2015 Volume 79 Issue 2 Pages 255-262
2015 Volume 79 Issue 2 Pages 255-262
Platelets initiate the formation of a thrombus at the site of an arterial injury, and the clotting cascade is activated as the thrombus matures. After coronary stent placement, dual antiplatelet therapy (DAPT) with aspirin and ticlopidine dramatically reduces the risk of stent thrombosis, compared with anticoagulation therapy, and has become the standard of care for prevention of stent thrombosis. Clopidogrel is a second-generation thienopyridine that eliminates the serious side effects of ticlopidine, and new P2Y12 receptor blockers have emerged to overcome the limitations of clopidogrel. Current guidelines recommend DAPT with aspirin and clopidogrel for 1 month after implantation of bare-metal stents, and for 6–12 months after implantation of drug-eluting stents (DES). In patients with acute coronary syndrome (ACS), DAPT administration for 12 months was shown to be superior to aspirin alone for the prevention of recurrent events. Treatment with aspirin and new P2Y12 receptor blockers has further reduced the rate of cardiovascular death, myocardial infarction or stroke after ACS compared with aspirin and clopidogrel. Nonetheless, long-term DAPT increases the risk of major bleeding, requiring a delicate balance between anti-ischemic benefit and bleeding risk. In summary, DAPT should be maintained for at least 6–12 months after implantation of DES, and for at least 12 months after ACS, unless contraindicated. (Circ J 2015; 79: 255–262)
Aspirin prevents serious vascular events in patients at high risk of atherosclerosis.1,2 The mechanism of aspirin’s antiplatelet action was first described in 1971,3 but it took a long time before aspirin’s value was accepted in cardiovascular medicine. Early aspirin trials were mostly of questionable statistical significance. Moreover, the first large randomized trial, the Aspirin Myocardial Infarction Study (AMIS), showed that aspirin (1,000 mg/day) did not prevent cardiac death in patients who had already had a myocardial infarction (MI).4 Adverse side effects, including peptic ulcer and gastrointestinal bleeding, were higher in the aspirin group than in the placebo group. Based on the AMIS results, aspirin was not routinely recommended for patients who had survived a MI. In the large, randomized International Study of Infarct Survival (ISIS)-2, however, aspirin (160 mg/day) demonstrated a dramatic 23% reduction in vascular mortality compared with placebo in patients with acute MI.5 Thereafter, the use of aspirin in real-world clinical practice rapidly increased in advance of controlled studies, reflecting the ISIS-2 trial’s strong positive results.6 Furthermore, in most clinical studies, aspirin has become the default therapy for arterial thrombosis. Current guidelines recommend the use of low-dose aspirin (75–325 mg/day) indefinitely for patients with established atherosclerotic cardiovascular disease.7
Platelets are critical during the initial development of arterial thrombi, and are activated when the vascular endothelium is damaged. Platelet activation can be triggered by many different agonists, including adenosine diphosphate, thromboxane A2, thrombin, etc. Adenosine diphosphate, as a P2Y12 receptor agonist, induces platelet degranulation and shape change, leading to amplification and stabilization of platelet aggregation.8 The central role of the P2Y12 receptor in platelet activation has made it a key target of antiplatelet agents. Ticlopidine, a first-generation thienopyridine, was discovered in 1972 and introduced as an antiplatelet agent in 1978. Despite its excellent antiplatelet effect, ticlopidine has rare but potentially fatal side effects, including aplastic anemia, neutropenia and thrombotic thrombocytopenic purpura, requiring routine hematological monitoring.9 Clopidogrel is a second-generation thienopyridine lacking the serious hematological side effects of ticlopidine and was approved for use in 1997. Clopidogrel has quickly replaced ticlopidine because of its equivalent efficacy and superior safety profile. P2Y12 receptor blocker development led to prasugrel and ticagrelor, which overcome the limitations of clopidogrel, such as delayed onset of action and high interindividual variability of antiplatelet response.10 Prasugrel is a third-generation thienopyridine with faster onset of action and less interindividual variability. It is more efficiently converted to an active metabolite than first- and second-generation thienopyridines. Thienopyridine derivatives exert their antiplatelet activity by irreversibly inhibiting the P2Y12 receptor. By contrast, ticagrelor is a new chemical class with faster onset of action and consistent platelet inhibition that does not require hepatic metabolism for its activity and causes reversible inhibition of the P2Y12 receptor.
P2Y12 Receptor Blockers vs. AspirinSeveral randomized trials have investigated whether P2Y12 receptor blockers are safe and more effective than aspirin in patients with atherosclerotic cardiovascular disease (Table 1).11–14 In the Ticlopidine Aspirin Stroke Study (TASS), 3,069 patients with transient ischemic attack or mild stroke were randomized to take either ticlopidine (500 mg/day) or aspirin (1,300 mg/day).11 The 3-year event rate for death from any causes or nonfatal stroke was significantly lower in the ticlopidine group compared with the aspirin group (17% vs. 19%, respectively, P=0.048). The rates of fatal and nonfatal stroke at year 3 were 10% for ticlopidine and 13% for aspirin (P=0.024); however, skin rash and gastrointestinal side effects with severe reversible neutropenia were more common in the ticlopidine group than in the aspirin group. These findings suggest that ticlopidine may be more effective than aspirin at preventing strokes in patients with recent ischemic stroke; however, in the African American Antiplatelet Stroke Prevention Study (AAASPS), in which 1,809 African American patients with noncardioembolic ischemic stroke were randomized to receive either ticlopidine (500 mg/day) or aspirin (650 mg/day),13 the 2 drugs were similar in terms of preventing recurrent strokes. The Clopidogrel versus Aspirin in Patients at Risk of Ischemic Events (CAPRIE) trial14 assessed the relative efficacy of clopidogrel (75 mg/day) and aspirin (325 mg/day) in reducing the risk of vascular death, MI or ischemic stroke (primary endpoints) in 19,185 patients with recent MI, recent ischemic stroke or symptomatic peripheral arterial disease. During a mean follow-up of 1.91 years, patients treated with clopidogrel had an annual 5.32% risk of primary endpoint compared with 5.83% with aspirin (relative-risk reduction 8.7%; 95% confidence interval [CI]; 0.3–16.5; P=0.043). The overall safety profile of clopidogrel was similar to that of aspirin. These findings show that clopidogrel is more effective than aspirin at preventing recurrent vascular events in patients with atherosclerotic vascular disease. Taken together, the thienopyridine derivatives seem to be slightly but significantly better than aspirin at preventing serious vascular events in patients with atherosclerotic cardiovascular disease. A large-scale randomized trial comparing the efficacy and safety of ticagrelor and aspirin in patients with acute stroke or transient ischemic attack is ongoing (Table 1) to assess the relative benefits of ticagrelor over aspirin in this population.
| Trial | Patients | n | Comparison | Follow-up (months) |
Primary endpoints |
Results | P value |
|---|---|---|---|---|---|---|---|
| TASS | TIA, stroke | 3,069 | Ticlopidine vs. aspirin |
36 | Death/stroke | RRR, 12% 95% CI (−2, 26) |
0.048 |
| AAASPS | Stroke | 1,470 | Ticlopidine vs. aspirin |
24 | Vascular death/MI/ stroke |
HR, 1.22 95% CI (0.94, 1.57) |
0.12 |
| STAMI | STEMI | 1,809 | Ticlopidine vs. aspirin |
6 | Death/MI/ stroke/angina |
8.0% vs. 8.0% | 0.966 |
| CAPRIE | Post-MI, stroke, PAD |
19,185 | Clopidogrel vs. aspirin |
23 | Vascular death/MI/ stroke |
RRR, 8.7% 95% CI (0.3, 16.5) |
0.043 |
| SOCRATES | TIA, stroke | 9,600 | Ticagrelor vs. aspirin |
3 | Death/MI/stroke | NCT01994720, ongoing |
AAASPS, African American Antiplatelet Stroke Prevention Study; CAPRIE, Clopidogrel versus Aspirin in Patients at Risk of Ischemic Events; CI, confidence interval; HR, hazard ratio; MI, myocardial infarction; NA, not available; PAD, peripheral artery disease; RRR, relative risk reduction; STEMI, ST-elevation myocardial infarction; TASS, Ticlopidine Aspirin Stroke Study; TIA, transient ischemic attack.
Stent thrombosis has been a serious concern since the introduction of coronary artery stents in 1986. In early clinical experience, the rates of stent thrombosis were unacceptably high, approaching 20%.15 Optimal stent deployment with adjunctive dual antiplatelet therapy (DAPT) resulted in a marked reduction in stent thrombosis and bleeding complications.16–18 In the Stent Anticoagulation Restenosis Study (STARS),17 1,653 patients with successful stent placement were randomly assigned to 1 of 3 regimens: aspirin alone, aspirin and warfarin, or aspirin and ticlopidine. Compared with aspirin alone (3.6%) and the combination of aspirin and warfarin (2.7%), the combination of aspirin and ticlopidine (0.5%) resulted in a lower rate of stent thrombosis within 30 days (P=0.001), demonstrating the superiority of aspirin and ticlopidine over aspirin and warfarin. DAPT with aspirin and clopidogrel for 1 month following bare-metal stent implantation was subsequently shown to be comparably effective and safer than aspirin and ticlopidine for the prevention of stent thrombosis,18 becoming the standard of care after coronary stent implantation.
DES have dramatically reduced the rate of restenosis and become the mainstream device for percutaneous coronary intervention (PCI); however, late stent thrombosis remains an important limitation of these devices,19 leading to the recommendation of DAPT with aspirin and clopidogrel for at least 6–12 months after placement.20,21 These recommendations were largely based on retrospective analyses with conflicting results,22–24 requiring adequately powered randomized controlled trials. Fortunately, several randomized controlled trials have been performed to determine the optimal duration of DAPT after DES implantation (Table 2).25–38
| Trial | No. of patients |
Stent type | Duration | Primary endpoints | HR (95% CI) | P value |
|---|---|---|---|---|---|---|
| ZEST/REAL-LATE | 2,701 | PES, SES, E-ZES | 12 vs. >12 months | Cardiac death/MI | 0.61 (0.30, 1.25) | 0.17 |
| DES LATE | 5,045 | Any DES | 12 vs. >12 months | Cardiac death/MI/stroke | 0.94 (0.66, 1.35) | 0.75 |
| ARTIC-interruption | 1,259 | Any DES | 12 vs. >12 months | Death/MI/stroke/ST/ urgent revascularization |
1.17 (0.68, 2.03) | 0.58 |
| DAPT | 9,961 | Any DES | 12 vs. 30 months | ST or | 3.45 (2.08–5.88) | <0.001 |
| Death/MI/stroke | 1.41 (1.18–1.69) | <0.001 | ||||
| PRODIGY | 2,013 | BMS, PES, E-ZES, EES | 6 vs. 24 months | Death/MI/stroke | 0.98 (0.74, 1.29) | 0.91 |
| EXCELLENT | 1,443 | SES vs. EES | 6 vs. 12 months | Cardiac death/MI/ target vessel revascularization |
1.14 (0.70, 1.86) | 0.60 |
| SECURITY | 1,399 | New DES | 6 vs. 12 months | Cardiac death/MI/stroke/ST/ BARC 3, 5 bleeding at 12 months |
NA | 0.469 |
| ISAR-SAFE | 4,005 | Any DES | 6 vs. 12 months | Death/MI/ST/stroke/ TIMI major bleeding |
NA | Pnoninferiority <0.001 |
| RESET | 2,117 | E-ZES vs. Any DES | 3 vs. 12 months | Cardiac death/MI/ST | NA | 0.84 |
| OPTIMIZE | 3,119 | E-ZES | 3 vs. 12 months | Death/MI/stroke/major bleeding | 1.03 (0.77, 1.38) | 0.84 |
| SMART-CHOICE | 5,100 | New DES | 3 vs. 12 months | Death/MI/stroke/ BARC bleeding 2, 3, 4, 5 |
NCT02079194, ongoing |
BARC, Bleeding Academic Research Consortium; BMS, bare-metal stent; DAPT, dual antiplatelet therapy; DES, drug-eluting stent; EES, everolimus-eluting stent; E-ZES, Endeavor zotarolimus-eluting stent; New DES, BiomatrixTM, EES (XienceTM, Promus elementTM), Endeavor Resolute-ZES, NoboriTM; PES, paclitaxel-eluting stents; SES, sirolimus-eluting stent; ST, stent thrombosis; TIMI, thrombolysis in myocardial infarction. Other abbreviations as in Table 1.
The benefit of DAPT beyond a 12-month period after PCI with DES was first tested in 2,701 patients who were free of major adverse cardiovascular events and major bleeding for at least 12 months after implantation.25 The cumulative risk of death from cardiac causes or MI at 24 months was 1.8% with DAPT, compared with 1.2% with aspirin monotherapy (hazard ratio [HR], 1.65; 95% CI: 0.80–3.36; P=0.17). No difference in the risk of bleeding complications was observed between the 2 groups. These findings were subsequently confirmed by an extended study26 showing that the use of DAPT for more than 12 months in stable patients with DES was not significantly more effective than aspirin monotherapy at preventing ischemic events. In the Assessment by a Double Randomization of a Conventional Antiplatelet Strategy versus a Monitoring-guided Strategy for Drug-Eluting Stent Implantation and of Treatment Interruption versus Continuation One Year after Stenting (ARCTIC)-Interruption trial,27 1,259 patients without contraindicated interruption of DAPT 12 months after DES implantation were randomly allocated to the continuation group or the interruption group. During a median follow-up of 17 months, no differences were observed in all-cause death, MI, stroke or transient ischemic attack, urgent coronary revascularization or stent thrombosis (HR, 0.85; 95% CI: 0.49–1.47; P=0.58); however, major or minor bleeding events were more common in the continuation group than in the interruption group (HR, 3.85; 95% CI: 1.10–14.29; P=0.04). In the DAPT study,28 9,961 patients who had been stable on DAPT for 12 months after DES implantation were randomly assigned to aspirin plus thienopyridine or aspirin plus placebo for 18 additional months. DAPT reduced the rates of stent thrombosis (HR, 0.29; 95% CI: 0.17–0.48; P<0.001) and major adverse cardiovascular and cerebrovascular events (HR, 0.71; 95% CI: 0.59–0.85; P<0.001) compared with aspirin monotherapy. A 50% reduction in MI (55% not related to stent thrombosis) was also observed, showing that DAPT prevents MI in arteries beyond the stented lesions; however, the rate of all-cause death was higher in the DAPT group compared with the aspirin monotherapy group (HR, 1.36; 95% CI: 1.00–1.85; P=0.05), and the rate of moderate or severe bleeding was also increased with DAPT (2.5% vs. 1.6%, P=0.001). Taken together, DAPT beyond 12 months after DES implantation may reduce both stent-related and other ischemic events beyond the stented region, but the benefits of DAPT are tempered by an increased risk of bleeding.
DAPT for 6 MonthsIn the Efficacy of Xience/Promus versus Cypher to Reduce Late Loss After Stenting (EXCELLENT) trial,29 1,443 patients who had received DES were randomized to receive 6 or 12 month DAPT. The rates of target vessel failure at 12 months were 4.8% in the 6-month DAPT group and 4.3% in the 12-month DAPT group (P=0.001 for noninferiority) with no difference in bleeding complications. Similarly, in the Prolonging Dual Antiplatelet Treatment after Grading Stent-Induced Intimal Hyperplasia Study (PRODIGY),30 2,013 patients were treated with 4 types of stents and then randomly allocated to receive 6 or 24 months of clopidogrel therapy in addition to aspirin. The rates of death of any cause, MI or cerebrovascular accident were not different between the 2 groups; however, DAPT for 24 months was associated with a significantly higher bleeding rate. These findings support the greater safety of 6-month DAPT compared with 12-month DAPT or longer after implantation of DES. In the Second-Generation Drug-Eluting Stent Implantation Followed by Six- versus Twelve-Month Dual Antiplatelet Therapy (SECURITY) trial,31 1,399 patients with newer-generation DES were randomized to receive 6-month or 12-month DAPT. No difference in efficacy or safety endpoints was observed between the 2 groups. In the Safety and Efficacy of Six-Month Dual Antiplatelet Therapy After Drug-Eluting Stenting (ISAR-SAFE) trial,32 4,005 patients undergoing DES implantation were randomized to either 6- or 12-month DAPT. This trial was prematurely terminated because of recruitment issues and low event rates. The rate of primary major adverse cardiac events was similar between the 6- and 12-month DAPT groups (1.5% vs. 1.6%, P<0.001 for noninferiority). TIMI major or minor bleeding events were similar between the 2 groups (0.3% vs. 0.7%, P=0.12), whereas Bleeding Academic Research Consortium ≥class 2 bleeding was lower in the 6-month DAPT group (1% vs. 2%, P=0.01) than in the 12-month group. Taken together, 6 months of DAPT may be noninferior to 12 months of DAPT in patients undergoing DES implantation, with a trend toward lower bleeding.
DAPT for 3 MonthsDES technology has rapidly advanced over the past decade, and late stent thrombosis may be less of a clinical issue with newer-generation DES than with older devices.34 Furthermore, stent thrombosis mostly occurs within 3 months after DES implantation and rarely continues to occur thereafter.35 Previous DES are no longer used in routine clinical practice, and a growing body of evidence suggests that 3 months of DAPT may be safe in stable patients treated with newer-generation DES.36,37 Recently, the Optimized Duration of Clopidogrel Therapy Following Treatment with the Zotarolimus-Eluting Stent in Real-World Clinical Practice (OPTIMIZE) trial also showed that 3 months of DAPT after Endeavor zotarolimus-eluting stent implantation was not inferior to 12 months of DAPT for net adverse clinical and cerebral events, without significantly increasing the risk of stent thrombosis.38 The 3-month DAPT regimen after newer-generation DES implantation may be considered in patients who are at low risk for stent thrombosis but at high risk for bleeding; however, both stented and non-stented lesions are involved in future coronary events, requiring a comprehensive approach.
Plaque rupture and subsequent thrombus formation are the major disease mechanisms in ACS. Platelets initiate the formation of a thrombus at the plaque rupture site, and then the clotting cascade is activated as the thrombus matures. Non-ST-elevation ACS (NSTE-ACS) is usually caused by incomplete coronary occlusion by thrombi, and ST-elevation MI by complete occlusion. The central role of platelets in the development of ACS was demonstrated by several randomized studies showing the unequivocal clinical benefit of antiplatelet therapy.39–47 Current guidelines recommend DAPT for at least 12 months in all ACS patients, regardless of initial treatment strategy, if not contraindicated.48,49
Previous DAPT in ACSThe Clopidogrel in Unstable Angina to Prevent Recurrent Events (CURE) trial39 compared the efficacy and safety of long-term DAPT to that of aspirin alone in 12,562 patients with NSTE-ACS (Table 3). Patients who had presented within 24 h after the onset of symptoms were randomized to receive clopidogrel (300 mg immediately followed by 75 mg once daily) or placebo in addition to aspirin for 3–12 months. The primary endpoint of death from cardiovascular causes, nonfatal MI or stroke occurred in 9.3% of the patients in the DAPT group and 11.4% of the patients in the aspirin group (relative risk, 0.80; 95% CI: 0.72–0.90; P<0.001). The rate of major bleeding was significantly higher in the DAPT group than in the aspirin group (3.7% vs. 2.7%; relative risk, 1.38; P=0.001), but the rate of life-threatening bleeding or hemorrhagic stroke was similar between the 2 groups. This study demonstrated the benefit of 12-month DAPT in patients with NSTE-ACS, providing a background for recommending DAPT to ACS patients. In this trial, however, most patients (79%) were treated with medication, and only 21% of the patients underwent PCI. The Clopidogrel for the Reduction of Events During Observation (CREDO) trial40 investigated the benefit of 12-month DAPT vs. 1-month DAPT after PCI in 2,116 patients with ACS (67.2%) or stable angina (32.8%). The rate of death, MI or stroke at 12 months was significantly lower in the long-term DAPT group compared with the short-term DAPT group (relative reduction, 26.9%; 95% CI: 3.9%–44.4%; P=0.02). The risk of major bleeding at 12 months increased, but not significantly (8.8% vs. 6.7%, respectively, P=0.07). These findings are consistent with the evidence from the CURE trial, suggesting that 12-month DAPT with aspirin plus clopidogrel significantly reduces the risk of recurrent ischemic events after PCI in patients with ACS.
| Trial | Patients | n | Anti-ischemic therapy |
Comparison | Follow-up (months) |
Primary endpoints |
Results | P value |
|---|---|---|---|---|---|---|---|---|
| CURE | NSTE-ACS | 12,562 | PCI, CABG, medication |
A+C vs. A | 3–12 (median, 9) |
CV death/MI/ stroke |
HR, 0.80 95% CI (0.72, 0.90) |
<0.001 |
| COMMIT/CCS-2 | Acute MI | 45,852 | Medication | A+C vs. A | 1 | Death/MI/Stroke | RRR, 9% (3, 14%) |
0.002 |
| Death | RRR, 7% (1, 13%) |
0.03 | ||||||
| CREDO | ACS, SA | 2,116 | PCI | A+C for 12 months vs. A+C for 1 month → A |
12 | CV death/MI/ stroke |
RRR, 26.9% (3.9, 44.4%) |
0.02 |
| TRITON-TIMI 38 | ACS | 13,608 | PCI | A+P vs. A+C | 14.5 | CV death/MI/ stroke |
HR, 0.81 95% CI (0.73, 0.90) |
<0.001 |
| TRILOGY- ACS | NSTE-ACS | 7,243 | Medication | A+P vs. A+C | 17 | CV death/MI/ stroke |
HR, 0.91 95% CI (0.79, 1.05) |
0.211 |
| PLATO | ACS | 18,624 | PCI, CABG, medication |
A+T vs. A+C | 6–12 (median, 9.2) |
CV death/MI/ stroke |
HR, 0.84 95% CI (0.77, 0.92) |
<0.001 |
| CHARISMA subgroup |
Post-MI, Stroke, PAD |
9,478 | Medication | A+C vs. A | 27.6 | CV death/MI/ stroke |
HR, 0.83 95% CI (0.72, 0.96) |
0.01 |
| PEGASUS | Post-MI | 21,000 | Medication | A+T (60 mg bid) vs. A+T (90 mg bid) vs. aspirin |
12–44 | CV death/MI/ stroke |
NCT01225562, ongoing |
|
| SMART-DATE | ACS | 3,000 | PCI | A+C for 6 months → A vs. A+C for 12 months |
6–18 | Death/MI/stroke ST/BARC 3,4,5 |
NCT01701453, ongoing |
|
| TALOS-AMI | Acute MI | 3,288 | PCI | A+T for 1 month → A+T vs. A+C |
12 | CV death/MI/ stroke/BARC bleeding 2,3,5 |
NCT02018055, ongoing |
|
| ISAR-REACTS5 | ACS | 4,000 | PCI | A+T vs. A+P | 12 | Death/MI/stroke | NCT01944800, ongoing |
|
| GLOBAL-LEADES | STEMI | 16,000 | PCI | A+T for 1 month → T vs. A+C for 12 months → A |
24 | Death/MI | NCT01813435, ongoing |
A, aspirin; ACS, acute coronary syndrome; C, clopidogrel; CABG, coronary artery bypass graft surgery; CV, cardiovascular; NSTE, non-ST-elevation; P, prasugrel; PCI, percutaneous coronary intervention; SA, stable angina; T, ticagrelor. Other abbreviations as in Tables 1,2.
Despite previous DAPT, patients with ACS still have high rates of recurrent major vascular events after the index event, requiring new approaches with more potent antiplatelet agents. Several randomized trials have been performed to compare the safety and efficacy of new DAPT (aspirin and new P2Y12 receptor blockers) with previous DAPT (aspirin and clopidogrel) in patients with ACS.42–44
The Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel-Thrombolysis in Myocardial Infarction 38 (TRITON-TIMI 38)42 randomized 13,608 moderate- to high-risk ACS patients scheduled for PCI to receive prasugrel (60 mg loading dose and then 10 mg/day) or clopidogrel (300 mg loading dose and then 75 mg/day) for 6–15 months. The rate of cardiovascular death, MI or stroke was 9.9% in the prasugrel group and 12.1% in the clopidogrel group (HR, 0.81; 95% CI: 0.73–0.90; P<0.001). Major bleeding occurred in 2.4% of the patients receiving prasugrel and 1.8% of the patients receiving clopidogrel (HR, 1.32; 95% CI: 1.03–1.68; P=0.03). Likewise, the rate of life-threatening bleeding was greater in the prasugrel group than in the clopidogrel group (1.4% vs. 0.9%, respectively, P=0.01), especially in patients with a history of transient ischemic attack/stroke, body weight <60 kg or age >75 years. Overall mortality did not differ between the 2 groups. In the Targeted Platelet Inhibition to Clarify the Optimal Strategy to Medically Manage Acute Coronary Syndromes (TRILOGY ACS) trial,43 7,243 patients under the age of 75 years with NSTE-ACS who were medically managed without revascularization were randomized to receive prasugrel (10 mg daily) vs. clopidogrel (75 mg daily). At a median follow-up of 17 months, no difference in safety and efficacy endpoints was observed between the 2 groups.
The Study of Platelet Inhibition and Patient Outcomes (PLATO) trial44 compared ticagrelor (180 mg loading dose and then 90 mg twice/day) to clopidogrel (300–600 mg loading dose and then 75 mg/day) for the prevention of cardiovascular events in 18,624 patients with ACS. At 12 months, the primary endpoint of death from vascular causes, MI or stroke occurred in 9.8% of the patients receiving ticagrelor compared with 11.7% of those receiving clopidogrel (HR, 0.84; 95% CI: 0.77–0.92; P<0.001). Death from any cause was also lower in the ticagrelor group compared with the clopidogrel group (4.5% vs. 5.9%, respectively, P<0.001). The rate of major bleeding was similar between the ticagrelor and clopidogrel groups (11.6% vs. 11.2%, respectively, P=0.43), but the rate of major bleeding not related to coronary artery bypass grafting was higher in the ticagrelor group (4.5% vs. 3.8%, P=0.03). These findings demonstrate that new DAPT with aspirin and ticagrelor is superior to previous DAPT in preventing recurrent vascular events in a broad population of patients presenting with ACS.
Residual Risk and Beyond 12 MonthsPatients with ACS remain at high risk of recurrent ischemic events despite evidence-based optimal therapy. The risk of cardiovascular death, MI or stroke is approximately 10% within 12 months after an index event, and thereafter 3–5% annually. Longer-duration DAPT beyond 12 months may improve outcomes for ACS patients; this has been an active area of investigation.
In the Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management and Avoidance (CHARISMA) subgroup study,45 9,478 patients with prior MI, stroke or peripheral artery disease were randomized to receive either previous DAPT (aspirin and clopidogrel) or aspirin for 27.6 months. The rate of cardiovascular death, MI or stroke was significantly lower in the DAPT group than in the aspirin group (HR, 0.83; 95% CI: 0.72–0.96; P=0.01). Severe bleeding was not different between the 2 groups, but moderate bleeding was significantly higher in the DAPT group (HR, 1.60; 95% CI: 1.16–2.20; P=0.004). These findings suggest that patients with prior MI may benefit from long-term DAPT beyond 12 months. In the Prevention of Thrombotic Events With Ticagrelor Compared to Placebo on a Background of Acetyl Salicylic Acid Therapy in Patients With History of Myocardial Infarction (PEGASUS) trial, approximately 21,000 patients with a history of spontaneous MI within 1 to 3 years and risk factors were randomized to ticagrelor (90 mg twice daily), ticagrelor (60 mg twice daily) or matching placebo, all with low-dose aspirin (75–150 mg/day). The primary endpoint is a composite of cardiovascular death, MI or stroke. The results will be available in the first quarter of 2015, providing useful information about the risks and benefits of long-term DAPT beyond 12 months in high-risk patients.
There is a significant association between the use of potent antiplatelet agents and excessive bleeding. The risk of major bleeding increases by 20–60% with the potency of antiplatelet therapy (Figure 1).39,42–45,50 DAPT duration also correlates with the risk of bleeding, limiting the benefits of long-term therapy. In general, prognosis in patients with CAD depends on clinical risk factors, left ventricular function and severity of disease;51 however, accurate models are not available to offer detailed guidance on the duration of DAPT for each individual patient.
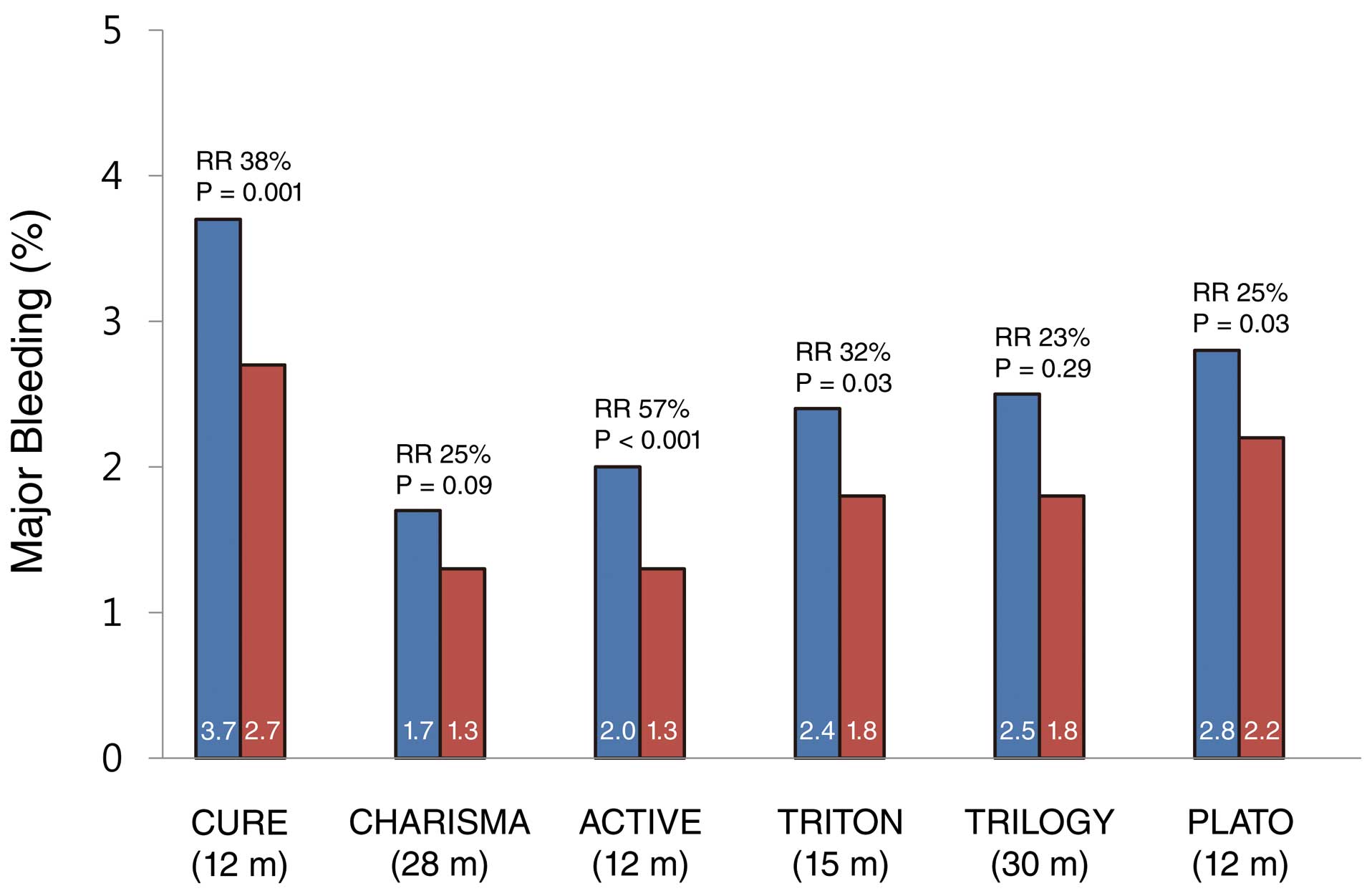
Incidence of major bleeding in large-scale randomized clinical trials comparing antiplatelet therapies (aspirin+clopidogrel vs. aspirin in the CURE, CHARISMA and ACTIVE trials; aspirin+prasugrel vs. aspirin+clopidogrel in the TRITON-TIMI38 and TRILOGY trials; aspirin+ticagrelor vs. aspirin+clopidogrel in the PLATO trial). Major bleeding events in the TRITON-TIMI 38, TRILOGY and PLATO trials were non-CABG-related TIMI major bleeding. Parentheses denote follow-up duration after randomization. CABG, coronary artery bypass graft surgery; TIMI, Thrombolysis in Myocardial Infarction; m, months; RR, relative risk.
Approximately 5% of the patients undergoing PCI are on oral anticoagulants for nonvalvular atrial fibrillation. These patients should receive DAPT for 1 month after implantation of bare-metal stents, and for 6–12 months after implantation of DES. Conventional triple therapy with aspirin, clopidogrel and warfarin remarkably increases the risk of major bleeding.52 In this clinical setting, antithrombotic regimens including new oral anticoagulants are now under investigation to reduce the bleeding risk.
Ethnic DifferencesThe response to DAPT may vary with ethnicity. East Asian patients, despite a higher prevalence of clopidogrel resistance, seem to have a lower rate of ischemic events after DES implantation than Western patients, suggesting ethnic differences in the response to DAPT.53 The maintenance dose of P2Y12 receptor blockers was determined from clinical trials conducted mostly in Caucasian populations. East Asians may require a lower dose of P2Y12 receptor blockers for similar protection from ischemic events. Further studies may be needed to clarify these issues.
Bioresorbable Vascular ScaffoldBioresorbable vascular scaffolds represent an emerging approach to the percutaneous treatment of CAD, providing transient vessel support and drug delivery to the vessel wall.54 Despite the potential advantages of this approach, concerns have been raised about the risk of stent thrombosis because of thick strut stents and incomplete stent apposition. The optimal DAPT regimens after implantation of bioresorbable vascular scaffolds remain to be determined.
DAPT is recommended for at least 6–12 months in patients with stable CAD undergoing DES placement, and for at least 12 months in all patients with ACS unless contraindicated (Figure 2). Prolonged DAPT beyond 12 months may be considered in patients at high risk for ischemic events. However, the benefits of long-term DAPT need to be balanced with the risks of bleeding, and therapy should be individualized for each patient.
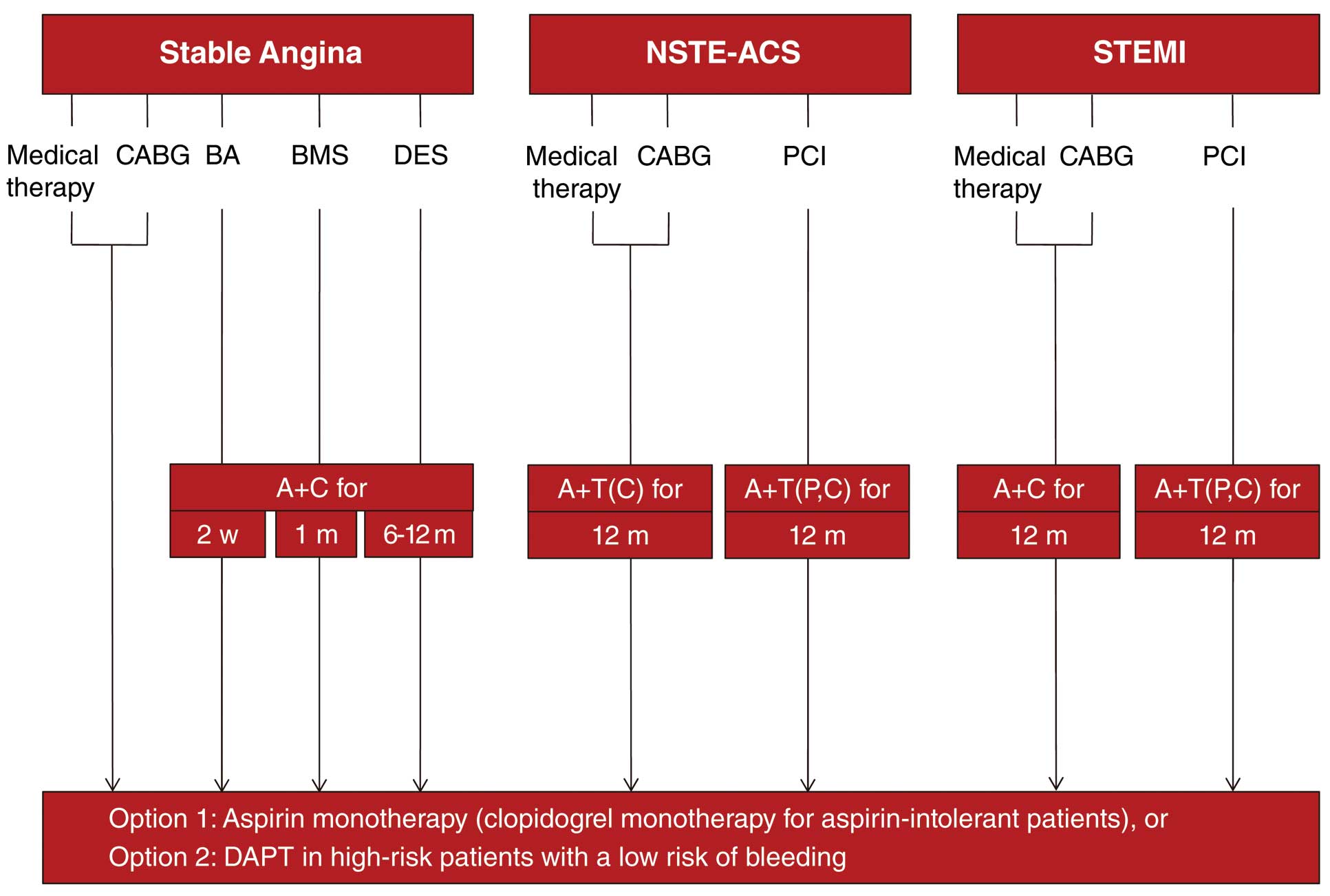
Schematic illustration of antiplatelet therapies for patients with coronary artery disease. A, aspirin; BA, balloon angioplasty; BMS, bare-metal stent; C, clopidogrel; CABG, coronary artery bypass graft surgery; DES, drug-eluting stent; m, months; NSTE-ACS, non-ST-elevation acute coronary syndrome; P, prasugrel; PCI, percutaneous coronary intervention; STEMI, ST-elevation myocardial infarction; T, ticagrelor; w, weeks.
This study was supported by a grant (2014-217) from the Asan Institute for Life Sciences, Seoul, Korea.