2015 Volume 79 Issue 11 Pages 2499-2508
2015 Volume 79 Issue 11 Pages 2499-2508
Background: Atherosclerosis is a progressive inflammatory disease that can lead to sudden cardiac events by plaque rupture and subsequent thrombosis. Factor Xa (FXa) not only occupies a crucial position in the coagulation cascade responsible for thrombin generation, but also has pro-inflammatory effects. The hypothesis that Fondaparinux, the selective FXa inhibitor, attenuates plaque progression and promotes stability of atherosclerotic lesions was assessed.
Methods and Results: Fondaparinux (5 mg/kg body weight/day) or 0.9% saline was intraperitoneally administered for 4 weeks to apolipoprotein E-deficient mice (n=12 per group) with established atherosclerotic lesions in the innominate arteries. Fondaparinux did not remarkably decrease the progression of atherosclerosis development in apolipoprotein E-deficient mice, but increased the thickness of fibrous cap (P=0.049) and decreased the ratio of necrotic core (P=0.001) significantly. Moreover, Fondaparinux reduced the staining against Mac-2 (P=0.017), α-SMA (P=0.002), protease-activated receptor (PAR)-1 (P=0.001), PAR-2 (P=0.003), CD-31 (P=0.024), MMP-9 (P=0.000), MMP-13(P=0.011), VCAM-1 (P=0.041) and the mRNA expression of inflammatory mediators (P<0.05) significantly, such as interleukin (IL)-6, MCP-1, IFN-γ, TNF-α, IL-10 and Egr-1.
Conclusions: Fondaparinux, the selective FXa inhibitor, can promote the stability of atherosclerotic lesions in apolipoprotein E-deficient mice, possibly through inhibiting expression of the inflammatory mediators in plaque and reduced synthesis of MMP-9 and MMP-13. (Circ J 2015; 79: 2499–2508)
Cardiovascular diseases are the leading cause of morbidity and mortality worldwide. The most important contributors to cardiovascular diseases are acute coronary syndromes, which have a strong association with the formation and disruption of unstable atherosclerotic plaques. Unstable atherosclerotic plaque is generally characterized by thin fibrous cap, large lipid core, active inflammation (monocyte/macrophage and sometimes T-cell infiltration), paucity of smooth muscle cells (SMCs) and inadequate or impaired elastin and collagen proteins.1 The rupture or erosion of unstable atherosclerotic plaque initiates the thrombotic process by exposing the pro-thrombotic content of necrotic cores to circulating thrombocytes,2 which triggers platelet aggregation with the subsequent coagulation cascade resulting in thrombin formation. The intrinsic and the extrinsic pathway of the coagulation cascade converge at the activation of coagulation protease factor X (FX) to FXa, leading to the production of thrombin and the ensuing acute vascular occlusion. Moreover, increasing evidence showed that FXa also participates in atherosclerotic heart disease in ways that do not directly involve thrombus formation; it mediates intracellular signaling via activation of protease-activated receptors (PARs).3 These signaling events concern proliferation,4 fibrosis,5 angiogenesis,6 tissue remodeling,7 inflammation5 and atherosclerosis.8
Editorial p 2329
Fondaparinux, the selective FXa inhibitor, is a synthetic antithrombin-III (AT-III) binding pentasaccharide, which inhibits FXa by enhancing the reactivity of AT-III to FXa. The binding of fondaparinux to AT-III potentiates the neutralization of FXa by 300-fold.9 Its role in the prevention or treatment of thromboembolic diseases has already been proved in clinical studies.10–12 In addition to the anticoagulant effect, as presented by several studies, fondaparinux furthermore has a positive effect on modification of inflammatory responses in the mouse models of myocarditis,13 kidney ischemia-reperfusion injury14 and metabolic syndrome.15 Moreover, it has been shown that fondaparinux can suppress fibrin deposition and angiogenesis effectively.16
Anticoagulants are commonly used in cardiovascular events; different anticoagulants have different outcomes. Fondaparinux can indirectly inhibit FXa, but not thrombin, while appropriate thrombin level is protective.17 FXa is no more a passive bystander in the coagulation cascade, but the potential therapeutic target. Hence, in the present study, we assess the effects of fondaparinux, the specific indirect FXa inhibitor, on the development and stability of atherosclerotic lesions in apolipoprotein E-deficient (ApoE–/–) mice.
The research was approved by the local Animal Ethics Committee of Southeast University (Nanjing, Jiangsu, P. R. China). The housing and care of animals and all the procedures done in the study were performed in accordance with the guidelines and regulations of the local Animal Ethics Committee of Southeast University.
Animal Model and Drug TreatmentMale ApoE–/– mice were purchased from The Jackson Laboratory and placed on a lard-containing diet comprising 21% lard and 0.15% added cholesterol at 6 weeks of age. All mice were housed in the same room and exposed to the same light-dark cycle and diet. Mice were housed at no more than four mice per cage. At 20 weeks of age, mice were divided into 2 groups (12 mice per group): disease control group and fondaparinux treatment group. Fondaparinux was intraperitoneally administered at a dose of 5 mg·kg–1·day–1 according to body weight from week 20 to 24.14 In the control group, the same volume of 0.9% saline was intraperitoneally injected with the same intervals.
Animal Sacrifice and Preparation of TissueAt the age of 24 weeks, mice were heavily sedated, before blood was collected from the inferior vena cava and saved in heparin vials. Blood samples were centrifuged at 1,500 rpm for 10 min at 4℃ to obtain plasma, which was stored at –20℃ until analysis. Mice were euthanized by exsanguination and perfused with 10 ml of phosphate-buffered saline at physiological pressure via the left ventricle, and thoracic aortas were ligated distal of the brachiocephalic artery and removed for subsequent gene expression analysis. Then, mice were perfused with 4% buffered formalin and the innominate arteries were dissected out, embedded in paraffin and serially sectioned (5 μm). Every fifth section was stained with hematoxylin and eosin. To identify vascular calcification, van Kossa stain was performed on adjacent slices.18 To detect intraplaque hemorrhage, additional sections were evaluated following Prussian blue stain. To detect collagen, Masson stain was performed on adjacent slices.
Determination of Plasma Lipid LevelsPlasma concentrations of total cholesterol, triglycerides, high-density lipoprotein (HDL) and low-density lipoprotein (LDL) cholesterol were determined enzymatically in heparinized plasma.
Evaluation of Plaque Composition and Lesion SizeFive consecutive sections per animal were used for analysis. Sections were selected by identifying the slide with the largest and most complex lesions. Two slides prior and 2 slides after the identified slide were also selected for analysis. Samples were analyzed by 2 independent blinded investigators and indices of atherosclerotic plaque were evaluated, which included the total lesion area, maximum lesion thickness, percentage of stenosis, thickness of fibrous cap, ratio of necrotic core, ratio of collagen to total lesion area, presence of cholesterol crystals, medial erosion (defined as infiltration of plaque tissue into the media), lateral xanthomas (defined as the presence of aggregates of macrophage-derived foam cells situated on the lateral margins of the plaques), intraplaque hemorrhage (defined as the presence of hemosiderin) and calcification (defined on the basis of positive staining with the van Kossa stain). These data were determined by using computer-assisted morphometry (Olympus, Tokyo, Japan) and used for subsequent statistical comparison.
ImmunohistochemistryFive slides per animal were acquired, as previously described, and analyzed by means of immunohistochemistry. Tissue sections were dewaxed and rehydrated. Endogenous peroxidase activity was inhibited by incubation with peroxoblock (Invitrogen, Karlsruhe, Germany). After sections were blocked with 20% (vol/vol) goat serum in PBS, they were incubated overnight at 4℃ either with an anti-Mac-2 mouse macrophage antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-smooth muscle alpha actin antibody (Santa Cruz Biotechnology), anti-PAR-1 antibody (Santa Cruz Biotechnology), anti-PAR-2 antibody (Santa Cruz Biotechnology), anti-CD 31 antibody (which is regarded as evidence of neovascularization; Abcam, Cambridge, England), anti-MMP-9 antibody (Santa Cruz Biotechnology), anti-MMP-13 antibody (Santa Cruz Biotechnology), anti-VCAM-1 antibody (Santa Cruz Biotechnology) or anti-Factor X antibody (Santa Cruz Biotechnology) according to the manufacturers’ protocols. Sections then were incubated with the biotinylated secondary antibodies, rinsed 3 times with phosphate-buffered saline, and incubated for 10 min with streptavidin at room temperature. AEC-chromogen substrate (Invitrogen) was used for visualization. The extent of positive staining within the lesions was determined by using computer-assisted morphometry (Olympus). Specimens were scored according to the intensity of the dye color and the number of positive cells. The intensity of the dye color was graded as 0 (no color), 1 (light yellow), 2 (light brown) or 3 (brown); the number of positive cells was graded as 0 (<5%), 1 (5–25%), 2 (25–50%), 3 (51–75%) or 4 (>75%). The two grades were multiplied together and specimens were assigned to one of 4 levels: 0–1 score (–), 2 scores (+), 3–4 scores (++), more than 5 scores (+++).19
Quantitative Real-Time Reverse Transcriptase-Polymerase Chain Reaction (PCR)Quantitative real-time reverse transcriptional-PCR (RT-PCR) was performed, as previously described.6 Total RNA was extracted from thoracic aortas and reverse transcribed with a RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, Waltham, MA, USA). Inflammatory cytokine gene primers were designed as follows:
TNF-α:
Forward: 5’-ACAGAAAGCATGATCCGCGA-3’
Reverse: 5’-CGATCACCCCGAAGTTCAGT-3’
IFN-γ:
Forward: 5’-ACTGTGATTGCGGGGTTGTA-3’
Reverse: 5’-ACATTCGAGTGCTGTCTGGC-3’
Interleukin (IL)-6:
Forward: 5’-GAGACTTCCATCCAGTTGCCT-3’
Reverse: 5’-TGGGAGTGGTATCCTCTGTGA-3’
IL-10:
Forward: 5’-AGGCGCTGTCATCGATTTCT-3’
Reverse: 5’-ATGGCCTTGTAGACACCTTGG-3’
Egr-1:
Forward: 5’-GCTCCTTCAGCACCTCAACT-3’
Reverse: 5’-GGAGGCAGGGATGGTAAGTG-3’
MCP-1:
Forward: 5’-CCTTTAGCTTGCCCCCATTC-3’
Reverse: 5’-GCTGCTGGAGGTTAGGTCTTTAT-3’
Quantitative RT-PCR was performed for each gene on a light cycler according to the manufacturer’s protocol.
Statistical AnalysisStatistical analysis was performed by using SPSS 17.0. All data were represented as mean±SEM. Significant differences between means were determined with a Student’s t-test or one-factor ANOVA. Ratio data were analyzed using a Chi-squared test. The statistical significance level was P<0.05.
Fondaparinux led to a dose-dependent decrease in thrombin generation (Figure 1A), but there was no significant difference on decrease between fondaparinux group (from 1 mg/kg to 5 mg/kg) and the control group. Compared to the control group, the endogenous thrombin potential showed a mean reduction of 18.01±3.14% with the applied dose of 5 mg/kg (1,241±87 nmol vs. 1,510±51 nmol of the control group, P=0.06). A statistically significant decrease was observed from the dose of 7 mg/kg (1,058±68 nmol vs. 1,510±51 nmol of the control group, P=0.01). In order to avoid the anticoagulant effect of fondaparinux, we chose the dose of 5 mg/kg. During the entire experimental period, no major bleeding complications were observed and no mice died. The administration of fondaparinux did not cause significant differences in body weight, total cholesterol, triglycerides, LDL-cholesterol and HDL-cholesterol between the 2 groups (Table 1).
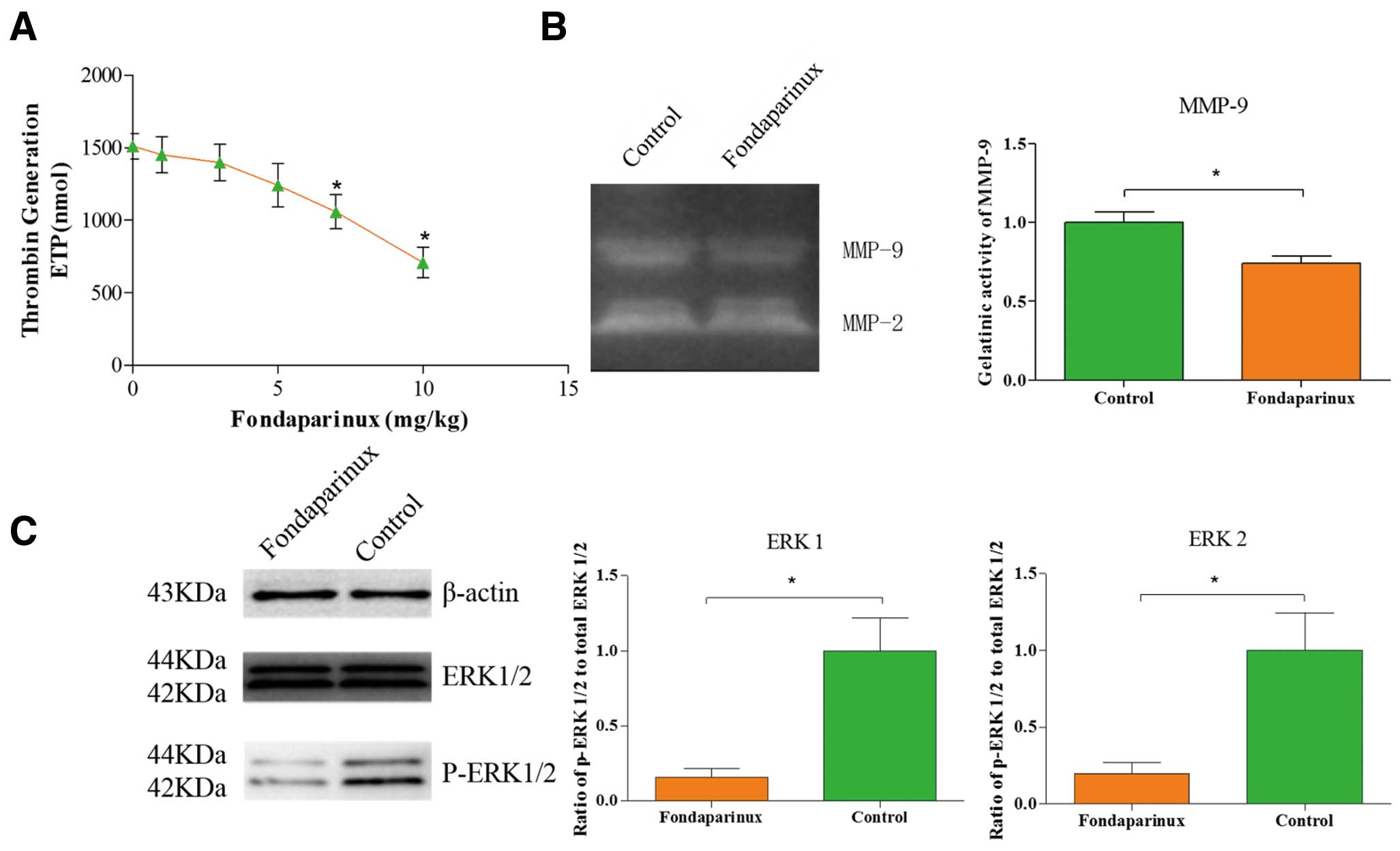
Some effects of fondaparinux. (A) Effect of fondaparinux on thrombin generation. Fondaparinux led to a dose-dependent decrease in thrombin generation; there was no significant difference on decrease between the fondaparinux group (from 1 mg/kg to 5 mg/kg) and the control group; a statistically significant decrease was observed at the doses of 7 mg/kg and 10 mg/kg. (B) Activity assay of MMP9. Administration of fondaparinux at a dose of 5 mg·kg–1·day–1 significantly decreased the enzyme activity of MMP-9. Data represent relative fold of enzyme activity as compared to the control group. (C) Inhibition of protease-activated receptors (PAR)-1/2 signal transduction by fondaparinux. Western blot analysis of phospho-ERK1/2 indicates that signal transduction was reduced significantly in the fondaparinux group. ERK1/2 phosphorylation is used as a surrogate marker for PAR-1 and PAR-2 signaling. Protein loading was verified and normalized using β-actin. Data represent mean±SEM. *P<0.05.
| Control (n=12) | Fondaparinux (n=12) | P value | |
|---|---|---|---|
| Body weight (g) | 28.67±0.75 | 28.38±0.35 | 0.730 |
| Total cholesterol (mmol/L) | 15.11±1.58 | 15.47±1.52 | 0.871 |
| Triglycerides (mmol/L) | 3.10±0.68 | 2.66±0.61 | 0.633 |
| LDL-cholesterol (mmol/L) | 6.26±0.56 | 6.25±0.62 | 0.989 |
| HDL-cholesterol (mmol/L) | 3.29±0.22 | 3.18±0.30 | 0.769 |
LDL, low-density lipoprotein; HDL, high-density lipoprotein. Differences were not significant between the study groups. Data represent mean±SEM. *P<0.05.
Quantification of atherosclerotic lesions in brachiocephalic arteries showed that a 4-week fondaparinux administration did not remarkably decrease the progression of atherosclerosis development in ApoE–/– mice (Figures 2A–C). Morphometric analysis of atherosclerotic plaques also revealed that administration of fondaparinux did not cause significant differences in the presence of cholesterol crystals, medial erosion, lateral xanthomas, intraplaque hemorrhage or calcification between the 2 groups (Table 2; Figure 3). However, administration of fondaparinux increased the thickness of the fibrous cap remarkably (56.78±6.18 μm vs. 42.35±2.62 μm of the control group, P=0.049; Figure 2D); moreover, the ratio of necrotic core was significantly decreased owing to the fondaparinux administration (34.8±0.91% vs. 41.32±1.44% of the control group, P=0.001; Figure 2E).
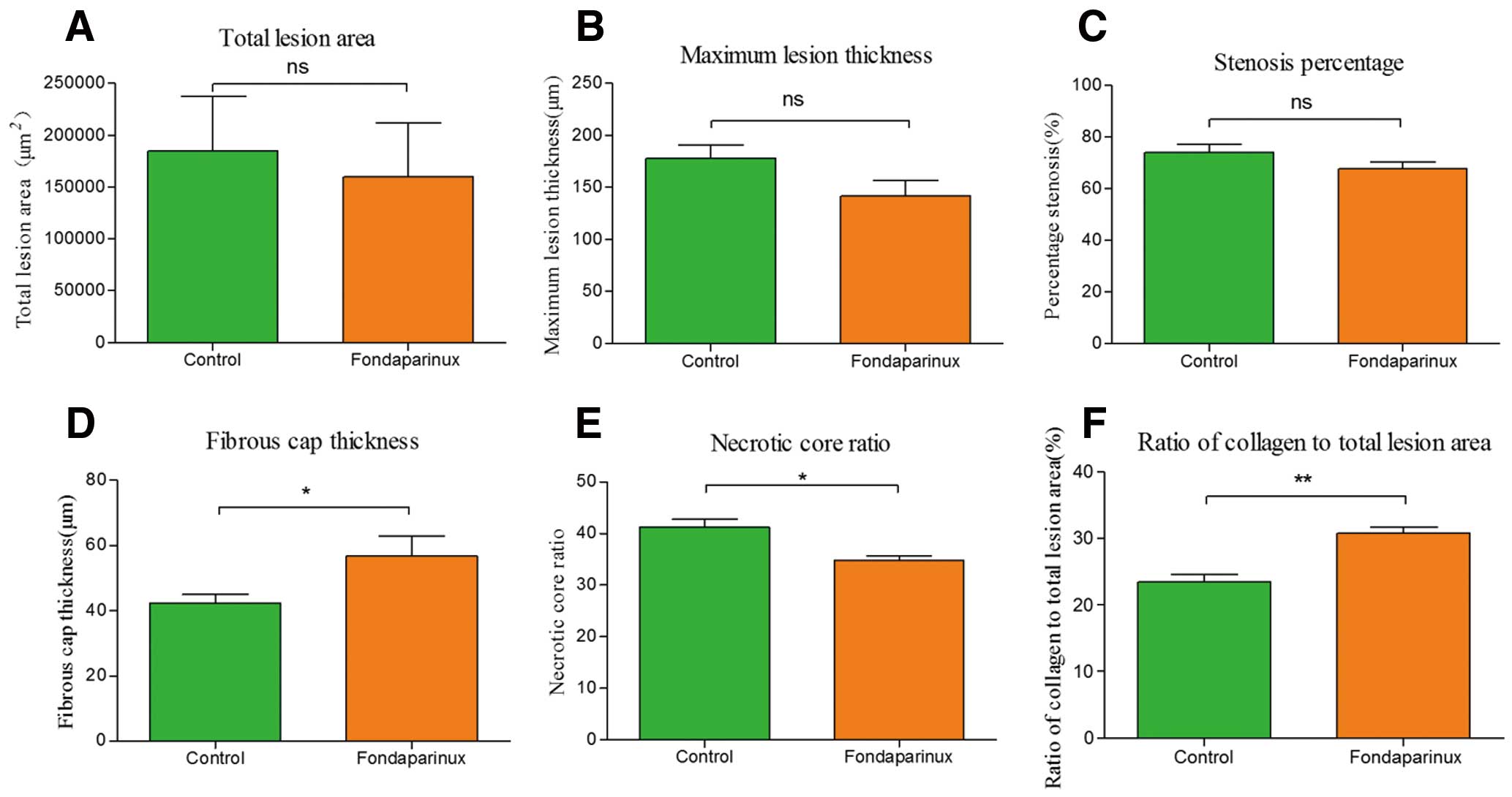
Morphometric analysis data. Administration of fondaparinux at a dose of 5 mg·kg–1·day–1 did not significantly reduce the total lesion area (A), maximum lesion thickness (B) or percentage of stenosis (C). Thickness of the fibrous cap (D) was significantly increased; the ratio of necrotic core (E) was remarkably reduced in fondaparinux-treated mice. The ratio of collagen to total lesion area (F) had a significant reduction in the control group, as compared to the fondaparinux-treated mice. Data represent mean±SEM. *P<0.05.
| Control (n=12) | Fondaparinux (n=12) | P value | |
|---|---|---|---|
| Cholesterol crystals | 8/12 | 8/12 | 1 |
| Medial erosion | 2/12 | 0/12 | 0.478 |
| Lateral xanthomas | 5/12 | 3/12 | 0.667 |
| Intraplaque hemorrhage | 1/12 | 0/12 | 1 |
| Calcification | 4/12 | 1/12 | 0.317 |
Differences were not significant between the study groups. *P<0.05.
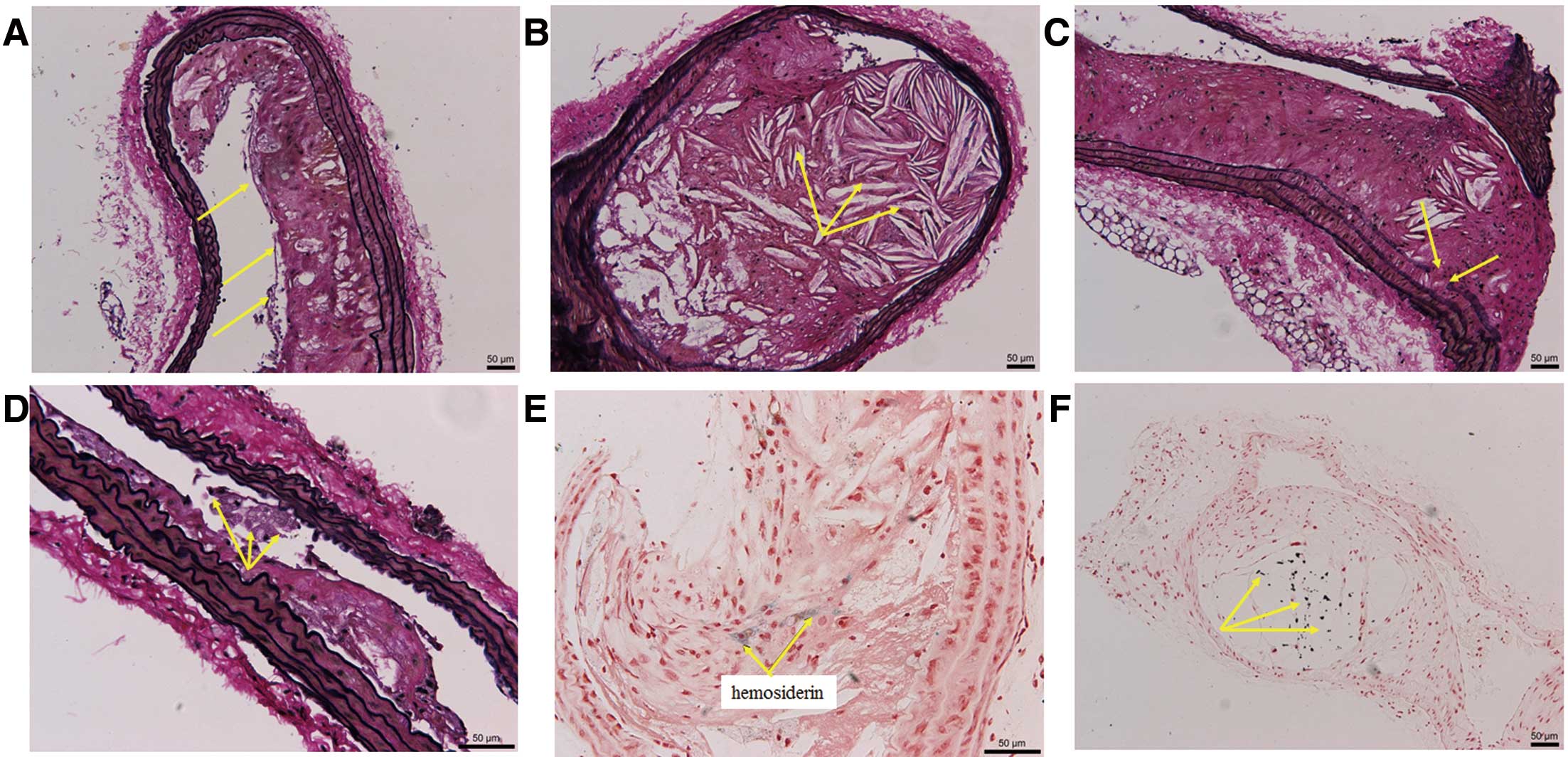
Representative lesion morphology. Special staining of the brachiocephalic artery of control mice show features of unstable atherosclerotic lesions with a thin protective fibrous cap (A) and a large necrotic core with cholesterol crystals (B), partially eroding into the media (C). Lateral xanthomas (D), defined as the presence of aggregates of macrophage-derived foam cells situated on the lateral margins of the plaques were observed in both groups with no significant difference. Intraplaque hemorrhage, with the presence of hemosiderin (E) was only observed in the control group. Calcification (F) was black or black-grey signals under van Kossa stain, predominantly located within the necrotic core regions of unstable lesions.
Analysis of plaque composition by immunohistochemistry showed a significant decrease of staining against Mac-2 (3.89±0.41 vs. 5.24±0.35 of the control group, P=0.017), α-SMA (5.67±0.54 vs. 8.10±0.52 of the control group, P=0.002), PAR-1 (5.19±0.43 vs. 7.78±0.56 of the control group, P=0.001) and PAR-2 (3.13±0.30 vs. 4.77±0.40 of the control group, P=0.003) in the fondaparinux group (Figure 4). Furthermore, immunohistochemistry confirmed that fondaparinux treatment resulted in a significantly reduced neovascularization, as measured by staining against CD-31 (4.33±0.53 vs. 6.33±0.60 of the control group, P=0.024; Figure 5A), compared with control mice. It also demonstrated a significantly reduced expression of MMP-9 (4.22±0.33 vs. 7.45±0.44 of the control group, P=0.000; Figure 5B), MMP-13 (3.83±0.17 vs. 4.67±0.21 of the control group, P=0.011; Figure 5C) and VCAM-1 (6.22±0.60 vs. 8.67±0.93 of the control group, P=0.041; Figure 5D) in the fondaparinux group. There was no statistical significant difference in the staining against Factor X between the 2 groups.
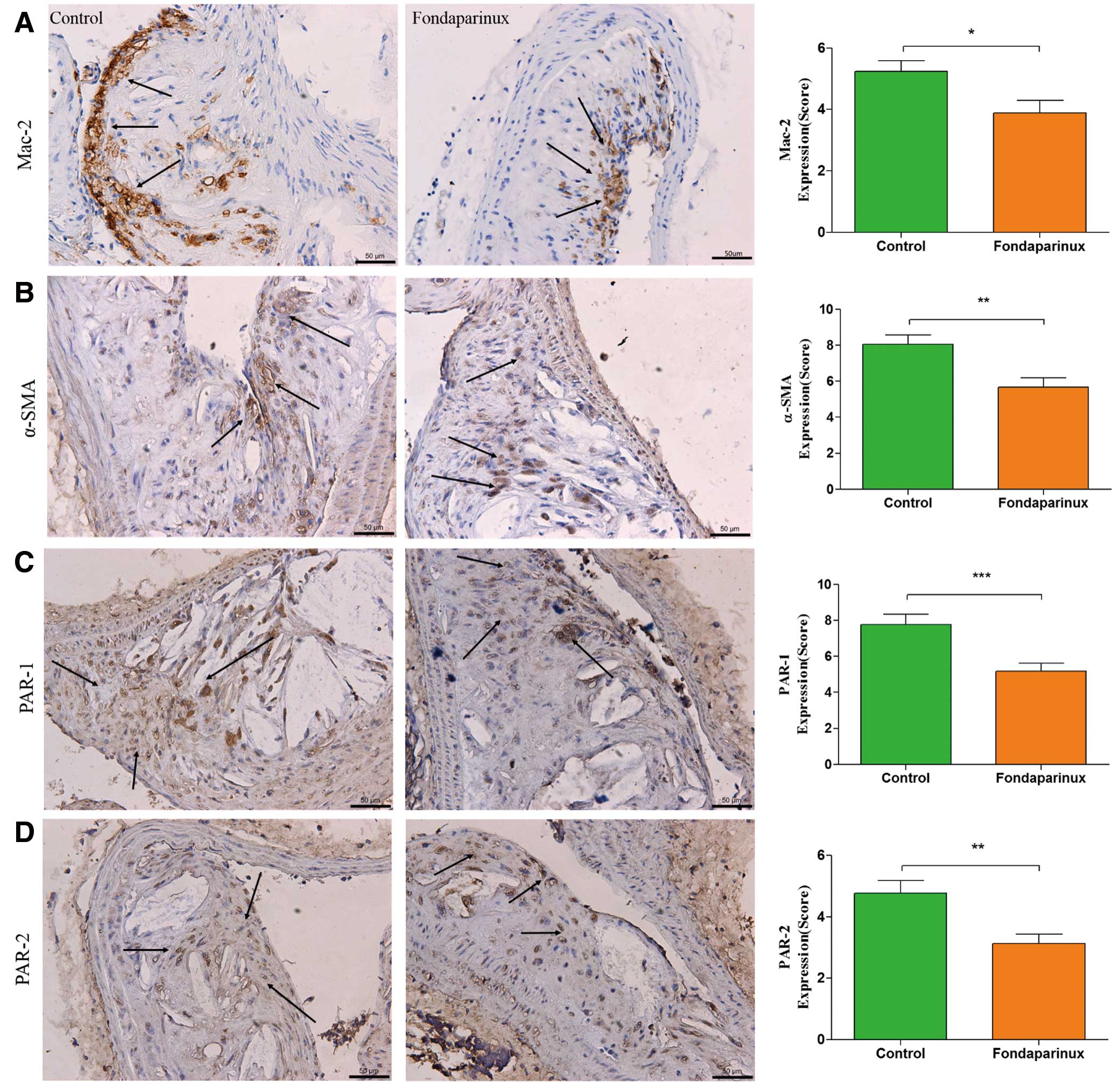
Immunohistochemistry. Immunohistochemistry staining with antibody against Mac-2 (A), which demonstrates the presence of macrophages within the atherosclerotic lesion, showed a significant reduction in the fondaparinux group. Staining for α-SMA (B) showed a significant reduction in the fondaparinux group, as compared to the control group. Immunohistochemistry staining with antibodies against protease-activated receptors (PAR)-1 (C) and PAR-2 (D) showed a significant reduction in the fondaparinux group. The arrows show positive stained areas within the atherosclerotic lesions. Data represent mean±SEM. *P<0.05.
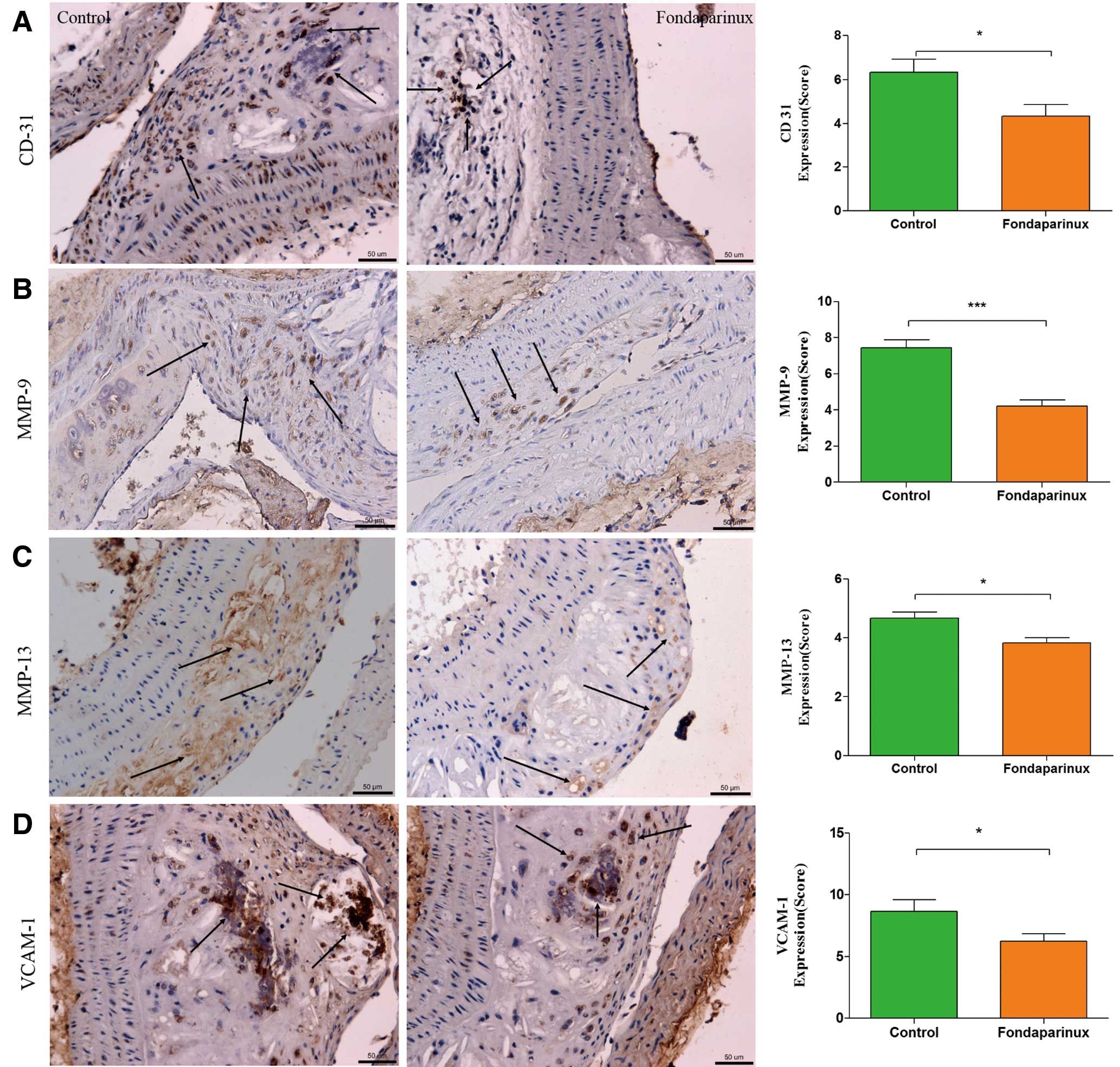
Immunohistochemistry. Immunohistochemistry staining with antibody against CD 31 (A), which is regarded as evidence of neovascularization, showed a significant reduction in the fondaparinux group, as compared to the control group. Staining for MMP-9 (B), MMP-13 (C) and VCAM-1 (D) showed a significant reduction in the fondaparinux group. VCAM-1-positive staining was mainly localized in the necrotic core regions. The arrows show positive stained areas within the atherosclerotic lesions. Data represent mean±SEM. *P<0.05.
As shown in Figure 6, the mRNA expression of inflammatory mediators, such as IL-6, MCP-1, IFN-γ, TNF-α, IL-10 and Egr-1, had a significant reduction in the group of fondaparinux-treated mice compared with controls.
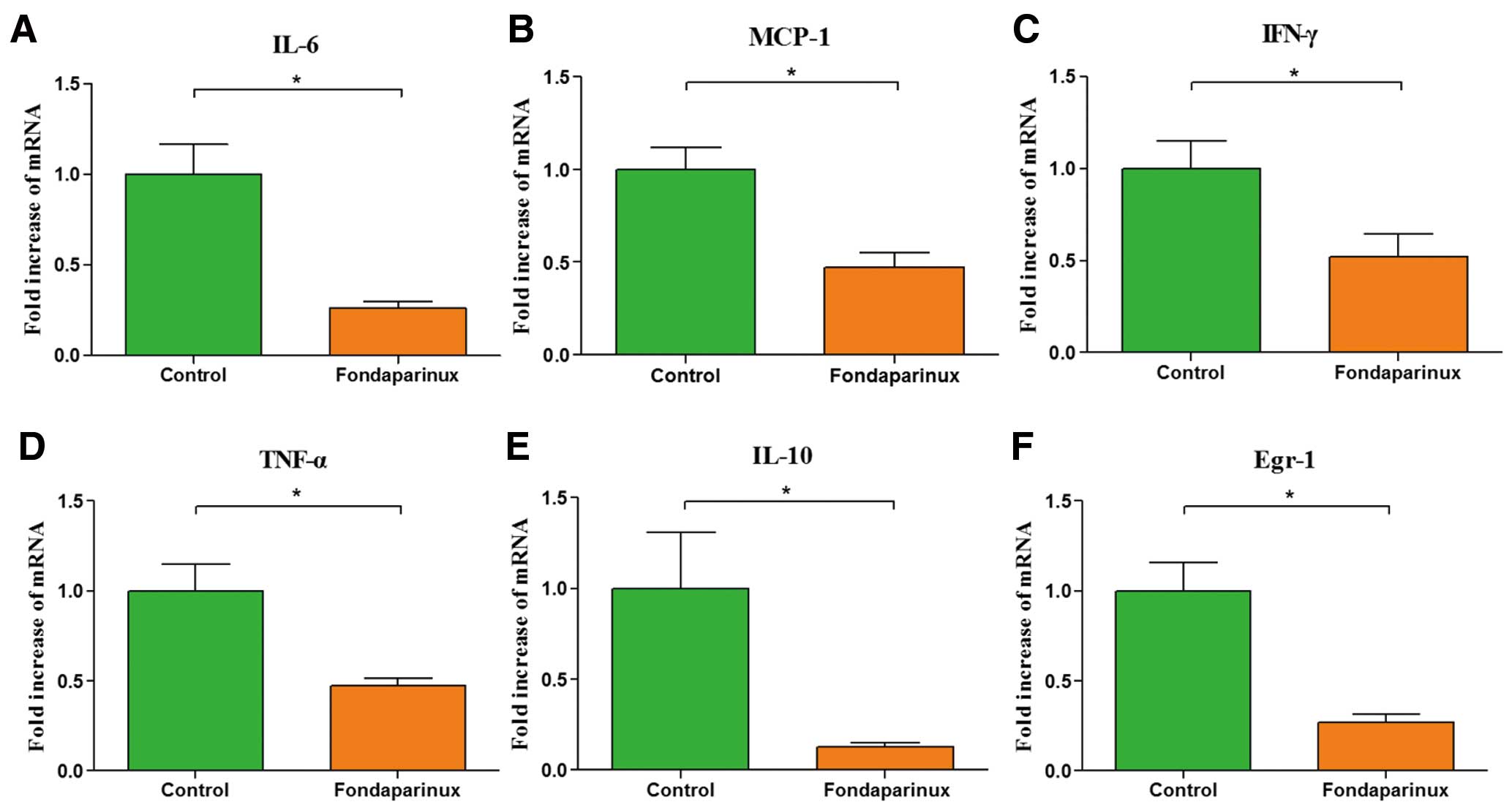
Real time reverse transcriptional-polymerase chain reaction of inflammatory mediators. Administration of fondaparinux at a dose of 5 mg·kg–1·day–1 significantly decreased mRNA expression of interleukin (IL)-6 (A), MCP-1 (B), IFN-γ (C), TNF-α (D), IL-10 (E) and Egr-1 (F) in aortic tissue of fondaparinux-treated mice, as compared to the control group. Data represent relative fold of mRNA expression as compared to the control group. Data represent mean±SEM. *P<0.05.
Atherosclerosis, characterized by the accumulation of lipids and fibrous elements in the arteries, is a progressive inflammatory disease that can lead to sudden cardiac events by plaque rupture and subsequently occlusive vascular thrombosis. Thus, local thrombin generation is considered to be detrimental during atherogenesis; anticoagulation is generally perceived as the standard of care in acute coronary syndrome. However, recent studies20,21 have demonstrated that direct thrombin inhibitors may not only exert protective effects, because they can increase the frequency of cardiovascular events. Two recent meta-analyses have confirmed that the direct thrombin inhibitor, dabigatran, increases the risk of myocardial infarction or acute coronary syndrome.22,23 In contrast, the FXa inhibitors could reduce the frequency of cardiovascular events consistently.24,25 Based on the central and upstream position of FXa in the coagulation cascade, it gets more and more attention, therefore targeting FXa may be an exciting new therapeutic strategy in the treatment of atherosclerosis.
In the present study, there was no significant difference in the lesion thickness, the total lesion area or the stenosis percentage between fondaparinux-treated and control groups. It means that fondaparinux did not affect the progression of atherosclerotic lesions. However, the administration of fondaparinux enhanced the thickness of the fibrous cap and reduced the ratio of necrotic core. Furthermore, fondaparinux reduced the expression of macrophages in the atherosclerotic plaque; meanwhile, the expression of inflammatory mediators in the aortic tissue of fondaparinux-treated mice, such as IL-6, MCP-1, IFN-γ, TNF-α, IL-10 and Egr-1, were decreased significantly. The vulnerability and composition of plaque rather than the lesion size are the principal reasons of plaque rupture and sudden cardiac events.26 Thus, the findings in our study demonstrate that administration of fondaparinux can promote the stability of atherosclerotic plaque.
The integrity and strength of the fibrous cap depends on the synthesis and degradation of extracellular matrix. The dynamic regulation of collagen levels in the fibrous cap plays an important role in the process; new collagen is mainly produced by SMCs; meanwhile, collagen degradation is due to the expression of active MMPs. The collagen metabolism disorders, including reduced collagen synthesis and increased collagen degradation, cause thinning of the fibrous cap. There is increasing evidence that increased collagen degradation plays an important role in the process of attenuating the fibrous cap.27,28 In the study by Libby, it was found that high expression of active MMPs increases collagen degradation in vulnerable plaque and decreases the thickness of the fibrous cap.29 The expression level of MMPs is associated with stability of plaques.30 In our study, although the expression of α-SMA was reduced in the fondaparinux group, there was more significantly reduced staining against MMP-9 and MMP-13 (Figures 5B,C) in the fondaparinux group. Moreover, we detected the enzyme activity of MMP-9 using zymography. As shown in Figure 1B, we found that fondaparinux reduced the enzyme activity of MMP9 significantly (0.74±0.04 vs. 1.00±0.07 of the control group, P=0.032). The lower activity and expression of MMPs may be the main reason for the thickening of the fibrous cap. To strengthen our theory, we further detected the expression of collagen, as shown in Figure 2F; the ratio of collagen to total lesion area had a significant reduction (30.79±0.84% vs. 23.52±1.04% of the control group, P=0.006) in the control group. It is consistent with our speculation.
FXa is well known as a crucial player in the process of hemostasis. It occupies a central position in the coagulation cascade by converting prothrombin into thrombin. More recently, increasing evidence suggests that FXa has a non-hemostatic function; it mediates intracellular signaling on a wide range of cell types by activating PAR-1 or PAR-2. FXa-induced PAR signaling links blood coagulation activation with pathophysiology. It has been found that FX coexists with macrophages and SMCs in atherosclerotic lesion.31 Fondaparinux did not significantly reduce FX deposition in this study; it is suspected that fondaparinux performed suppression on FXa activity, rather than reduction of FX quantity. PAR-2 expression is enhanced in human coronary atherosclerotic lesions.8 In the present study, the expression levels of PAR-1 and PAR-2 in atherosclerotic lesions decreased significantly in the fondaparinux-treated group. Moreover, we examined the phosphorylation of ERK1/2, which is widely used as a surrogate marker for PAR-1 and PAR-2 signaling. As shown in Figure 1C, the PAR-1 and PAR-2 signaling was reduced significantly in the fondaparinux group. Therefore, we speculate that FXa affects the stability of the atherosclerotic lesion via the activation of PAR-1 or PAR-2.
Angiogenesis in the atherosclerotic plaque has been intensively investigated as a possible risk factor of the vulnerable plaque. The process of angiogenesis has been implicated to play a major role in the development of intraplaque hemorrhage and thrombosis.32 The neovascularization within the atherosclerotic plaque is a key element in the plaque stability. Fondaparinux has been demonstrated to have anti-vasculogenic33 and anti-angiogenic16,34 effects through inhibiting PAR-1-mediated signaling.34 Both PAR-1 and PAR-2 play roles in hypoxia-driven angiogenesis, and PAR-2 signaling is sufficient for the pro-angiogenic effect.35 In the present study, fondaparinux suppressed angiogenesis; meanwhile, the expression and signaling of PAR-1 and PAR-2 were reduced in the fondaparinux group. Taken together, it is tempting to speculate that fondaparinux might contribute to the stability of the plaque by inhibiting angiogenesis through PAR-1/2 regulation.
Atherosclerosis is the result of a chronic inflammatory response in the arterial wall, and is related to the uptake of LDL by macrophages and their subsequent transformation in foam cells.36 Sustained inflammation plays a vital role in the process of plaque formation and rupture. FXa has been proved to be associated with pro-inflammatory responses; it can activate NF-κB, induce the release of IL-6, IL-8 and MCP-1 via the activation of PAR-1 or PAR-2.37,38 MCP-1 relates to leukocyte recruitment. The interaction between MCP-1 and its receptor, CCR2, affects monocyte recruitment in atherosclerosis.39 The FXa inhibitors decrease the expression of pro-inflammatory cytokines, such as TNF-α, INF-γ, IL-6 and GM-CSF, which contributes to monocyte-macrophage maturation.40 It is consistent with the result of our study, in the present study, fondaparinux reduced the expression of macrophage in the atherosclerotic plaque. Monocyte recruitment occupies a crucial position in the pro-inflammatory response of atherogenesis. Moreover, fondaparinux decreased the level of pro-inflammatory mediators in aortic tissue. The result suggests that the modification of inflammatory mediators in plaque may provide a possible mechanism for the plaque-stabilizing effects of FXa inhibition with fondaparinux.
Cellular adhesion molecules are strongly associated with atherogenesis, in our study, fondaparinux reduced the expression of VCAM-1 significantly. Considering the significant reduction in TNF-α expression, we deduce that fondaparinux leads to a reduced VCAM-1 expression through the downregulation of TNF-α, which is normally upregulated during vascular inflammation. VCAM-1 is a major cytokine in monocyte adhesion, and triggers the transformation from monocytes into macrophages within the vascular wall.41 VCAM-1 is mainly expressed by macrophages in advanced atherosclerotic lesion42 and increases atherosclerotic lesion.43 Therefore, we infer that the reduction of VCAM-1 expression may contribute to the reduced aggregation of macrophages, and may therefore be responsible for the reduced lesion inflammation and the necrotic cores in the fondaparinux group compared with the control.
IL-10 is one of the most important anti-inflammatory cytokines, secreted by lymphocytes of the Th2 subtype and also in large amounts by macrophages. The in vitro and in vivo studies have shown a protective role of IL-10 in both atherogenesis and plaque instability.44,45 In our study, the expression of IL-10 is reduced in the fondaparinux group, there could be following reasons through analysis: on the one hand, in the context of atherosclerosis, activated macrophages participate critically in every stage of lesion progression, from early fatty streak formation to the acute onset of plaque rupture and thrombosis.46 Macrophage accumulation is one of the characteristic features of vulnerable plaques; it has been proven that macrophages were the main cells that were immunoreactive for IL-10.47 Mallat et al have also identified that immunoreactive IL-10 was mainly observed in macrophages of advanced carotid plaques.48 In our study, the expression of macrophage was decreased in the fondaparinux group; these results indicate one possible mechanism of the reduced expression of IL-10. In contrast, inflammation is a critical process of atherogenesis and plaque instability; IL-10 is one of the most important mediators that physiologically limits and downregulates inflammation.44,45 In addition, inflammatory factors such as C-reactive protein and TNF-α, which are rich in unstable coronary plaques,49,50 also upregulate IL-10 production.51,52 IL-10 appears to act in a feedback loop to inhibit continued pro-inflammatory cytokine production. IL-10 expression in macrophages might vary with inflammatory conditions. In our study, the pro-inflammatory cytokines, such as IL-6, MCP-1, IFN-γ, TNF-α and Egr-1 were reduced in the fondaparinux group; the attenuation of inflammation may be the reason of the reduced expression of IL-10.
Outward remodeling is one criterion for defining vulnerable plaques;53 meanwhile, it leads to narrowing of the lumen during restenosis, intimal hyperplasia and atherogenesis. Accumulation of myofibroblasts takes an important place in vascular remodeling. As a powerful fibroblasts chemoattractant, FXa provokes proliferative responses in fibroblasts and contributes to fibroblast differentiation into myofibroblasts, which typically express α-SMA.5,54 Alpha-SMA is proved to be associated with PAR-2 expression in renal interstitial fibrosis.55 All these suggest that FXa can mediate vascular remodeling via PAR-2 activation. Though the presence of SMC is generally considered to enhance stability in atherosclerotic lesions, uncontrolled proliferation of SMCs can contribute to restenosis after vascular injury. In the present study, there was a significantly reduced staining against α-SMA in atherosclerotic lesions of fondaparinux-treated mice, which suggests that fondaparinux may promote the stability of atherosclerotic lesions by inhibiting vascular remodeling during atherogenesis.
In conclusion, the present study demonstrates that the FXa inhibitor, fondaparinux, can promote the stability of atherosclerotic lesions in ApoE–/– mice, possibly through inhibiting expression of the inflammatory mediators in plaque, and reduced synthesis of MMP-9 and MMP-13. Targeting FXa may be a potential therapeutic strategy for patients with established atherosclerotic disease.
This work was supported by the Natural Science Foundation of Jiangsu Province, China (grant number 7790000048). We are grateful to Dr Zhou Meng for his valuable technical assistance.
The author(s) declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.