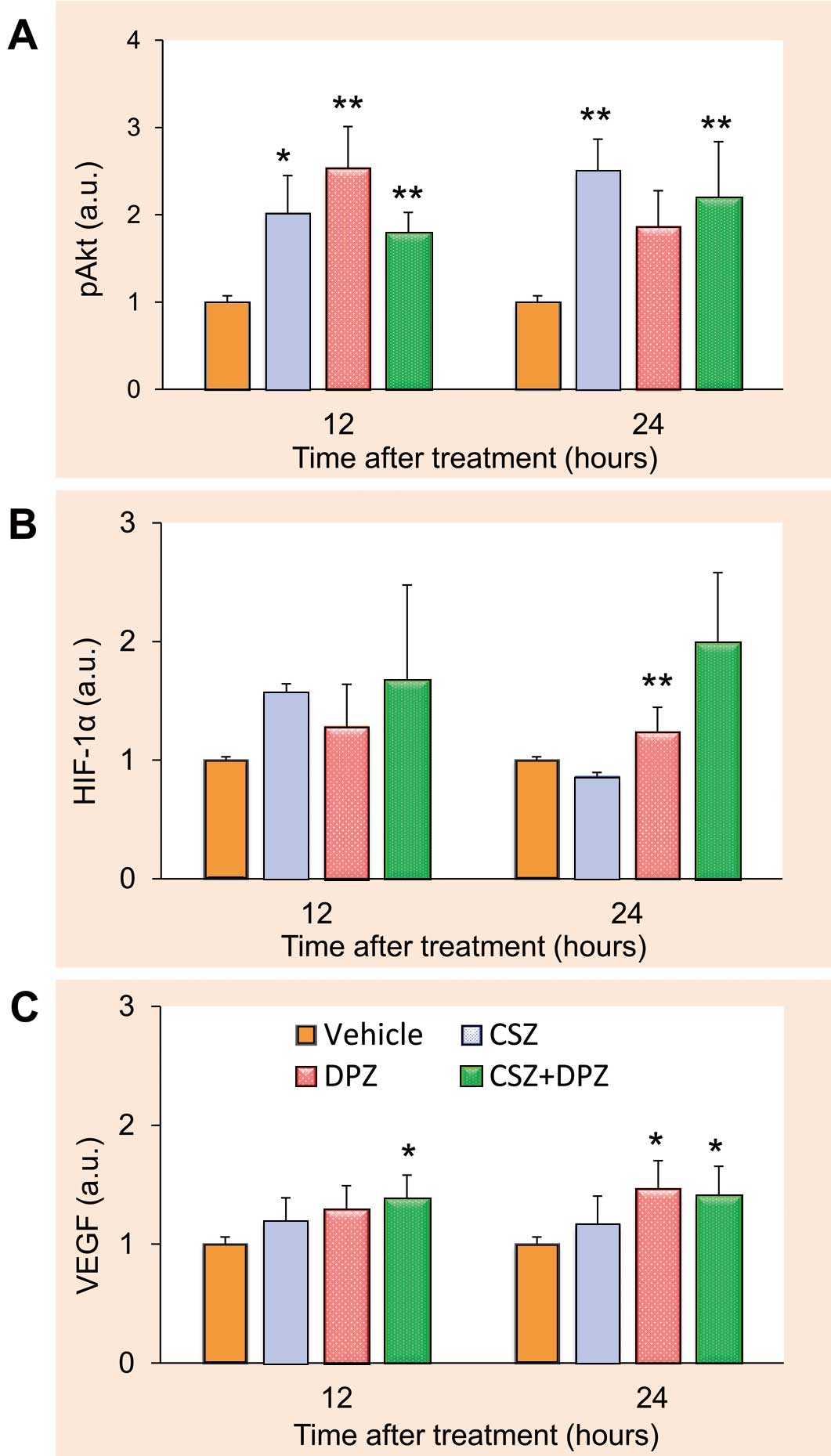
Effects of cilostazol (CSZ), donepezil (DPZ), and their combination (CSZ+DPZ) on the expression of pAkt (A), hypoxia inducible factor (HIF)-1α (B), and vascular endothelial growth factor (VEGF) protein (C) in human aortic endothelial cells (n=8 for each treatment group). Values are normalized by amount of α-tubulin. *P<0.05, **P<0.01 from control. a.u., arbitrary units. See text for details.
There were no effects of CSZ, DPZ or the combination therapy on heart rate or blood pressure. The effects of treatment on thermal laterality are shown in Figure 2, where values were calculated by subtraction of nonischemic skin temperature from ischemic skin temperature. At 3 weeks after ligation, laterality was attenuated by 16±9.8% in the CSZ group and significantly decreased to 46±9.1% in the CSZ+DPZ group. These results suggested that the combination treatment was more effective in improving hindlimb ischemia than conventional treatment with CSZ alone.
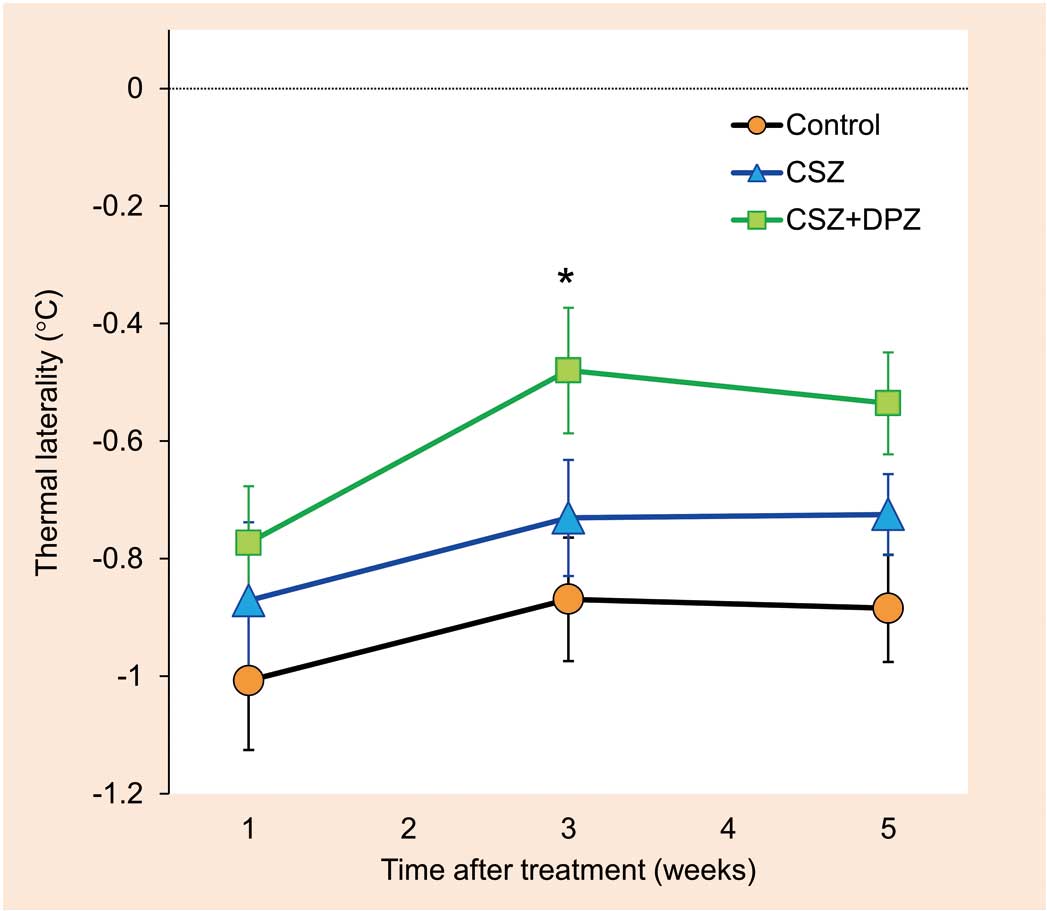
Effects of cilostazol (CSZ) alone and in combination with donepezil (DPZ) on thermal laterality at 1, 3, and 5 weeks after treatment. n=15 for each treatment group. *P<0.05 from control. See text for details.
Some of the small round-shaped cells that were located adjacent to capillary endothelial cells among muscle fibers were labeled with Pax7 (Figure 3A). In terms of their morphology and immunohistochemistry, these Pax7-positive cells were regarded as satellite cells. VEGF signals were often observed in satellite cells as well as endothelial cells. The ratio of VEGF-positive to Pax7-positive cells was significantly higher in the CSZ (69±4.5%) and CSZ+DPZ (91±3.5%) groups than in the control group (53±6.7%) (Figure 3B). A significant difference in the number of VEGF-positive cells per single muscle fiber was observed between the control and CSZ groups. Furthermore, the combination therapy increased the number of VEGF-positive cells per single muscle fiber (P<0.01 from control, P<0.05 from CSZ).
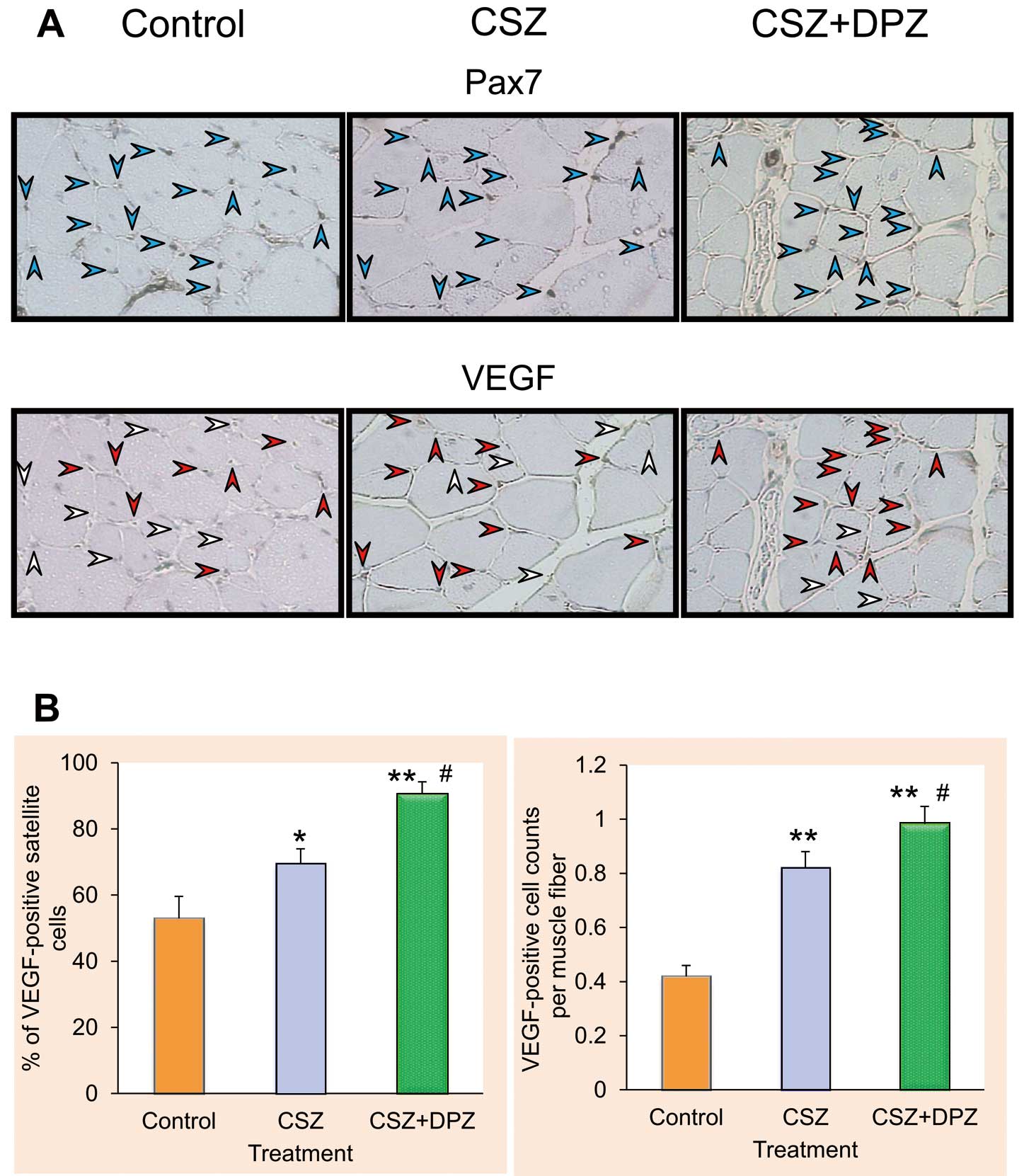
Effects of cilostazol (CSZ) alone and in combination with donepezil (DPZ) on the expression of Pax7 and vascular endothelial growth factor (VEGF) in ischemic muscle tissues. (A) Microscopic images of transverse sections of ischemic femoral quadriceps. Blue arrowheads indicate Pax7 immunosignals; red arrowheads indicate VEGF-positive and Pax7-positive cells, white arrowheads indicate VEGF-negative and Pax7-positive cells. (B) Percentages of VEGF-positive cells to Pax7-positive cells and the cell counts of VEGF positives per single muscle fiber (n=20 for each treatment group). *P<0.05 and **P<0.01 from control, #P<0.05 from CSZ. See text for details.
The effects of CSZ and DPZ on VEGF expression in ischemic muscle are shown in Figure 4. Compared with the control group, VEGF mRNA expression in the ischemic hindlimb was significantly high in the CSZ (1.97±0.10 arbitrary units [a.u.]) and CSZ+DPZ (2.24±0.20 a.u.) groups. Western blot analysis also showed a significant upregulation of VEGF protein in the CSZ (1.54±0.14 a.u.) and CSZ+DPZ (1.63±0.16 a.u.) groups.
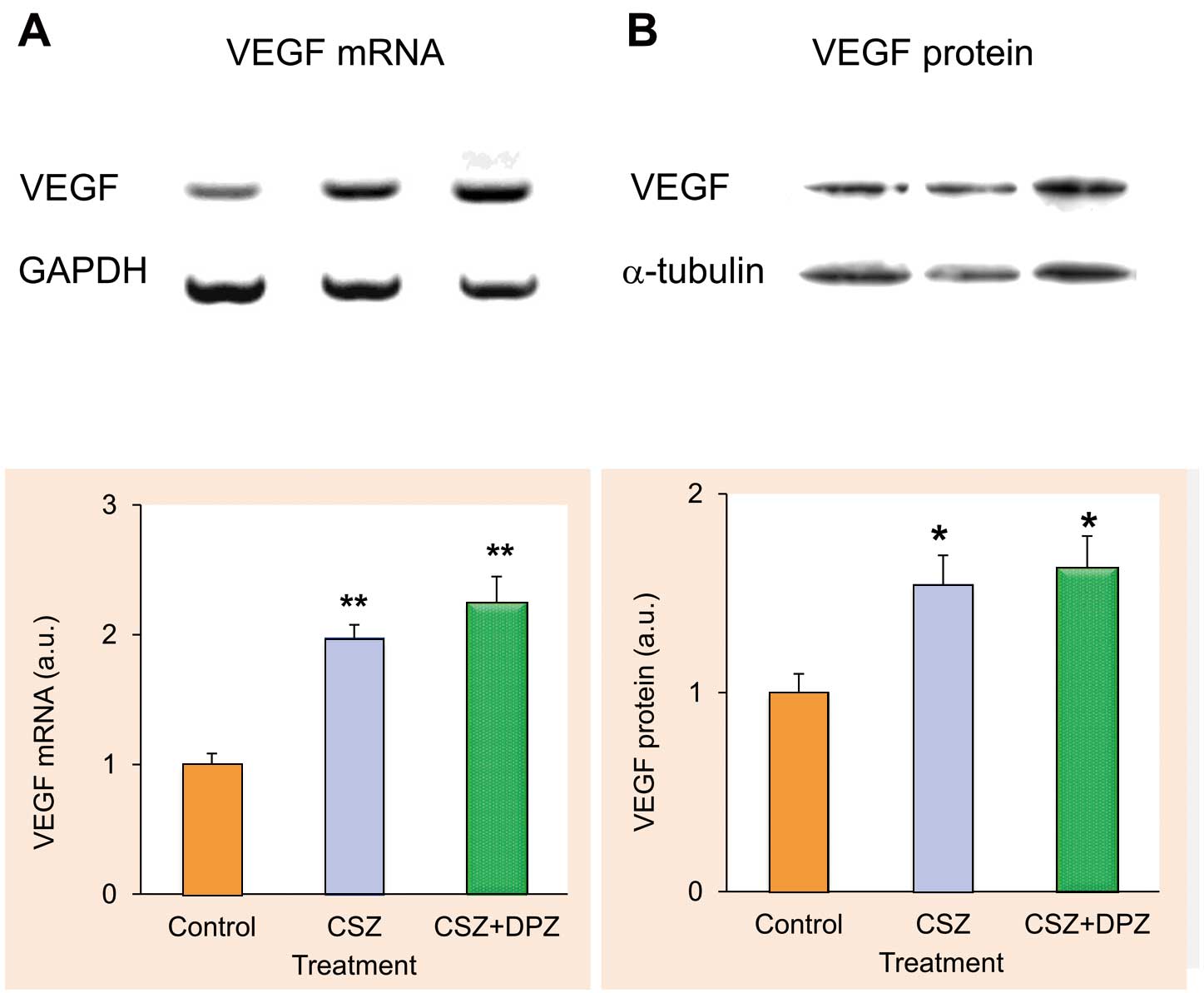
Effects of cilostazol (CSZ) alone and in combination with donepezil (DPZ) on the expression of vascular endothelial growth factor (VEGF) mRNA (A) and protein (B). n=8 for each treatment group. For mRNA measurement, values are normalized by amount of GAPDH; for protein measurement, values are normalized by amount of α-tubulin. *P<0.05 and **P<0.01 from control. a.u., arbitrary units. See text for details.
Compared with the vehicle group, VEGF protein expression was significantly enhanced in the CSZ (1.74±0.22 a.u.), DPZ (2.08±0.31 a.u.), and CSZ+DPZ (1.60±0.27 a.u.) groups at 24 h after treatment (Figure 5A). Protein expression for a myogenic factor, IL-1β, was significantly upregulated in the CSZ (2.28±0.50 a.u.), DPZ (5.51±1.07 a.u.), and CSZ+DPZ (3.48±0.63 a.u.) groups (Figure 5B). Similarly, FGF2 expression was significantly higher in the CSZ+DPZ group (2.84±0.57 a.u.) than in the vehicle group (Figure 5C).
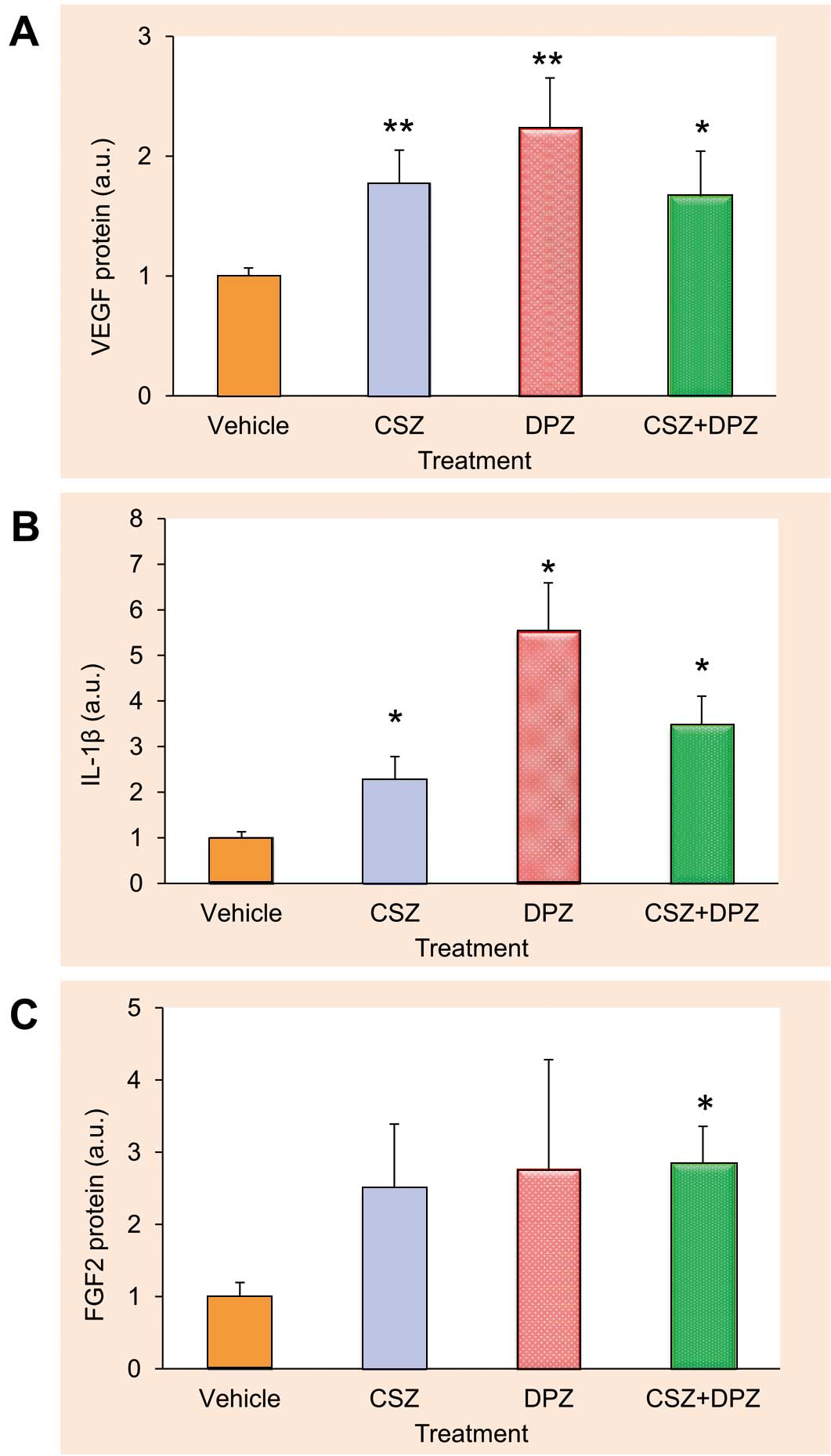
Effects of cilostazol (CSZ), donepezil (DPZ), and their combination (CSZ+DPZ) on protein expression of VEGF (A, n=10 for each treatment group), interleukin (IL)-1β (B, n=7 for each treatment group), and fibroblast growth factor (FGF) 2 (C, n=7 for each treatment group) in satellite cells. Values are normalized by amount of α-tubulin. *P<0.05 and **P<0.01 from control. a.u., arbitrary units. See text for details.
Primary cultured murine satellite cells treated with DPZ (1 μmol/L) showed increased HIF-1α protein expression (1.23±0.04 a.u.) even under normoxic conditions; however, this increment in the HIF-1α protein level was blunted with a PI3K/Akt inhibitor, LY294002 (50 μmol/L) (0.49±0.04 a.u.) (Figure 6).
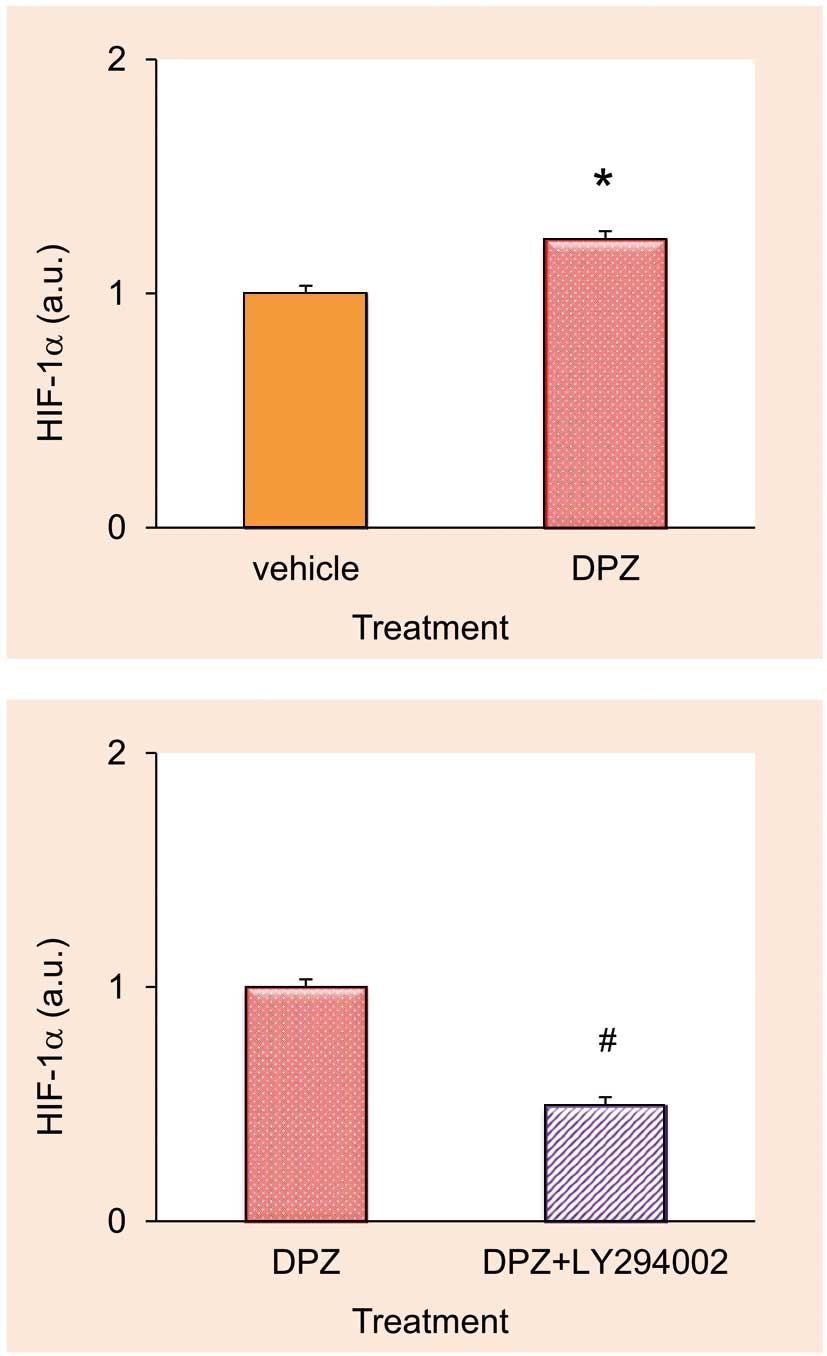
Effects of donepezil (DPZ) and its combination with LY294002 on hypoxia inducible factor (HIF)-1α protein expression. Statistical significance was observed between DPZ alone and LY294002+DPZ (n=4 for each treatment group). *P<0.05 from control, #P<0.05 from DPZ alone. a.u., arbitrary units. See text for details.
Figure 7 shows the muscle mass ratio of the ischemic to nonischemic hindlimbs. In the DPZ group, the mean muscle mass ratio remained >1 after 3 weeks of treatment. Multiple regression analysis revealed a statistically significant relationship between muscle mass ratio and DPZ treatment (P<0.01), suggesting that DPZ attenuated ischemia-induced muscle atrophy and induced super-restoration in the affected limb.
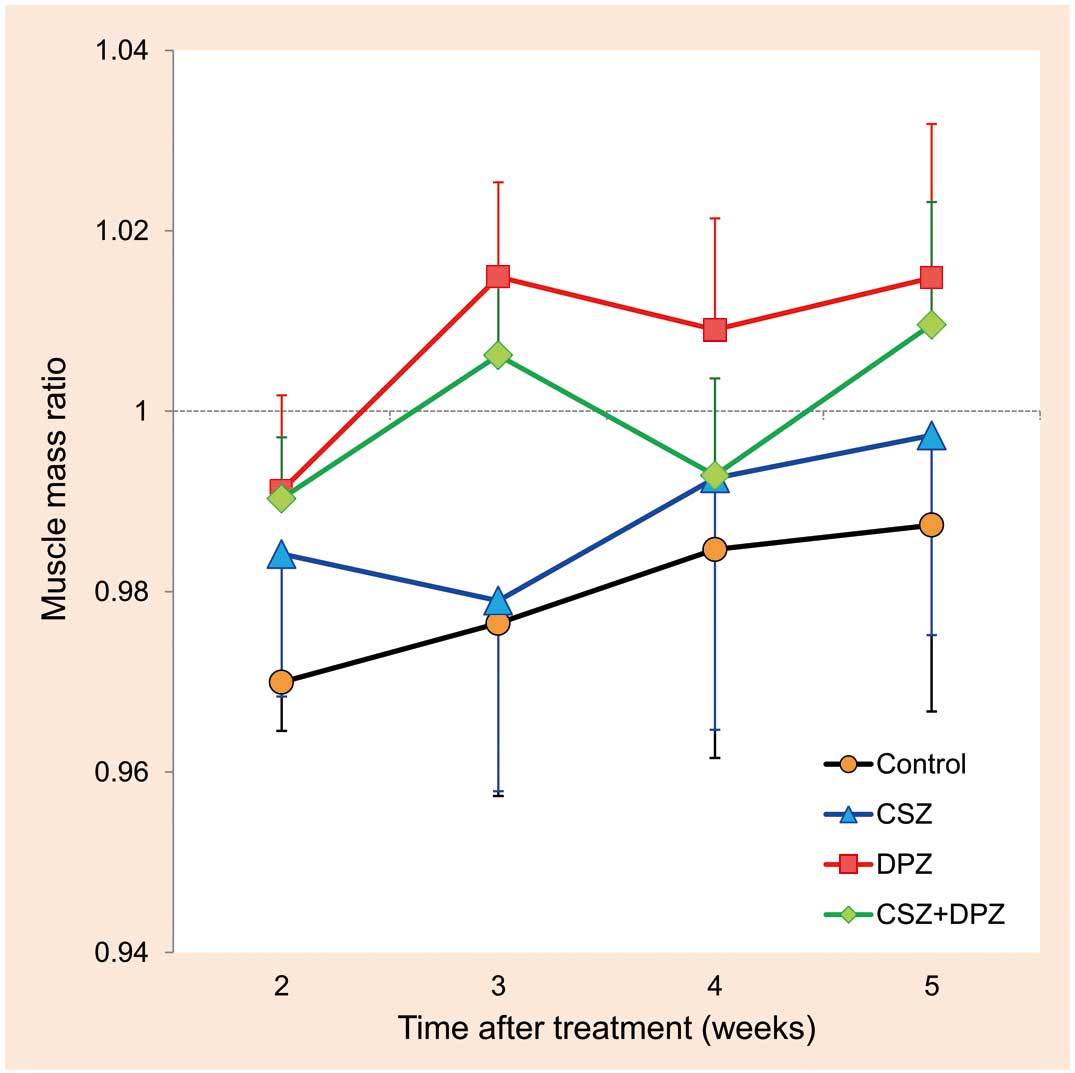
Effects of cilostazol (CSZ), donepezil (DPZ), and their combination (CSZ+DPZ) on ischemic muscle atrophy. Values are the muscle mass ratio of the ischemic to nonischemic hindlimb. A statistically significant relationship between muscle mass ratio and DPZ treatment was observed (P<0.01). n=6 for each treatment group. See text for details.
The inhibitory effect of DPZ on muscle atrophy was canceled by a selective tyrosine kinase inhibitor of the VEGF receptor, axitinib, as evaluated by thermal laterality: DPZ 126±19% vs. DPZ+axitinib 5±18% (P<0.05). Representative images are shown in Figure 8.
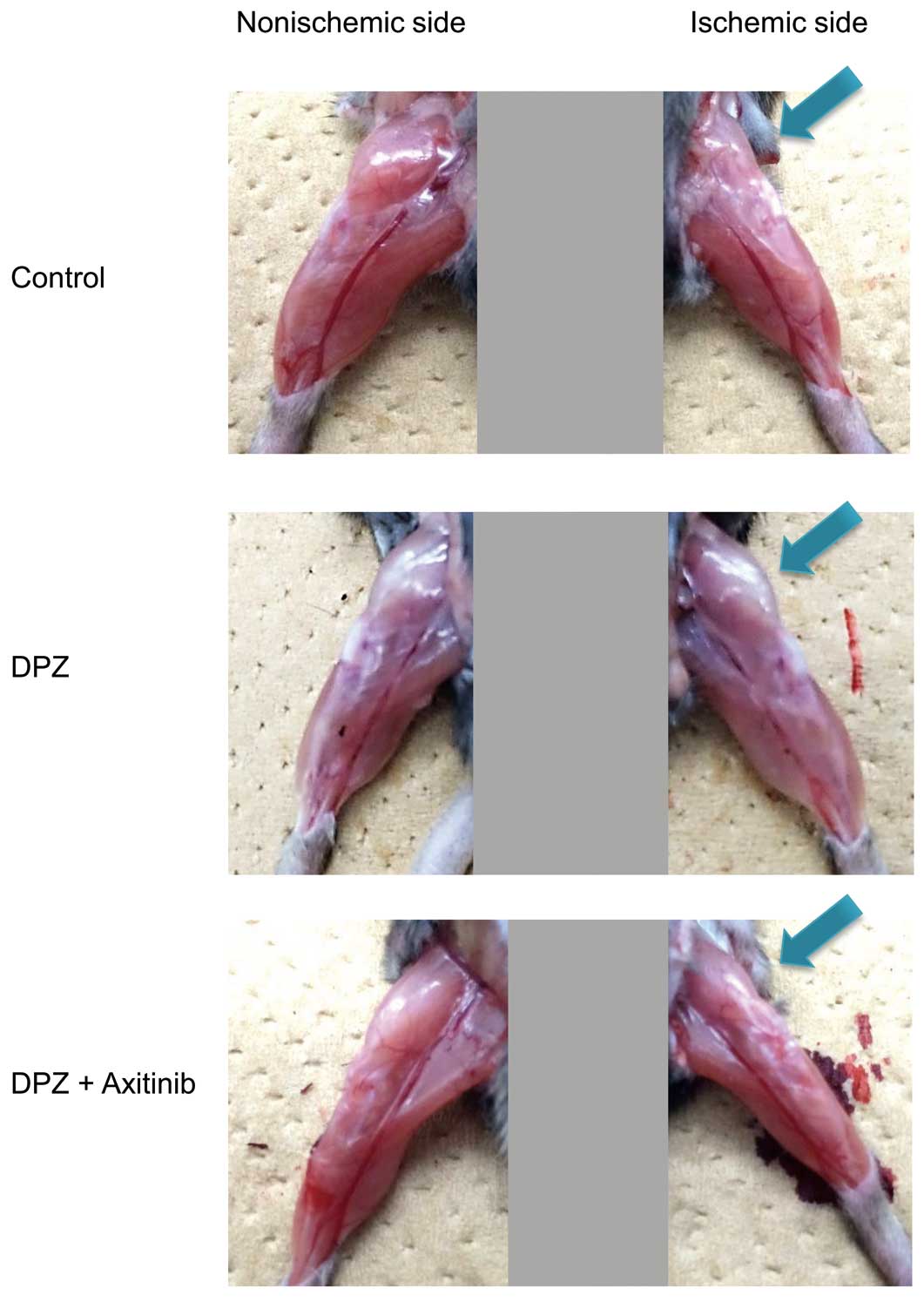
Effects of axitinib on the donepezil-induced anti-muscle atrophy effect. Representative images suggest that axitinib inhibits the effect of donepezil.
Our previous study8 confirmed that the angiogenic actions of DPZ target vascular endothelial cells, leading to suppression of ischemia-induced muscle atrophy. In the present study, therefore, we investigated the effects of DPZ on satellite cells and ischemic muscle atrophy with careful consideration of the following points. First, although a large dosage of DPZ (5 mg·kg–1·day–1) was used in our previous studies, we selected a clinical dosage of 0.2 mg·kg–1·day–1 in the current in vivo study. Second, a synergistic effect of DPZ with other conventional PAD drugs was investigated for clinical application of DPZ to PAD. Monotherapy with DPZ was not investigated in the present study, because maintenance of skin temperature and upregulation of VEGF expression in DPZ-treated ischemic hindlimbs has been reported.8
DPZ alone and in combination with CSZ increased VEGF protein expression in HAECs. Phosphorylation of Akt was also increased by CSZ, DPZ, and their combination. Moreover, HIF-1α protein expression was elevated, particularly by DPZ, via a PI3K/Akt pathway, which sustained protein stabilization. These results suggest that the combination therapy had a synergistic angiogenic effect on endothelial cells. Such effects of DPZ on endothelial cells were confirmed in the in vivo study. Mice treated with the combination therapy showed the least laterality in skin temperature, suggesting that DPZ protected the affected hindlimb from ischemia. Immunohistochemical studies indicated that the number of VEGF-positive satellite cells in the ischemic skeletal muscle was increased by CSZ, and more effectively when combined with DPZ. These results suggest that satellite cells were recruited to the ischemic muscle, and were activated to produce VEGF protein, by CSZ and DPZ.
Differences among treatments over the time course of changes in skeletal muscle mass of hindlimbs after ischemic insult were revealed by the CT scan study. DPZ prevented loss of skeletal muscle and induced super-restoration. Such a beneficial effect of DPZ could be the result from its action on satellite cells, and thus, DPZ is expected to be a novel therapeutic agent against ischemic muscle atrophy.
To clarify the cellular mechanism of the beneficial effect of DPZ on ischemic muscle atrophy, we investigated the effect of DPZ on primary cultured murine satellite cells. DPZ also upregulated expression of all angiogenic factors (ie, VEGF, IL-1β, and FGF2) in satellite cells in vitro. DPZ-induced upregulation of angiogenic and myogenic factors was potentiated by CSZ. These results suggested that DPZ improves ischemic muscle atrophy by activating the angiomyogenic properties of satellite cells. Therefore, DPZ could be a promising option for concomitant drug therapy with CSZ.
In contrast to our previous studies, a recent study by Miyazaki et al reported that DPZ attenuated angiogenesis through inhibition of the PI3K/Akt pathway in a murine hindlimb ischemia model.24 They administered 10 mg·kg–1·day–1 of DPZ to mice, which is 100-fold higher than the suggested clinical dosage, and the dose was higher than what they referred to as the usual dose for rodents.25 Therefore, their experimental findings should be interpreted carefully. In our studies, we never used a dosage larger than 5 mg·kg–1·day–1, because chronic overdosage of DPZ resulted in growth retardation and weight loss because of anorexia and diarrhea (unpublished observations). In addition, we had already investigated VEGF expression in vitro and in vivo with higher doses of DPZ (10 μmol/L) in both HAECs and murine ischemic skeletal muscles (10 mg·kg–1·day–1), and confirmed that even at these higher doses DPZ did not attenuate angiogenesis at all. Our in vivo study with 10 mg·kg–1·day–1 DPZ did not alter the expression of angiogenic factors and its-related cytokine, unlike Miyazaki et al reported. Indeed, the reason for the discrepancy between the 2 studies is unexplainable, but we believe that appropriate selection of dosage is important in translational research.
From the current study, it could not be completely concluded that myogenesis is a cause or consequence of angiogenesis during DPZ treatment. However, as with the previous study by Kuwabara et al,26 we speculate that a muscle-derived angiogenic factor plays an important role in the hindlimb ischemia model, which is also supported by Tateno K et al.11 Therefore, we speculate that DPZ could inhibit skeletal muscle loss in hindlimb ischemia to sustain muscle volume and stimulate myogenesis through increased Pax7-positive cells, both resulting in activation of the angiogenic mechanism of skeletal muscle, that is, satellite cells. Because the DPZ effects appear evident within the first week, much earlier than the time point when a non-treated ischemic hindlimb shows a nadir of subjected skin temperature, this result also suggests that DPZ salvages skeletal muscle and accelerates angiogenesis.
As shown by our previous studies, it is unlikely that the angiogenic effect of DPZ on endothelial cells results from its inhibitory action on cholinesterase. In our preliminary study, DPZ (1 μmol/L) upregulated angiomyogenic factors in primary cultured satellite cells even with an excessive concentration of physostigmine (100 μmol/L). Therefore, the angiomyogenic effect of DPZ on satellite cells is independent of cholinesterase inhibition. Such a mechanism of action is a pharmacologic merit for the clinical use of DPZ for PAD. If angiomyogenic effect of DPZ on satellite cells is dependent of cholinesterase inhibition alone, it must require a large dosage of DPZ in skeletal muscle. But we consider “NOT”. As described above, the effect of donepezil is independent of cholinesterase inhibition. Therefore, we can expect promising effect of clinical dosage of DPZ against PAD patients without concerns about a cholinesterase inhibitor’s adverse effect. Recent clinical studies by us27,28 and Nordström et al29 support our proposal for the clinical use of DPZ for PAD. These studies showed that a clinical dosage of DPZ for Alzheimer’s disease reduced the risk for myocardial infarction, which shares risk factors with PAD. Taken together, these experimental and clinical findings suggest favorable effects of DPZ on PAD, and thus provide the rationale for a clinical study of DPZ to treat PAD.
Study LimitationsA murine hindlimb ischemia is a model for acute limb ischemia, not for chronic PAD; there are few suitable small animal models of human PAD. Therefore, our present findings should be applied to clinical situations with careful consideration. In the near future, a case-control study is expected to be performed.
The present study demonstrated that a clinical dosage of DPZ can suppress ischemic muscle atrophy by activating the angiomyogenic properties of satellite cells. Therefore, DPZ, based on this novel concept consolidated by our basic studies, could be a potential adjunct to conventional drug therapy against PAD.
We especially thank all members of Institute for Laboratory Animal Research in Kochi University for their care of the experimental animals. This work was supported by Grants-in-Aid from the Japan Society for the Promotion of Science (21590283, 23590678), and in part by a grant from the Smoking Research Foundation.
None.
None declared.
This work was supported by Grants-in-Aid from the Japan Society for the Promotion of Science (21590283, 23590678), and in part by a grant from the Smoking Research Foundation.