Abstract
Background:
In patients with chronic heart failure (HF) the Metabolic Exercise Cardiac Kidney Indexes (MECKI) score, is a predictor of cardiovascular death and urgent heart transplantation. We investigated the relationship between age, exercise tolerance and the prognostic value of the MECKI score.
Methods and Results:
We analyzed data from 3,794 patients with chronic systolic HF. The primary endpoint was a composite of cardiovascular death and urgent heart transplantation. Older patients had higher prevalence of comorbidities and lower exercise performance compared with younger subjects (peak V̇O2, 925 vs. 1,351 L/min; P<0.0001; V̇E/V̇CO2
slope, 33.2 vs. 28.3; P>0.0001). The rate of the primary endpoint was 19% in the highest age quartile and 14% in the lowest quartile. At multivariable analysis, the independent predictors of the primary endpoint were left ventricular ejection fraction (LVEF), eGFR, peak V̇O2, serum Na+
and the use of β-blockers in patients aged ≥70 years, and LVEF, eGFR and peak V̇O2
in younger subjects. The MECKI risk score increased across age subgroups, but on receiver operating characteristic curve analysis its prognostic power was similar in both patients aged ≥70 and <70 years.
Conclusions:
Older patients with HF are a high-risk population with lower exercise performance. The MECKI score increased according to age and maintained its prognostic value also in older patients. (Circ J 2015; 79: 2608–2615)
Heart failure (HF) is one of the most important causes of death and the leading cause of hospitalization in patients aged >65 years.1,2
The annual incidence of HF doubles every decade in patients aged >65 years, with a prevalence reaching 10% in the patients aged >80 years.3
Age is also one of the major determinants of prognosis in patients with HF and is associated with a wider range of comorbidities that contribute to worsen outcome.4,5
Editorial p 2547
Aging is associated with several changes in the cardiovascular system and skeletal muscle that may influence the pathophysiology and clinical presentation of HF and affect exercise performance.6,7
Cardiopulmonary exercise test (CPET) is widely used to assess exercise capacity and prognosis in HF patients,1,2,8
but few data regarding its value in older patients are currently available. Recently, the metabolic exercise and cardiac and kidney indexes (MECKI) score, a prognostic score combining data from CPET with clinical, laboratory and echocardiographic measurements, was validated in patients with chronic systolic HF.9
This score includes 6 variables: hemoglobin (Hb), serum sodium, estimated glomerular filtration rate (eGFR; estimated by means of the Modification of Diet in Renal Disease equation, MDRD), left ventricular ejection fraction (LVEF), peak oxygen consumption (calculated as % of predicted value, based on age and gender) and the slope of the minute ventilation to carbon dioxide production ratio (V̇E/V̇CO2
slope). In a previous study this score had a strong association with risk of death or need for urgent heart transplantation.9
The present study is a subanalysis of the effects of age on the predictive capacity of exercise-derived HF prognostic variables.
Methods
Patients
The study cohort consisted of 3,794 patients with systolic HF recruited and prospectively followed up in 14 Italian HF centers. Patients were enrolled as part of the MECKI score research group database. Accordingly, data for some of these patients have been previously reported.9–12
At enrollment, clinical history, laboratory, electrocardiographic, echocardiographic, and CPET data were collected. Study inclusion/exclusion criteria and follow-up have been previously reported.9
Briefly, we evaluated chronic HF patients with New York Heart Association functional class I–III and present or previous history of systolic HF with former documentation of LV systolic dysfunction (LVEF <40%), on stable evidence-based pharmacological therapy since ≥3 months before enrollment. Patients with comorbidities affecting exercise capacity or with exercise-induced angina or signs of acute myocardial ischemia were excluded.
Measurements
Standard clinical, laboratory, echocardiographic and CPET measurements were collected.9
eGFR was calculated using the MDRD formula.13
LV volumes and EF were measured on echocardiography according to standard recommendations.14
MECKI score was calculated with the subsequent algorithm: exp(k)/(1+exp(k)) where k=10.3464–0.0262×V̇O2peak(%pred)+0.0472×V̇E/V̇CO2slope–0.1086×Hb(g/dl)–0.0615×Na+(mmol/L)–0.0699×LVEF(%)–0.0136×MDRD(ml/min).9
CPET was performed using an electronically braked cycle-ergometer or a treadmill. For comparison with cycle-ergometer, treadmill peak V̇O2
data were reduced by 10%.15
Procedures and measurements for CPET have been previously described in detail.9
Follow-up
Follow-up was performed according to the local HF program in a theoretically endless fashion. Follow-up ended with the last clinical evaluation in the center where the patient had been recruited or with the patient’s death or urgent heart transplantation. For patients who did not present for the planned follow-up visit, the subject or the family were contacted via telephone to collect data regarding prognosis, and a subsequent follow-up visit was rescheduled. If the patient died outside the hospital where they were followed up, we obtained medical records of the event and the cause of death. Patients who died for non-cardiovascular reasons were considered censored at the time of the event. The primary outcome of the study was a composite of cardiovascular death, including stroke, and urgent cardiac transplant.
Data Management and Analysis
The details regarding data management and data quality control have been reported previously.9
In brief, quality control was set up at Centro Cardiologico Monzino, where P.A. was the director of the center and responsible for data collection, while individual investigators were responsible for their own records. All investigators were experts on CPET and in the management of patients with HF. Quality data control included the control center staff as well as external experts (M.P. and D.M.) not involved in the recruitment of patients. Data collection was computerized. All computerized data were stored on a secure network that limited access to authorized individuals. An institutional review committee approved the study, and the subjects gave informed consent.
For the purposes of this subanalysis, data are presented with regard to the whole group and also according to age quartile (<50; 50–<60; 60–<70; ≥70 years).
Continuous variables are shown as median (IQR), and discrete variables as frequency (percentage). Differences between groups were compared using ANOVA test for continuous variables, while categorical variables were compared using chi-squared test. In a Cox regression model we calculated the hazard ratio (HR) of the MECKI score according to age quartile. Associations between variables and primary endpoint were assessed using Cox proportional hazards models. Two stepwise multivariate models were developed to evaluate the association with variables and prognosis, in patients aged <70 and ≥70 years, respectively. The variables included in the models were the following: anaerobic threshold (AT), β-blockers, body mass index (BMI), cardiac resynchronization therapy (CRT), etiology, atrial fibrillation (AF), LVEF, gender, Hb, peak heart rate, implantable cardioverter defibrillator, MDRD, sodium, pacemaker, periodic breathing, peak respiratory rate, peak ventilation, V̇E/V̇CO2
slope, V̇O2
at AT, ad peak V̇O2% of predicted.
Receiver operating characteristic (ROC) curves were used to compare the performance of the MECKI score to the multivariate model in patients aged <70 and ≥70 years. Moreover, a second ROC analysis was carried out to directly evaluate the performance of the MECKI score in the 2 age subgroups.
P<0.05 was considered statistically significant. Statistical analysis was performed using SAS (version 9.2 SAS Institute, Cary, NC, USA).
Results
Follow-up
The median follow-up was 1,117 days (IQR, 574–1,792) for the whole group. During follow-up 654 patients (17%) died of cardiovascular causes or underwent urgent heart transplantation. The number of events rose across subgroups from 14% in younger patients (<50 years) to 19% in the ≥70-year quartile. Specifically, cardiovascular death occurred in 373 (37.5/ year) and 184 (51.9/year) younger and elderly patients, respectively. Urgent cardiac transplant occurred in 94 cases in the younger group and in 1 case in the elderly group.
Baseline Characteristics
We analyzed 3,794 patients enrolled in the MECKI HF Italian registry. The clinical characteristics of the whole group and according to age quartile are listed in
Table 1. The median age was 62 years (IQR, 53–70 years). Median peak V̇O2
was 14.2 ml·kg–1·min–1
(IQR, 11.5–17.3) with a percent of predicted value of 53.3% (IQR, 42.8–64.9).
Table 1.
Clinical Patient Characteristics vs. Age Quartile
| |
Overall
(n=3,794) |
Age (years ) |
P-value |
| <50 (n=725) |
50–<60 (n=999) |
60–<70 (n=1,080) |
≥70 (n=990) |
| Age (years) |
62 (53–70) |
44 (37–48) |
56.0 (53.0–58.0) |
65 (63–67) |
74 (72–77) |
<0.0001 |
| Sex (% male) |
3,177 (84) |
587 (81) |
862 (86) |
912 (84) |
816 (82) |
0.0145 |
| Weight (kg) |
76 (67–85) |
80 (68–90) |
78 (69–88) |
75 (67–83) |
74 (65–82) |
<0.0001 |
| BMI (kg/m2) |
26.2 (23.9–29.1) |
26.4 (23.6–29.7) |
26.9 (24.4–30.0) |
26.1 (23.9–28.8) |
25.7 (23.5–28.1) |
<0.0001 |
| NYHA class |
2 (2–3) |
2 (2–3) |
2 (2–3) |
2 (2–3) |
2 (2–3) |
<0.0001 |
| SBP (mmHg) |
120 (110–130) |
115 (100–125) |
120 (110–130) |
120 (110–130) |
120 (110–130) |
<0.0001 |
| DBP (mmHg) |
70 (70–80) |
70 (70–80) |
75 (70–80) |
70 (70–80) |
70 (65–80) |
0.001 |
| HR at rest (beats/min) |
70 (62–78) |
71 (65–80) |
70 (62–78) |
68 (60–76) |
68 (60–76) |
<0.0001 |
| QRS duration (ms) |
117 (90–140) |
105.5 (90.0–130.0) |
110 (90–145) |
120 (90–145) |
120 (90–140) |
<0.0001 |
| Etiology (%) |
| Idiopathic |
1,636 (43) |
477 (66) |
448 (45) |
430 (40) |
281 (28) |
<0.0001 |
| Ischemic |
1,790 (47) |
173 (24) |
479 (48) |
561 (52) |
577 (58) |
|
| Valvular |
122 (3) |
10 (1) |
23 (2) |
32 (3) |
57 (6) |
|
| Other |
242 (6) |
63 (9) |
47 (5) |
57 (5) |
75 (8) |
|
| AF |
598 (16) |
60 (8) |
106 (11) |
197 (18) |
235 (24) |
<0.0001 |
| PM |
721 (19) |
97 (13) |
149 (15) |
205 (19) |
270 (28) |
<0.0001 |
| ICD |
952 (25) |
177 (25) |
254 (26) |
280 (26) |
241 (25) |
0.8178 |
| CRT |
367 (10) |
61 (9) |
94 (10) |
99 (10) |
113 (12) |
0.1584 |
| Hemoglobin (g/dl) |
13.6 (12.5–14.6) |
14.0 (12.8–15.0) |
14.0 (12.8–14.9) |
13.7 (12.5–14.6) |
13.0 (12.0–14.1) |
<0.0001 |
| Lymphocytes (%) |
27.0 (21.0–33.3) |
29.0 (23.5–35.7) |
27.8 (22.6–33.3) |
26.8 (21.0–32.5) |
25.7 (20.0–33.0) |
<0.0001 |
| Serum creatinine (mg/dl) |
1.1 (0.9–1.3) |
1.0 (0.9–1.2) |
1.1 (0.9–1.3) |
1.1 (1.0–1.4) |
1.2 (1.0–1.5) |
<0.0001 |
| eGFR (ml/min/1.73 m2) |
69.4 (54.1–84.0) |
83.8 (70.8–97.6) |
72.9 (60.2–85.2) |
66.7 (53.1–79.2) |
58.1 (44.4–70.9) |
<0.0001 |
| Na++ (mmol/L) |
140 (138–141) |
139 (137–141) |
140 (137–141) |
140 (138–141) |
140 (138–142) |
0.015 |
| K+ (mmol/L) |
4.3 (4.0–4.6) |
4.2 (4.0–4.5) |
4.3 (4.0–4.6) |
4.3 (4.0–4.6) |
4.3 (4.0–4.6) |
0.009 |
| Uric acid (mg/dl) |
6.3 (5.1–7.7) |
6.0 (5.0–7.4) |
6.2 (5.1–7.5) |
6.3 (5.2–7.7) |
6.6 (5.3–8.0) |
0.008 |
| Cholesterol (mg/dl) |
181 (156–210) |
181 (158–217) |
180 (155–212) |
182 (154–212) |
183 (157–203) |
0.572 |
| BNP |
346.0
(127.0–876.0) |
269.5
(80.0–920.0) |
389.0
(135.0–962.5) |
410.5
(139.5–1,021.0) |
334
(141–706) |
0.004 |
| LVEF (%) |
30 (25–38) |
30 (25–37) |
29 (23–35) |
30 (25–37) |
34 (27–40) |
<0.0001 |
| EDV (ml) |
170 (127–218) |
175 (132–223) |
182 (134–226) |
169 (126–217) |
158 (122–206) |
<0.0001 |
| ESV (ml) |
115 (82–157) |
120 (86–166) |
123 (89–165) |
114 (84–158) |
105 (75–145) |
<0.0001 |
| sPAP (mmHg) |
35 (28–44) |
32 (26–42) |
35 (27–44) |
35 (28–45) |
36 (30–45) |
<0.0001 |
Data given as median (IQR) or n (%). BMI, body mass index; BNP, brain natriuretic peptide; CRT, cardiac resynchronization therapy; DBP, diastolic blood pressure; EDV, end-diastolic volume; eGFR, estimated glomerular filtration rate; ESV, end-systolic volume; HR, heart rate; ICD, implantable cardioverter defibrillator; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; PM, pacemaker; SBP, systolic blood pressure; sPAP, systolic pulmonary artery pressure.
Compared to patients in the lowest quartile of age (<50 years), patients in the highest quartile (≥70 years) were more likely to have an ischemic etiology of HF and had a higher prevalence of comorbidities such as atrial fibrillation AF (24% vs. 8%; P<0.0001), lower Hb (13.0 vs. 14.0 g/dl; P<0.0001), and lower eGFR (58.1 vs. 83.8 ml/min/1.73m2; P<0.0001). The proportion of patients with a pacemaker or CRT was higher in older patients. Elderly patients also had lower lymphocyte count (expressed as percent of whole white cell count) and increased uric acid.
At echocardiography, patients in the highest age quartile had higher LVEF (34% vs. 30%; P<0.0001), smaller LV volumes and increased systolic pulmonary artery pressure (36 mmHg vs. 32 mmHg; P<0.0001). With regards to HF treatment, older patients were less likely to receive evidence-based medications for HF, namely, β-blockers (76% vs. 88%; P<0.0001) and angiotensin-converting enzyme inhibitors (70% vs. 84%; P<0.0001), and have a higher proportion of subjects on diuretics, amiodarone and statins (Table 2).
Table 2.
Pharmacological Treatment vs. Age Quartile
| |
Overall
(n=3,794) |
Age (years) |
P-value |
| <50 (n=725) |
50–<60 (n=999) |
60–<70 (n=1,080) |
≥70 (n=990) |
| β-blocker |
3,135 (83) |
641 (88) |
868 (87) |
874 (81) |
752 (76) |
<0.0001 |
| ACEI |
2,960 (78) |
609 (84) |
834 (83) |
823 (76) |
694 (70) |
<0.0001 |
| ARB |
585 (15) |
83 (11) |
116 (12) |
185 (17) |
201 (20) |
<0.0001 |
| Anti-aldosterone |
1,951 (51) |
332 (46) |
548 (55) |
583 (54) |
488 (49) |
0.0004 |
| Diuretic |
3,057 (81) |
527 (73) |
820 (82) |
864 (80) |
846 (85) |
<0.0001 |
| Digoxin |
999 (26) |
222 (31) |
302 (30) |
276 (26) |
199 (20) |
<0.0001 |
| Amiodarone |
985 (26) |
148 (20) |
252 (25) |
288 (27) |
297 (30) |
0.0002 |
| Statin |
1,156 (30) |
129 (18) |
293 (29) |
360 (33) |
374 (38) |
<0.0001 |
| Allopurinol |
781 (21) |
102 (14) |
187 (32) |
230 (35) |
262 (35) |
<0.0001 |
| Anti-platelets |
1,925 (51) |
264 (36) |
505 (19) |
569 (21) |
587 (26) |
<0.0001 |
| OAT |
1,154 (30) |
167 (23) |
303 (30) |
367 (34) |
317 (32) |
<0.0001 |
Data given as n (%). ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; OAT, oral anticoagulant therapy.
Peak exercise performance decreased steadily across the age subgroups, with patients in the highest age quartile having lower peak V̇O2
expressed as absolute value (925 vs. 1,351 L/min; P<0.0001) and percent of predicted value (52.2% vs. 56.5%; P=0.005). AT was identified in fewer patients in the older groups and all submaximum exercise parameters were similarly reduced in older patients, with lower V̇O2, work rate and heart rate at AT. The V̇E/V̇CO2
slope was increased in the older subgroup (33.2 vs. 28.3; P<0.0001). Last, the percentage of patients with respiratory oscillations increased progressively with age from 10% to 26% (P<0.0001) in older patients (Table 3).
Table 3.
CPET Parameters vs. Age Quartile
| |
Overall
(n=3,794) |
Age (years) |
P-value |
| <50 (n=725) |
50–<60 (n=999) |
60–<70 (n=1,080) |
≥70 (n=990) |
| Peak V̇O2 (L/min) |
1,070
(836–1,375) |
1,351
(1,038–1,725) |
1,146
(899–1,430) |
1,036
(821–1,297) |
925
(739–1,126) |
<0.0001 |
| Peak V̇O2/kg (ml·kg−1·min−1) |
14.2 (11.5–17.3) |
17.1 (13.6–20.8) |
14.8 (12.0–17.8) |
13.9 (11.5–16.5) |
12.5 (10.3–15.0) |
<0.0001 |
| Peak V̇O2 (% of predicted) |
53.3 (42.8–64.9) |
56.5 (43.7–68.0) |
53.4 (42.9–64.6) |
52.8 (42.5–65.3) |
52.2 (42.7–62.9) |
0.005 |
| Peak HR (beats/min) |
120 (105–137) |
132 (118–148) |
124 (110–138) |
118 (105–133) |
111 (97–127) |
<0.0001 |
| Peak HR (% of predicted) |
76.3 (67.3–86.3) |
74.5 (66.3–83.6) |
75.7 (67.4–85.2) |
77.3 (67.8–87.3) |
77.0 (66.9–88.7) |
0.001 |
| Peak work rate (W) |
79 (60–100) |
93 (65–127) |
80 (60–109) |
78 (60–100) |
69 (50–88) |
<0.0001 |
Peak O2 pulse
(ml·beats−1·min−1) |
9.1 (7.1–11.4) |
10.3 (8.0–12.8) |
9.4 (7.4–11.8) |
8.8 (6.9–11.1) |
8.4 (6.6–10.5) |
<0.0001 |
| Peak TV (L) |
1.4 (1.1–1.7) |
1.6 (1.3–2.0) |
1.5 (1.2–1.8) |
1.4 (1.1–1.7) |
1.3 (1.0–1.5) |
<0.0001 |
| Peak RR (beats/min) |
31.0 (27.0–36.0) |
31.0 (27.2–36.0) |
30.6 (27.0–35.2) |
31.0 (27.0–35.9) |
31.0 (27.0–36.0) |
0.506 |
| Peak V̇E (L/min) |
44.8 (36.4–54.7) |
50.0 (40.0–60.1) |
46.9 (38.0–56.9) |
44.3 (36.5–53.1) |
40.3 (32.8–49.6) |
<0.0001 |
| Peak RER |
1.10 (1.04–1.18) |
1.12 (1.05–1.20) |
1.11 (1.05–1.19) |
1.11 (1.05–1.17) |
1.09 (1.03–1.16) |
<0.0001 |
| Identified AT |
3,117 (82) |
634 (87) |
852 (85) |
898 (83) |
733 (74) |
<0.0001 |
| V̇O2 at AT (L/min) |
746 (587–952) |
876 (667–1,122) |
785 (615–1,006) |
713 (558–888) |
673 (540–826) |
<0.0001 |
| V̇O2 at AT/kg (ml·kg−1·min−1) |
9.8 (8.0–12.0) |
11.0 (8.8–13.5) |
10.0 (8.0–12.2) |
9.5 (7.8–11.5) |
9.2 (7.6–11.0) |
<0.0001 |
| V̇O2 at AT (% of peak) |
67.8 (58.5–77.7) |
63.9 (55.6–74.3) |
66.8 (58.3–76.5) |
68.7 (59.2–78.3) |
70.9 (61.4–80.6) |
<0.0001 |
| HR at AT (beats/min) |
95 (84–110) |
105 (93–116) |
96 (86–110) |
93 (82–107) |
89 (79–102) |
<0.0001 |
| Work rate at AT (W) |
47 (34–60) |
52 (38–75) |
50 (35–65) |
45 (35–60) |
40 (30–55) |
<0.0001 |
O2 pulse at AT
(ml·beats−1·min−1) |
7.9 (6.2–9.9) |
8.6 (6.6–10.5) |
8.1 (6.4–10.2) |
7.7 (5.9–9.6) |
7.4 (5.8–9.2) |
<0.0001 |
| V̇E/V̇CO2_slope |
31.1 (27.4–37.0) |
28.3 (25.1–33.0) |
30.8 (27.0–35.9) |
32.0 (28.0–37.4) |
33.2 (29.3–39.0) |
<0.0001 |
V̇O2/Work slope
(ml/min−1/W−1) |
9.5 (8.2–10.9) |
10.0 (9.0–11.4) |
9.6 (8.6–11.0) |
9.4 (8.1–10.8) |
9.0 (7.8–10.4) |
<0.0001 |
| Periodic breathing (%) |
651 (17) |
71 (10) |
139 (14) |
179 (17) |
262 (26) |
<0.0001 |
Data given as median (IQR) or n (%). AT, anaerobic threshold; CPET, cardiopulmonary exercise test; HR, heart rate; RER, respiratory exchange ratio; RR, respiratory rate; TV, tidal volume; V̇CO2, carbon dioxide consumption; V̇E, ventilation; V̇O2, oxygen uptake.
MECKI score increased with age from 0.04 (IQR, 0.02–0.09) to 0.07 (IQR, 0.03–0.14; P<0.0001) in the first and the last quartile, respectively (Table 4). In the Cox models for age quartiles, MECKI score had similar HR, showing that its prognostic power was similar across the age classes (P for interaction=0.734;
Table 5).
Table 4.
MECKI Score and Primary Endpoint vs. Age Quartile
| |
Overall
(n=3,794) |
Age <50
(n=725) |
Age 50–<60
(n=999) |
Age 60–<70
(n=1,080) |
Age ≥70
(n=990) |
P-value |
| Follow-up duration (days) |
1,117
(574–1,792) |
1,014
(534–1,776) |
1,174
(561–1,876) |
1,093
(571–1,737) |
1,178
(617–1,790) |
0.091 |
| MECKI score |
0.060
(0.026–0.129) |
0.041
(0.019–0.091) |
0.058
(0.027–0.126) |
0.066
(0.030–0.140) |
0.069
(0.029–0.137) |
<0.0001 |
CV death or urgent heart
transplantation |
654 (17) |
98 (14) |
179 (18) |
192 (18) |
185 (19) |
0.0288 |
Data given as median (IQR) or n (%). CV, cardiovascular; MECKI, metabolic exercise and cardiac and kidney indexes.
Table 5.
Hazard Ratio for MECKI Score vs. Age Quartile
| |
Hazard ratio |
95% CI |
P-value |
| Age <50 (n=725) |
1.816 |
1.488 |
2.217 |
<0.0001 |
| Age 50–<60 (n=999) |
1.598 |
1.424 |
1.793 |
<0.0001 |
| Age 60–<70 (n=1,080) |
1.704 |
1.543 |
1.883 |
<0.0001 |
| Age ≥70 (n=990) |
1.699 |
1.518 |
1.901 |
<0.0001 |
CI, confidence interval. Other abbreviation as in Table 4.
In a multivariate model we analyzed the predictors of cardiovascular death or urgent heart transplantation in patients <70 years and ≥70 years, excluding MECKI score (Table 6). In patients aged <70 years, the independent predictors of death or urgent heart transplantation were LVEF, eGFR and peak V̇O2
(expressed as percentage of the predicted value). In the older group (age ≥70 years), the independent predictors of the primary endpoint were LVEF, eGFR, peak V̇O2
(expressed as percentage of the predicted value), serum Na++
and the use of β-blockers.
Table 6.
Multivariate Indicators of the Primary Endpoint
| Parameter |
Hazard ratio |
95% CI |
P-value‡ |
| Patients aged <70 years |
| LVEF (%) |
0.951 |
0.935 |
0.968 |
<0.0001 |
| eGFR (ml/min) |
0.989 |
0.983 |
0.996 |
0.0017 |
| Peak V̇O2 (% of predicted) |
0.972 |
0.962 |
0.982 |
<0.0001 |
| Patients aged ≥70 years |
| β-blocker (%) |
0.529 |
0.334 |
0.838 |
0.0067 |
| LVEF (%) |
0.972 |
0.950 |
0.996 |
0.0215 |
| eGFR (ml/min/1.73 m2) |
0.978 |
0.966 |
0.990 |
0.0005 |
| Na++ (mmol/L) |
0.905 |
0.856 |
0.956 |
0.0004 |
| Peak V̇O2 (% of predicted) |
0.970 |
0.951 |
0.988 |
0.0016 |
†Cardiovascular death or urgent heart transplantation (and excluding MECKI score). ‡Chi-squared test. Abbreviations as in Tables 1,3,4.
On ROC curves analysis the present multivariate risk model was compared with the MECKI score model. In patients aged <70 years the area under the curve (AUC) for the multivariate model and for the MECKI model was 0.734 (95% confidence interval (CI), 0.704–0.764) and 0.735 (95% CI, 0.706–0.765), respectively, which was not significantly different (P=0.857, χ2
test;
Figure 1A). Similarly, in patients aged ≥70 years the two models did not differ significantly (multivariate model: AUC, 0.712; 95% CI, 0.668–0.757 vs. MECKI model: AUC, 0.693; 95% CI, 0.646–0.740; P=0.172, χ2
test;
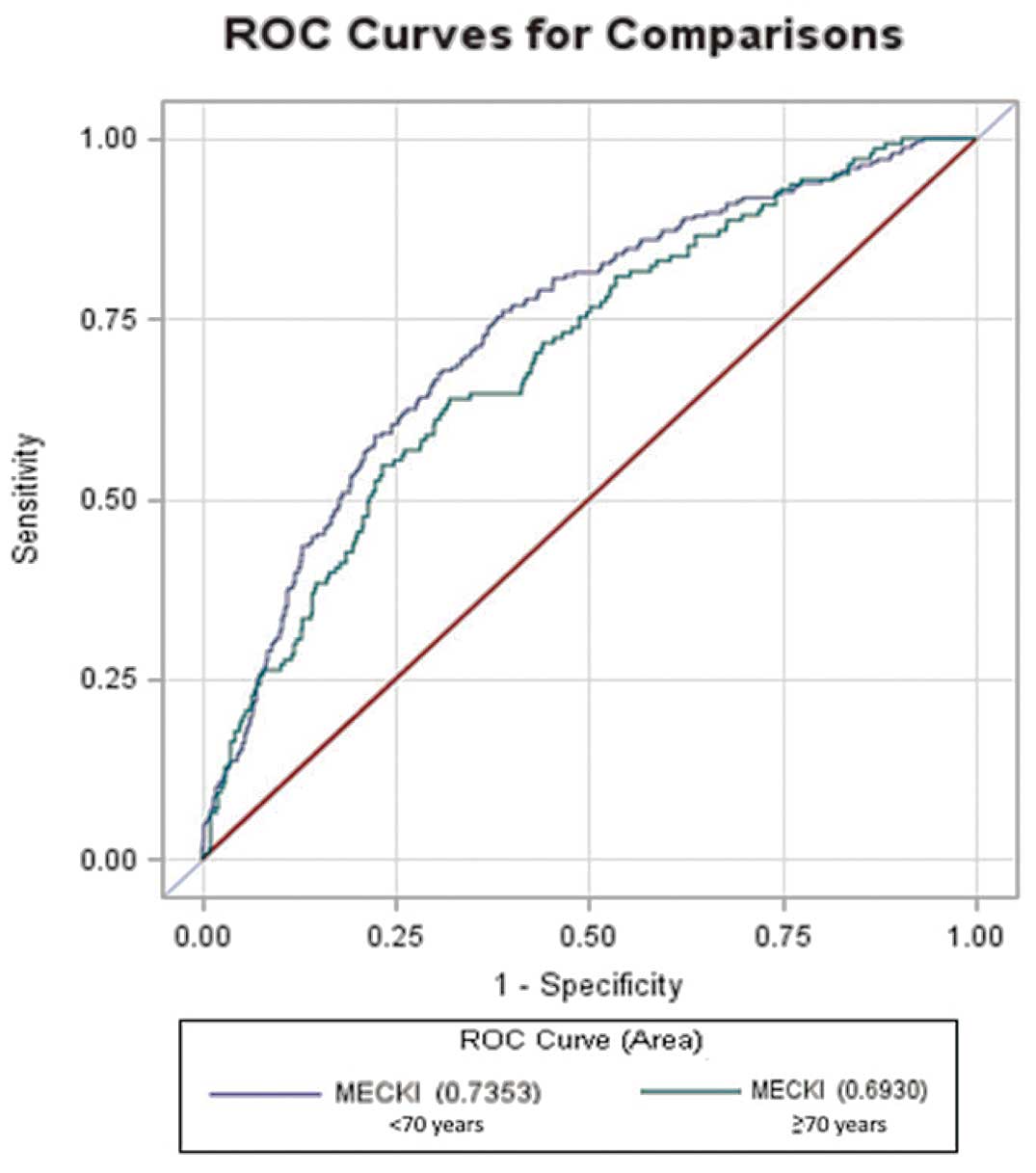
Figure 1B). In the direct comparison of the MECKI score model in patients aged <70 or ≥70 years, no significant difference was found (Figure 2), confirming that MECKI score can be applied in both age subgroups. Also, using only cardiovascular death as the endpoint, AUC did not differ in the younger group, being 0.724 for cardiovascular death and 0.735 for the composite endpoint of cardiovascular death and urgent cardiac transplant.

Discussion
In the present study, in patients with chronic HF, exercise tolerance decreased according to age. Peak V̇O2, however (expressed as percentage of the predicted value), retained its prognostic value in the elderly population. When we applied the MECKI risk score, we observed a similar trend. Indeed, the MECKI score increased across age subgroups, but its predictive power was constant for both younger and older patients.
Aging is characterized by a progressive worsening of exercise capacity,6,16,17
and cardiac dysfunction further impairs this physiological impairment.18
Elderly patients with HF have a blunted hemodynamic response to exercise due to both reduced stroke volume and chronotropic incompetence that leads to suboptimal exercise performance.12,19–21
In addition, older patients frequently have a sedentary lifestyle, which favors muscle bulk loss, and a higher prevalence of comorbidities, namely AF, renal dysfunction, chronic obstructive pulmonary disease, peripheral vascular disease, and orthopedic disorders that further limit exercise tolerance.11,18,22–26
There is limited experience of CPET in elderly patients with HF in clinical practice. Although the strong prognostic value of CPET parameters in large HF series is well known,27,28
few clinical studies have evaluated its prognostic role in older patients. Scardovi et al showed that CPET was safe and feasible in HF patients aged ≥70 years, reporting also a high proportion of subjects who reached a significant respiratory exchange ratio and a detectable AT.29
In the present study AT was detectable in 74% of patients aged ≥70 years, with a reduction of V̇O2
at AT in comparison with other subgroups, showing that submaximum exercise parameters are reduced in older patients and may be a marker of worse prognosis, as confirmed by a recent study.30
In a subanalysis of the Heart Failure: A Controlled Trial Investigating Outcomes of Exercise TraiNing (HF-ACTION), the authors observed a decrease in peak V̇O2
and a concomitant increase in V̇E/V̇CO2
in patients aged ≥70 years, compared with younger patients. The multivariable model of this study showed that age was the strongest independent predictor of peak V̇O2
even after adjustment for concomitant diseases. Our data are consistent with these results.31
Interestingly, we observed that peak V̇O2, despite its reduction in the elderly population, remained a strong predictor of cardiovascular events. Furthermore, we showed that the MECKI score increased significantly across age subgroup, resulting in worse outcome.
Similar risk models, which also included CPET variables, have been published previously, but the specific characteristics of the cohorts used to develop these scoring systems limit their validation in a broad range of HF patients. HF survival score was designed at an early phase of the β-blocker era, using a relatively young population (mean age, 50 years), which differs significantly from the current population of patients with HF.32
The risk score model of the HF-ACTION trial included CPET data, namely exercise duration, serum urea nitrogen, female sex, and BMI, but it was developed using a selected population of HF patients who met the eligibility criteria of the trial.33
Conversely, the MECKI score is based on a multicenter registry and the patients’ characteristics are closer to the current clinical practice. In addition, this model includes data regarding renal function and hemoglobin, both known as important prognostic factors in patients with HF.
Although the original MECKI score model was developed in patients of all ages, in the present study the comparison of MECKI score with the multivariate models and the performance of MECKI score to predict cardiovascular events were similar in both younger and older patients, showing that MECKI score can be applied independently of age in a broad population of patients with HF. However, grouping patients according to age may affect predictive models and several variables may gain or lose their prognostic role. Notably, in our multivariate models the main difference between younger and older patients was the strong prognostic role of β-blocker treatment in the elderly population. This is likely related to the lower percentage of elderly patients receiving β-blockers, leading to a significant difference between β-blocker treated and untreated patients. In future, a MECKI score algorithm selected for specific populations may need to be implemented.
Finally, it is recognized that elderly patients rarely undergo cardiac transplant. Accordingly, in our study, only younger patients underwent cardiac transplant, and this may have affected the primary endpoint. The composite endpoint, however, which has been used in several previous MECKI score studies, was pre-specified, and the exclusion of urgent heart transplantation from the predictive models had little if any effect on the present findings.
Conclusions
Elderly patients with chronic systolic HF are a high-risk population with several comorbidities and lower exercise performance compared with younger patients. Exercise tolerance is a strong predictor of cardiovascular events in all age subgroups. MECKI risk score was increased in older patients, but its prognostic value was maintained independently of patient age, with a similar predictive power across age groups. Our study confirmed that MECKI score can be applied to a broad range of patients with chronic HF.
Appendix
Other MECKI Score Group members are:
Centro Cardiologico Monzino, IRCCS, Milan: Fabrizio Veglia, Valentina Mantegazza, Anna Apostolo, Mauro Contini, Pietro Palermo, Stefania Farina, Alice Bonomi, Erika Bertella
“Sapienza” University of Rome, Rome: Matteo Casenghi Division of Cardiology Rehabilitation, “S. Maugeri” Foundation, IRCCS, Scientific Institute of Veruno, Veruno: Pantaleo Giannuzzi, Andrea Giordano, Alessandro Mezzani
“Federico II” University, Naples: Pasquale Perrone-Filardi, Stefania Paolillo, Paola Gargiulo
Division of Cardiac Rehabilitation, Azienda Ospedali Riuniti, Ancona: Francesca Pietrucci
Cardiology Division, Santo Spirito Hospital, Rome: Angela B. Scardovi, Roberto Ricci, Alessandro Ferraironi
Department of Cardiology, San Luca Hospital, Istituto Auxologico Italiano, Milan: Gabriella Malfatto; Sergio Caravita
Cardiology SUN, Monaldi Hospital, Naples: Teo Roselli, Andrea Buono, Raffaele Calabrò, Daniele Masarone, Giuseppe Pacileo
CNR-Milan, Milan: Renata De Maria
Division of Cardiology, “S. Maugeri” Foundation, IRCCS, Institute of Cassano Murge, Bari: Andrea Passantino, Daniela Santoro, Saba Campanale, Domenica Caputo
“S. Maugeri” Foundation, IRCCS, Institute of Tradate, Department of Medicine and Cardiorespiratory Rehabilitation, Unit of Cardiac Rehabilitation, Tradate: Raffaella Vaninetti, Donatella Bertipaglia
Ospedali Riuniti and University of Trieste, Trieste: Marco Confalonieri, AnnaMaria Iorio, Giafranco Sinagra, Emanuela Berton, Chiara Torregiani
“Gabriele Monasterio” Foundation, CNR-Toscana, Pisa: Luigi E Pastormerlo, Michele Emdin
Division of Cardiology, University of Civil Hospital, Brescia: Marco Metra, Valentina Carubelli, Carlo Lombardi
University of Verona, Verona: Corrado Vassanelli, Elisa Battaia
Division of Cardiology, Salvatore Maugeri Foundation, IRCCS, Institute of Milan: Giovanni Marchese
Ospedale Cà Granda A.O. Niguarda, Milan: Davide Girola, Maria Frigerio, Fabrizio Oliva
San Camillo-Forlanini Hospital, Rome: Federica Re.
References
- 1.
McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology: Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J 2012; 33: 1787–1847.
- 2.
Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, et al. 2013 ACCF/AHA guideline for the management of heart failure: Executive summary: A report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2013; 128: 1810–1852.
- 3.
Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, et al. Executive summary: Heart disease and stroke statistics – 2014 update: A report from the American Heart Association. Circulation 2014; 129: 399–410.
- 4.
Mogensen UM, Ersboll M, Andersen M, Andersson C, Hassager C, Torp-Pedersen C, et al. Clinical characteristics and major comorbidities in heart failure patients more than 85 years of age compared with younger age groups. Eur J Heart Fail 2011; 13: 1216–1223.
- 5.
Murad K, Kitzman DW. Frailty and multiple comorbidities in the elderly patient with heart failure: Implications for management. Heart Fail Rev 2012; 17: 581–588.
- 6.
Fleg JL, Morrell CH, Bos AG, Brant LJ, Talbot LA, Wright JG, et al. Accelerated longitudinal decline of aerobic capacity in healthy older adults. Circulation 2005; 112: 674–682.
- 7.
Lazzarini V, Mentz RJ, Fiuzat M, Metra M, O’Connor CM. Heart failure in elderly patients: Distinctive features and unresolved issues. Eur J Heart Fail 2013; 15: 717–723.
- 8.
JCS Joint Working Group. Guidelines for rehabilitation in patients with cardiovascular disease (JCS 2012): Digest version. Circ J 2014; 78: 2022–2093.
- 9.
Agostoni P, Corrà U, Cattadori G, Veglia F, La Gioia R, Scardovi AB, et al. Metabolic exercise test data combined with cardiac and kidney indexes, the MECKI score: A multiparametric approach to heart failure prognosis. Int J Cardiol 2013; 167: 2710–2718.
- 10.
Magri D, Agostoni P, Corra U, Passino C, Scrutinio D, Perrone-Filardi P, et al. Deceptive meaning of oxygen uptake measured at the anaerobic threshold in patients with systolic heart failure and atrial fibrillation. Eur J Prev Cardiol 2015; 22: 1046–1055.
- 11.
Scrutinio D, Agostoni P, Gesualdo L, Corra U, Mezzani A, Piepoli M, et al. Renal function and peak exercise oxygen consumption in chronic heart failure with reduced left ventricular ejection fraction. Circ J 2015; 79: 583–591.
- 12.
Magrì D, Corrà U, Di Lenarda A, Cattadori G, Maruotti A, Iorio A, et al. Cardiovascular mortality and chronotropic incompetence in systolic heart failure: The importance of a reappraisal of current cut-off criteria. Eur J Heart Fail 2014; 16: 201–209.
- 13.
Hillege HL, Nitsch D, Pfeffer MA, Swedberg K, McMurray JJ, Yusuf S, et al. Renal function as a predictor of outcome in a broad spectrum of patients with heart failure. Circulation 2006; 113: 671–678.
- 14.
Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification. Eur J Echocardiogr 2006; 7: 79–108.
- 15.
Wasserman K, Hansen J, Sue DY, Stringer WW, Sietsema KE, Sun XG, et al. Principles of exercise testing and interpretation including pathophysiology and clinical applications. Philadelphia, PA: Lippincott Williams & Wilkins, 2012.
- 16.
Hollenberg M, Yang J, Haight TJ, Tager IB. Longitudinal changes in aerobic capacity: Implications for concepts of aging. J Gerontol A Biol Sci Med Sci 2006; 61: 851–858.
- 17.
Borlaug BA. Mechanisms of exercise intolerance in heart failure with preserved ejection fraction. Circ J 2014; 78: 20–32.
- 18.
Haykowsky MJ, Kouba EJ, Brubaker PH, Nicklas BJ, Eggebeen J, Kitzman DW. Skeletal muscle composition and its relation to exercise intolerance in older patients with heart failure and preserved ejection fraction. Am J Cardiol 2014; 113: 1211–1216.
- 19.
Brubaker PH, Joo KC, Stewart KP, Fray B, Moore B, Kitzman DW. Chronotropic incompetence and its contribution to exercise intolerance in older heart failure patients. J Cardiopulm Rehabil 2006; 26: 86–89.
- 20.
Miura Y, Fukumoto Y, Miura T, Shimada K, Asakura M, Kadokami T, et al. Impact of physical activity on cardiovascular events in patients with chronic heart failure: A multicenter prospective cohort study. Circ J 2013; 77: 2963–2972.
- 21.
Nakanishi M, Takaki H, Kumasaka R, Arakawa T, Noguchi T, Sugimachi M, et al. Targeting of high peak respiratory exchange ratio is safe and enhances the prognostic power of peak oxygen uptake for heart failure patients. Circ J 2014; 78: 2268–2275.
- 22.
Okita K, Kinugawa S, Tsutsui H. Exercise intolerance in chronic heart failure: Skeletal muscle dysfunction and potential therapies. Circ J 2013; 77: 293–300.
- 23.
Agostoni P, Emdin M, Corrà U, Veglia F, Magrì D, Tedesco CC, et al. Permanent atrial fibrillation affects exercise capacity in chronic heart failure patients. Eur Heart J 2008; 29: 2367–2372.
- 24.
Guazzi M, Myers J, Vicenzi M, Bensimhon D, Chase P, Pinkstaff S, et al. Cardiopulmonary exercise testing characteristics in heart failure patients with and without concomitant chronic obstructive pulmonary disease. Am Heart J 2010; 160: 900–905.
- 25.
Kato J, Koike A, Hoshimoto-Iwamoto M, Nagayama O, Sakurada K, Sato A, et al. Relation between oscillatory breathing and cardiopulmonary function during exercise in cardiac patients. Circ J 2013; 77: 661–666.
- 26.
Makita S. Significance of oscillatory breathing on cardiopulmonary exercise testing in chronic heart failure. Circ J 2013; 77: 598–599.
- 27.
Ingle L. Prognostic value and diagnostic potential of cardiopulmonary exercise testing in patients with chronic heart failure. Eur J Heart Fail 2008; 10: 112–118.
- 28.
Corrà U, Giordano A, Mezzani A, Gnemmi M, Pistono M, Caruso R, et al. Cardiopulmonary exercise testing and prognosis in heart failure due to systolic left ventricular dysfunction: A validation study of the European Society of Cardiology Guidelines and Recommendations (2008) and further developments. Eur J Prev Cardiol 2012; 19: 32–40.
- 29.
Scardovi AB, Coletta C, De Maria R, Perna S, Aspromonte N, Feola M, et al. The cardiopulmonary exercise test is safe and reliable in elderly patients with chronic heart failure. J Cardiovasc Med (Hagerstown) 2007; 8: 608–612.
- 30.
Magri D, Agostoni P, Corra U, Passino C, Scrutinio D, Perrone-Filardi P, et al. Deceptive meaning of oxygen uptake measured at the anaerobic threshold in patients with systolic heart failure and atrial fibrillation. Eur J Prev Cardiol 2015; 22: 1046–1055.
- 31.
Forman DE, Clare R, Kitzman DW, Ellis SJ, Fleg JL, Chiara T, et al. Relationship of age and exercise performance in patients with heart failure: The HF-ACTION study. Am Heart J 2009; 158(4 Suppl): S6–S15.
- 32.
Aaronson KD, Schwartz JS, Chen TM, Wong KL, Goin JE, Mancini DM. Development and prospective validation of a clinical index to predict survival in ambulatory patients referred for cardiac transplant evaluation. Circulation 1997; 95: 2660–2667.
- 33.
O’Connor CM, Whellan DJ, Wojdyla D, Leifer E, Clare RM, Ellis SJ, et al. Factors related to morbidity and mortality in patients with chronic heart failure with systolic dysfunction: The HF-ACTION predictive risk score model. Circ Heart Fail 2012; 5: 63–71.