2015 Volume 79 Issue 12 Pages 2641-2647
2015 Volume 79 Issue 12 Pages 2641-2647
Background: The aim of this study was to investigate the accuracy of optical frequency domain imaging (OFDI) in lipid-rich plaque detection and determine the causes of “misinterpretation,” and evaluate whether these limitations can be overcome with intravascular ultrasound (IVUS) using ex-vivo human coronaries.
Methods and Results: The OFDI images and corresponding 218 histological segments were evaluated. Segments with a poor signal and diffuse borders on OFDI, classified as lipid-rich plaques, were compared with the histological segments and IVUS images. Using histological images as the gold standard, the sensitivity, specificity, positive predictive value, and negative predictive value of OFDI for the detection of lipid-rich plaques were 93%, 93%, 85%, and 97%, respectively. The causes of false-positive diagnosis of lipid-rich plaque (11 segments) were superficial macrophage infiltration causing signal attenuation (8/11 segments, 73%) and tangential signal dropout of light (3/11 segments, 27%), whereas the cause of false-negative diagnosis was thickening of the fibrous cap (5 segments, 100%). Simultaneous IVUS helped to correct the misinterpretation of OFDI results and improved the diagnostic accuracy; the sensitivity, specificity, positive predictive value, and negative predictive value of OFDI with adjunct use of IVUS were 96%, 99%, 99%, and 98%, respectively.
Conclusions: OFDI occasionally over- or underestimates the existence of lipid-rich plaques, which may be overcome with adjunctive usage of IVUS. (Circ J 2015; 79: 2641–2647)
Intravascular imaging modalities have helped us understand the progression of coronary artery disease and have given insight to the strategy of percutaneous coronary intervention (PCI). Optical coherence tomography (OCT) enables visualization of the detailed features of the coronary artery wall, including characteristics of atherosclerotic plaque such as fibrous lesions, lipid-rich plaques, calcification, thrombus, and dissection. In particular, OCT has the advantage of being capable of detecting the signs of “vulnerable plaque” and the thickness of a fibrous cap over the necrotic core because of its high resolution (10–15 μm).1 Kubo et al reported that the identification of plaque rupture, fibrous cap erosion, intracoronary thrombus, and thin-cap fibroatheroma (TCFA) in patients with acute myocardial infarction was more accurate with OCT than with intravascular ultrasound (IVUS).2 Furthermore, it has been reported that OCT can predict no-reflow after PCI in patients with acute coronary syndrome by stratifying the arc of lipid-rich plaque.3 However, the accuracy of this device to detect lipid-rich plaque remains unclear, since van Soest et al showed that the finding of lipid-rich plaques in OCT could be wrongly interpreted, although they did not show the incidence of these misinterpretations.4
In the current study of ex vivo human coronary artery segments, using a recently developed optical frequency domain imaging (OFDI) system (Terumo Corporation, Tokyo, Japan),5 the second-generation OCT, we investigated (1) the accuracy of detection for lipid-rich plaques (ie, the frequency of misinterpretation), (2) the causes of “misinterpretation,” and (3) whether these limitations can be overcome with adjunct use of IVUS.
The institutional ethics committee approved the experimental protocol. Images were acquired from 253 segments of 38 coronary arteries obtained at autopsy from 15 cadavers (Table 1). The coronary arteries were dissected from the heart and fixed in 10% buffered formalin solution and stored more than 48 h.
| Patient clinical characteristics | n=15 |
| Age, years | 70±13 |
| Male | 11 (73%) |
| Hypertension | 15 (100%) |
| Dyslipidemia | 9 (67%) |
| Diabetes mellitus | 8 (60%) |
| Smoker | 7 (53%) |
| Hemodialysis patient | 4 (27%) |
| Cause of death | n=15 |
| Myocardial infarction | 7 (47%) |
| Heart failure | 2 (13%) |
| Unknown sudden death | 2 (13%) |
| Gastric cancer | 1 (7%) |
| Pneumonia | 1 (7%) |
| SMA occlusion | 1 (7%) |
| Acute limb ischemia | 1 (7%) |
| Segment characteristics | n=218 |
| LAD/LCX/RCA/LMCA | 70/13/122/13 |
| Proximal/distal (%) | 66/34 |
LAD, left anterior descending; LCX, left circumflex artery; LMCA, left main coronary artery; OFDI, optical frequency domain imaging; RCA, right coronary artery; SMA, superior mesenteric artery.
The samples were pinned on a rubber sheet and immersed in a waterbath with 0.9% saline solution. A 0.014-inch guidewire was introduced into the vessel followed by an IVUS catheter (frequency 40 MHz, VISIWAVE, Terumo). IVUS images of the entire vessel were acquired at a pullback rate of 0.5 mm/s (30 frames/s). Following the IVUS procedure, the catheter was removed, an OFDI catheter (LUNAWAVE, Terumo) was introduced in a similar manner, and sequential images were acquired at a pullback rate of 20 mm/s (158 frames/s).
HistologyAfter the intracoronary imaging, samples were cut into 3-mm segments and embedded in paraffin to obtain 4–6-μm thick sections, which were stained with hematoxylin & eosin (H&E) and Movat pentachrome stain.
Definition of Plaque AssessmentAn OFDI image of a signal-poor, high-attenuation lesion with a diffuse border was defined as a lipid-rich plaque.6 For lipid-rich plaques, the lipid arc and fibrous cap were measured. OFDI-derived TCFA was defined as lipid plaque with lipid arc >90° and a fibrous cap <65 μm measured at the thinnest part. On IVUS, an echolucent atheroma is considered to indicate a high lipid content plaque, whereas plaques with high and intermediate echogenicity are recognized as fibrous, as previously described.7 In histology, the plaque type was classified as fibrous, fibrocalcific, or lipid-rich. Plaques with a lipid pool and/or necrotic core were defined as “lipid-rich” plaques.8 The fibrous cap of lipid-rich plaques was measured and the plaque was classified as TCFA when the fibrous cap was <65 μm.
Coregistration of Histology and ImagingSingle OFDI and IVUS frames were extracted and digitized for direct comparison with the corresponding histological images. Adjustments were made using the luminal configuration or anatomical landmarks such as vessel branches, thus improving the accuracy of registration. We excluded 35 histological segments on the basis of cutting artifacts, coregistration difficulty, and presence of a large branch. Altogether, a total of 218 pairs of matched images were acquired from IVUS and OFDI with corresponding histological sections (Figure 1).

Lesion flowchart for the current study. IVUS, intravascular ultrasound; OFDI, optical frequency domain imaging.
Two experienced observers, unaware of the histological examination data, independently performed a qualitative analysis of OFDI and IVUS for each. They were instructed to provide a single diagnosis for each segment. The sections selected were taken approximately every 3 mm from each epicardial coronary artery and randomly shown to the observers. Both observers were also blinded to the clinical information. In the case of disagreement, the 2 observers reanalyzed the image and reached a consensus diagnosis. The first observer repeated a blinded analysis of the same images after an interval of 2 weeks.
Statistical AnalysisThe degree of agreement between the results obtained by the OFDI and IVUS readers was quantified by the κ test of concordance.9 A κ value of 0.61–0.80 indicates good agreement, and a value of 0.81–1.0 indicates excellent agreement. For estimation of predictive ability of each modality, the sensitivity, specificity, positive predictive value, and negative predictive value of the imaging modality were calculated for each tissue component.
We investigated 218 matched segments; the external elastic laminar area, lumen area, and percent stenosis by histology were 11.0±4.1 mm2, 3.4±1.9 mm2, and 69.2%, respectively. Of the 218 segments, 74 were classified as lipid-rich plaques on OFDI images. Among those, 63 segments (85%) showed necrotic cores and/or lipid-pools in the histological segments (Figure 2); the remaining 11 segments (15%) did not have lipid-rich contents. The remaining 144 segments were classified as a fibrous or calcified lesion on OFDI; however, 5 segments (3%) had lipid-rich plaque on histological examination, which had been misdiagnosed as fibrous plaque by OFDI (Table 2A). Accordingly, the sensitivity, specificity, positive predictive value, and negative predictive value of OFDI for the detection of lipid-rich plaques were 93%, 93%, 85%, and 97%, respectively. Of 16 OFDI-derived TCFA, 7 plaques (44%) were classified as TCFA by histological examination (Table 2B). The thickness of the caps of TCFA on OFDI and histology was 60.6±5.7 μm and 51.0±15.0 μm, respectively. The sensitivity, specificity, positive predictive value, and negative predictive value of OFDI for the detection of TCFAs were 100%, 96%, 44%, and 100%, respectively. The intra- and interobserver agreement for characterizing plaque type using OFDI was high (κ=0.83, 0.94, respectively).

Representative fibroatheroma with thick fibrous cap on OFDI (A), corresponding IVUS image (B), histological segment (Movat pentachrome stain, ×20) (C) and magnified image of the inset in C (×100) (D). IVUS, intravascular ultrasound; OFDI, optical frequency domain imaging.
| (A) | Histology (n=218) | ||
|---|---|---|---|
| Lipid (+) | Lipid (−) | ||
| OFDI | Lipid (+) | 63 | 11 |
| Lipid (−) | 5 | 139 | |
| IVUS | Lipid (+) | 44 | 15 |
| Lipid (−) | 24 | 135 | |
| OFDI + IVUS | Lipid (+) | 65 | 1 |
| Lipid (−) | 3 | 149 | |
| (B) | Histology (n=218) | ||
| TCFA (+) | TCFA (−) | ||
| OFDI | TCFA (+) | 7 | 9 |
| TCFA (−) | 0 | 202 | |
| OFDI + IVUS | TCFA (+) | 7 | 2 |
| TCFA (−) | 0 | 209 | |
IVUS, intravascular ultrasound; TCFA, thin-cap fibroatheroma. Other abbreviations as in Table 1.
The causes of false-positive diagnosis of lipid-rich plaque by OFDI were superficial macrophage infiltration causing signal attenuation (8 of 11 segments, 73%, Figure 3) and tangential signal dropout of light because of an acute angle between the imaging catheter and the vessel surface (3/11 segments, 27%, Figure 4), whereas the cause of false-negative diagnosis was the limitation of OFDI in signal penetration (ie, the fibrous cap was too thick to diagnose with OFDI and the mean actual thickness of the fibrous cap measured on histological section was 661.3±201.1 μm, Figure 5).

Representative images of superficial shadowing caused by foamy macrophage infiltration. Dense macrophage infiltration located at 10–4 o’clock creates a highly light scattering layer that casts a dark shadow on the tissue behind (A). The lesion appears to be a thin-cap fibroatheroma; however, the IVUS image (B), and histological section (Movat Pentachrome stain, ×20) (C) show that the lesion is a stable fibrous plaque. (D) Magnified image of the inset in C (×100). IVUS, intravascular ultrasound; OFDI, optical frequency domain imaging.

Representative images of tangential signal dropout of light because of an acute angle between the imaging catheter and the vessel surface. Although a signal-poor lesion with a diffuse border appears to be located at 4–6 o’clock on the OFDI image (A) the histological segment (H&E, ×20) (C) shows that it is a stable fibrous plaque. The OFDI beam from the catheter strikes the vessel wall, but tangential signal dropout will occur when the angle between the beam and the surface of the vessel (angle θ in B) is small. IVUS, intravascular ultrasound; OFDI, optical frequency domain imaging.
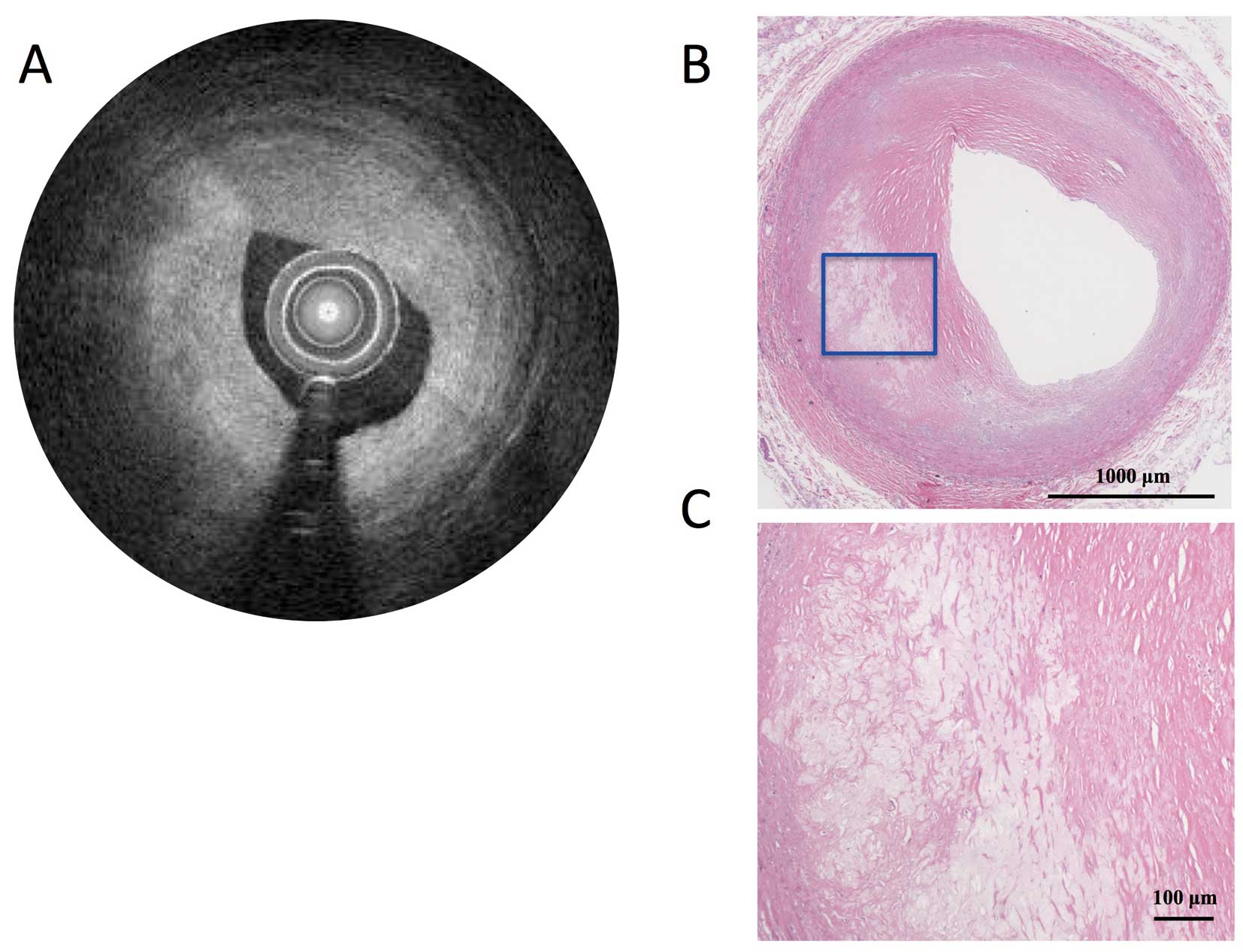
Representative images of a false-negative segment on OFDI because of the thickness of the plaque. The OFDI image (A) is interpreted as a fibrous plaque, but the histological section (H&E, ×20) (B) shows a lipid pool at 7–9 o’clock. (C) Magnified image of the inset in B (×100). IVUS, intravascular ultrasound; OFDI, optical frequency domain imaging.
Simultaneous use of IVUS helped to overcome misinterpretation by OFDI and improved diagnostic accuracy. For example, IVUS can identify intermediate-echoic plaques (ie, fibrous plaques) in the areas of attenuation on OFDI because of macrophage infiltration, which were diagnosed as lipid-rich plaques according to the OFDI definition of interpretation. Finally, IVUS compensates for the pitfalls of OFDI, and the sensitivity, specificity, positive predictive value, and negative predictive value for the detection of lipid-rich plaques increased up to 96%, 99%, 99%, and 98%, respectively (Table 3). The causes of false-positive diagnosis of TCFA with OFDI were same as for lipid-rich plaques, so the simultaneous use of IVUS also improved diagnostic accuracy, and the sensitivity, specificity, positive predictive value, and negative predictive value for the detection of TCFAs increased up to 100%, 99%, 78%, and 100%, respectively.
| Sensitivity | Specificity | PPV | NPV | |
|---|---|---|---|---|
| OFDI (%) | 93 | 93 | 85 | 97 |
| IVUS (%) | 65 | 90 | 75 | 85 |
| OFDI + IVUS (%) | 96 | 99 | 99 | 98 |
NPV, negative predictive value; PPV, positive predictive value. Other abbreviations as in Tables 1,2.
One of the goals of imaging is the identification of vulnerable plaque that may cause myocardial infarction or cardiac death, because the majority of coronary thrombosis-related cardiac deaths are the result of a ruptured plaque.10,11 It has been reported that OCT has advantages in identifying unstable plaque with a thin-fibrous cap compared with IVUS because of its higher resolution.2,12 These unstable lipid-rich plaques can cause not only coronary thrombotic events,13 but also complications during PCI such as the no-reflow phenomenon.3,14 However, the question then arises as to how accurate are the current imaging modalities in detecting “vulnerable plaques”? With improved penetration depth, the sensitivity for fibroatheroma detection by second-generation OCT has significantly increased (94–98%).15,16
The current study demonstrated a sensitivity of 93%, which is similar to a previous study using second-generation OCT. On the other hand, the positive predictive value in the present study was 85%, which means that 15% of the segments demonstrated a signal-poor lesion with a diffuse border on OFDI images and showed no sign of lipid-rich contents in the histological segments. The causes of “overestimation” were superficial shadowing (8 segments, 73%) and tangential signal dropout of light (3 segments, 27%). A previous study reported the causes of false-positive diagnosis of lipid-rich plaque with OCT as foamy macrophage accumulation, microcalcifications, hemosiderin accumulation on the luminal surface, and organized thrombus.17 However, microcalcifications, hemosiderin, and organized thrombus were not the causes of misdiagnosis in our study.
Superficial shadowing occurs because of a strongly scattering foamy macrophage concentration in the innermost intima, which creates a “pseudo-fibroatheroma” on OFDI. The presence of accumulated foamy macrophages on the luminal surface exhibits a distinct appearance, with a remarkably bright signal that causes high attenuation to the tissue behind with a delineated radial border;18 however, the concern is that foamy macrophages often accumulate on top of “true” unstable plaques, including TCFA and ruptured plaque.19 Therefore, it is occasionally difficult to distinguish between “pseudo-fibroatheroma” and true unstable plaques by OFDI/OCT alone.
In the current study, we demonstrated that concurrent use of IVUS may be helpful for avoiding such misinterpretations, because it appears that superficial macrophage infiltration does not affect the IVUS signals; therefore, it can identify the tissue type behind the “pseudo-fibroatheroma”.
On the other hand, tangential signal dropout occurs when the imaging beam strikes the tissue at an oblique angle. The beam does not reflect correctly, so the signal from that area is weaker than when the imaging beam strikes the interface perpendicularly, which causes the image to appear like a lipid-rich tissue covered by a fibrous cap. Special attention to the positioning of the OFDI catheter is required in such situations.
The negative predictive value for lipid-rich plaque detection was as high as 97%. However, there were some segments with “underestimation” of lipid-rich plaque, mainly caused by the thickness of the fibrous tissue over the lipid-rich area (ie, the fibrous cap). Although the penetration of OFDI (ie, second-generation OCT) is improved compared with the first-generation OCT, the signal still attenuates as plaque thickness increases.
We assume that experienced physicians may be able to distinguish the difference between true lipid-rich plaque and artifacts caused by these patterns; however, plaque volume and characteristics cannot be assessed because of the attenuated signals of OFDI. Therefore, adjunctive usage of IVUS does have potential for correct diagnosis of unstable lesions, as shown in the current study.
In the PROSPECT trial, patients with vulnerable plaque detected by VH-IVUS had more clinical events in a 3-year follow-up period.13 Although vulnerable plaques have a higher risk of future cardiac events, the strategy to prevent rupture of non-stenotic vulnerable plaques is controversial.20 Brugaletta et al recently presented the hypothesis that implanting bioresorbable scaffolds in lesions with a TCFA (so-called “plaque sealing”) might induce the growth of a neointimal layer, creating a fibrous cap over the treated plaque and preventing a future cardiac event.21 However, considering the event rate with current intracoronary devices, far more accurate detection of vulnerable plaque is necessary to prevent future cardiac events and benefit the patient’s prognosis. To achieve a higher frequency of diagnosis of vulnerable plaques, simultaneous usage of OFDI/OCT and IVUS is a possible option; however, it is currently unrealistic for economic reasons. Although usage of a combined IVUS-OCT catheter has been recently reported,22,23 there are some problems with pullback speed and blood clearance for human use. A new device with both OFDI and IVUS functions to enable simultaneous use of both modalities needs to be developed.
Study LimitationsFirst, the clinical applicability of our results is inherently limited by the ex vivo setting of our experiments, because the absence of cardiac motion, blood and body temperature has a documented effect on the OFDI signal.24 The occurrence of tangential signal dropout of light might be overestimated because the arterial wall changes shape beat by beat during pulsation so that the angle between the arterial wall and the OFDI imaging beam would be more perpendicularly in the in vivo setting. In addition, there might be a discrepancy in quantitative measurements between “histopathology” derived TCFA and “OFDI” derived TCFA because tissue shrinkage or swelling can occur during histopathological processing. Although the analysis was performed with formalin-fixed specimens, Okubo et al reported that formalin fixation does not significantly change the quantitative echo characteristics of the plaque tissue of human aortic walls,18,25 and there are several studies using a similar method to correlate histology and imaging devices, and we believe that we made the best effort to minimize these limitations.18,26,27 Second, although a multistep approach was used to achieve ideal coregistration of the OFDI and IVUS images with histological segments, there were no other indicators or computational methods to pin-point the precise location of the histological segments to the catheter-based signals. Also, the axial resolution of imaging (OFDI 15–20 μm; IVUS 100–200 μm) was obtained from a much thicker section than the corresponding histological sections (4–6 μm). Third, radiofrequency IVUS, including IB-IVUS and VH-IVUS, is more accurate in detecting plaque characteristics, because of the minimal inter- and intraobserver reproducibility for grayscale IVUS. However, we believe that grayscale IVUS is enough to overcome misinterpretation by OFDI because it can easily overcome the superficial shadowing on the OFDI image. Fourth, lack of immunochemical staining to identify macrophages/foam cells is an additional limitation. Finally, the study was performed in a limited number of samples, and therefore, further studies are required to confirm the significance and application of our findings in a larger cohort of patients.
OFDI is an optimal imaging technique for the detection of lipid-rich plaques, but misinterpretation occasionally occurs, which may be overcome by adjunctive use of IVUS.
The authors thank Yoshiko Ito from the Education and Research Support Center, Tokai University, for her valuable technical assistance.
None.
G.N. is a consultant for Terumo Corporation, St. Jude Medical, Abbott Vascular Japan, and Japan Stent Technology. All other authors have no conflict of interests.