Abstract
Background:
The effectiveness of statins remains to be examined in patients with heart failure (HF) with preserved ejection fraction (EF).
Methods and Results:
Among 4,544 consecutive HF patients registered in the Chronic Heart Failure Registry and Analysis in the Tohoku district-2 (CHART-2) between 2006 and 2010, 3,124 had EF ≥50% (HFpEF; mean age 69 years; male 65%) and 1,420 had EF <50% (HF with reduced EF (HFrEF); mean age 67 years; male 75%). The median follow-up was 3.4 years. The 3-year mortality in HFpEF patients was lower in patients receiving statins [8.7% vs. 14.5%, adjusted hazard ratio (HR) 0.74; 95% confidence interval (CI), 0.58–0.94; P<0.001], which was confirmed in the propensity score-matched cohort (HR, 0.72; 95% CI, 0.49–0.99; P=0.044). The inverse probability of treatment weighted further confirmed that statin use was associated with reduced incidence of all-cause death (HR, 0.71; 95% CI, 0.62–0.82, P<0.001) and noncardiovascular death (HR, 0.53; 95% CI, 0.43–0.66, P<0.001), specifically reduction of sudden death (HR, 0.59; 95% CI, 0.36–0.98, P=0.041) and infection death (HR, 0.53; 95% CI, 0.35–0.77, P=0.001) in HFpEF. In the HFrEF cohort, statin use was not associated with mortality (HR, 0.87; 95% CI, 0.73–1.04, P=0.12), suggesting a lack of statin benefit in HFrEF patients.
Conclusions:
These results suggest that statin use is associated with improved mortality rates in HFpEF patients, mainly attributable to reductions in sudden death and noncardiovascular death. (Circ J 2015; 79: 574–582)
Heart failure (HF) is a progressive disorder with high mortality and morbidity,1
and its prevalence has been rapidly increasing worldwide.2
Particularly, the obvious increase in the prevalence of HF with preserved ejection fraction (HFpEF) is now a serious healthcare problem all over the world.3
To date, however, no pharmacological strategy has been established for the treatment of HFpEF patients.4–8
Editorial p 508
Unlike in patients with HF with reduced ejection fraction (HFrEF), previous randomized clinical trials with cardioprotective drugs, including angiotensin-converting enzyme inhibitors (ACEIs),4
angiotensin II receptor blockers (ARBs),5,6
aldosterone antagonists7
and β-blockers,8
have failed to show the effectiveness of these drugs in HFpEF patients, which could be at least in part explained by differences in the characteristics of HFrEF and HFpEF patients. Indeed, as compared with HFrEF, HFpEF is characterized by higher age, higher prevalence of female sex, hypertension, and left ventricular diastolic dysfunction associated with myocardial hypertrophy and fibrosis, and lower incidence of cardiovascular death, but comparable all-cause death.9–11
Statins [3-hydroxy-3-methylgulutaryl coenzyme A reductase inhibitors] are cholesterol-lowering drugs with pleiotropic properties, such as antiinflammatory,12
antihypertrophic,13
antifibrotic and antioxidant effects,14,15
all of which could be theoretically beneficial for the management of HF. However, previous clinical trials have failed to show beneficial effects of statins in HFrEF patients,16–18
but the prognostic effect of statins in HFpEF patients remains to be elucidated. Therefore, in the present study, we aimed to examine whether statin use is associated with better clinical outcomes in HFpEF patients, using the database of the Chronic Heart Failure Registry and Analysis in the Tohoku district-2 (CHART-2), a multicenter prospective observational study of cardiovascular diseases (CVD) in Japan.19–22
Methods
Data Sources
The CHART-2 Study is a registry of Japanese patients with CVD.19–22
Between October 2006 and March 2010, 10,219 consecutive Japanese patients older than 20 years with ACC/AHA stages B–D of HF23
or coronary artery disease (CAD) were enrolled in both in- and outpatient settings.19
The ACC/AHA stages B–D of HF were defined as follows: Stage B, structural heart disease but without signs or symptoms of HF; Stage C, structural heart disease with prior or current symptoms of HF; Stage D, refractory HF requiring specialized interventions.23
The diagnosis of HF was based on the Framingham criteria.24
Written informed consent was provided by all patients before enrollment.19
Information on medical history and baseline demographics, including medications and echocardiographic data, was collected at the time of enrollment by the clinical research coordinators. Follow-up was at least twice yearly by review of medical records, surveys and telephone interviews conducted by clinical research coordinators. The CHART-2 Study was approved by the local ethics committees of the 24 participating hospitals (1 university hospital, 23 general hospitals) and registered in ClinicalTrials.gov (Identifier: NCT00418041).
Study Sample
Of 10,219 patients, 4,736 had a history of or current symptoms of HF (Stage C/D).19
After exclusion of 192 patients lacking EF data, we enrolled 3,124 patients with HFpEF (EF ≥50%, mean [SD] age 69.4 [12.2] years; male 65%) and 1,420 patients with HFrEF (EF <50%, mean [SD] age 67.4 [12.4] years; male 75%) in the present study (Figure S1).
Study Outcomes and Definitions
The study endpoints were death, mode of death and hospitalization for worsening HF. All events and mode of death were reviewed and assigned by consensus of at least 2 independent physicians from the members of the Tohoku Heart Failure Association (Appendix S1) after reviewing case reports, death certificates, and hospital records provided by the investigators. Cardiovascular death was defined as death attributed to cardiovascular origins. Noncardiovascular death was defined as death from noncardiovascular causes. For the mode of death, only the main mode was recorded. Hospitalization for worsening HF was defined as documentation of worsening HF requiring hospitalization. A patient admitted for worsening HF had to show signs and symptoms of HF and to require treatment with intravenous diuretics.
Statistical Analysis
Descriptive statistics, including mean±SD, median and frequencies for continuous and categorical data, are presented for all patients and by statin treatment category. The Wilcoxon rank sum and Pearson’s chi-square test were used to compare the characteristics of patients with and without statin therapy.
To reduce confounding effects related to differences in the patient’s background between those with and those without statin use, 3 propensity score (PS) methods were used in combination with Cox regression modeling. For the calculation of PS, we used a logistic regression model in which the treatment status of statins was regressed for the following 31 baseline characteristics: age, male sex, blood pressure, heart rate, body mass index, NYHA classification, smoking status (current and past smoker or nonsmoker), history of hospitalization for HF, hypertension, diabetes mellitus, dyslipidemia, atrial fibrillation, stroke, cancer, previous myocardial infraction (MI), CAD (3-vessel disease), hypertrophic cardiomyopathy, hemoglobin, B-type natriuretic peptide, potassium, estimated glomerular filtration rate, LVEF, left atrial dimension, loop diuretics, ACEI or ARB, β-blocker, aldosterone antagonist, calcium-channel blocker, antiplatelet or anticoagulant, percutaneous coronary intervention and coronary artery bypass grafting.9,11,25,26
Missing data for 31 variables were handled by estimating the variables for each missing variable based on the pattern for all available observations. Area under the curve (AUC) to show the performance of the PS for statin use was 0.78 [95% confidence interval (CI), 0.77–0.80]. Using the PS, patients were matched for 1:1 optimal match with a <0.001 caliper and no replacement. The distribution of PS in the total and matched cohorts is shown in
Figure S2. Kaplan-Meier curves were plotted to evaluate the association between statin use and all-cause death or hospitalization for worsening HF in the total cohort using the log-rank test and PS-matched cohort using the PS-stratified Cox analysis. To reduce confounding in the time-to-event observational data, the inverse probability of treatment weighted (IPTW) method was used.27
Using the same 31 variables for the PS calculation, case-weight estimation was done with a logistic regression model to predict the inverse probability of statin use. Finally, in order to examine the clinical effect of statins in the total cohort, we constructed 5 Cox proportional hazard models: unadjusted, age- and sex-adjusted, PSadjusted, PS-stratified and the IPTW analyses.27
The assumption of proportional hazards was tested for the model, and no significant departure was found.
PS methods are used to eliminate the effect of treatment-selection bias in an observational study by estimating the probability of assignment to treatments or exposure on the outcomes. PS matching is a statistical matching technique aiming to estimate the effect of an intervention by accounting for the covariates that predict receiving the treatment. Our PS-stratified model enabled us to compare treated and untreated patients within each of the strata providing similar estimates of the PS, which can reduce the imbalance in observed covariates and thus can verify the results of PS matching. The IPTW method has recently been developed to utilize all sample information with assigned weights by making an unbiased estimation of the true risk difference with the lowest standard error of the estimated risk difference, the lowest mean-squared error and approximately correct type I error rates. Although all 3 of these PS methods are useful to reduce selection bias in observational studies, we conferred the primary significance to the ITPW method in the present study, because it has been shown to have superior performance to other PS methods, including the PS-matching and PS-stratified methods.27
In the subgroup analysis, we examined interactions between selected baseline variables and statin use and reported the interaction P value. Baseline variables were selected from previous studies9,11,25,26
and by using the Cox proportional hazard model with a stepwise method; we included variables with P<0.2.
Consistency Analysis
In the previous studies, statins did not reduce all-cause death in HFrEF.16–18
Thus, in order to confirm consistency between those trials and the present study regarding the lack of stain benefit in HFrEF patients, we performed a statistical analysis of the HFrEF cohort derived from the same CHART-2 registry (EF <50%, n=1,420) and compared the results with those in the previous studies. In the HFrEF cohort, PS was calculated using the same 31 variables used in the HFpEF cohort and the ITPW models (Table S1). AUC for the PS was 0.80 (95% CI, 0.77–0.82). We performed all analyses using IBM SPSS Statistics 21.0 (IBM, Somers, NY, USA) and R 3.0.2. Two-sided probability values <0.05 and P<0.1 were considered to be statistically significant. All authors had a full access to the data and approved the manuscript as written.
Results
Baseline Characteristics of HFpEF Patients (Table 1)
Among the 3,124 HFpEF patients, 1,163 (37.2%) received statins and 1,961 (62.8%) did not (specific agents and doses are listed in
Table S2). In the total HFpEF cohort, there were several differences between the 2 groups. The patients taking statins were more likely to be male and less symptomatic, and had a higher prevalence of previous MI, medical treatment and a history of coronary intervention. Although the high-density lipoprotein cholesterol and triglyceride levels were comparable, low-density lipoprotein cholesterol (LDL-C) levels significantly differed between the patients with and without statin use (99 vs. 110 mg/dl). After PS matching, baseline characteristics became generally comparable between the 2 groups (Table 1). The patients excluded from the PS matching were younger and had a lower prevalence of smoking, hypertension, dyslipidemia and previous MI as compared with those included (Table S1).
Table 1.
Baseline Characteristics of the Total and Matched Cohorts of Patients With HFpEF
| |
Total cohort (n=3,124) |
Matched cohort (n=1,252) |
| Statin use |
P value |
Statin use |
P value |
| Yes (n=1,163) |
No (n=1,961) |
Yes (n=626) |
No (n=626) |
| Age, mean (SD), years |
69.0 (11.0) |
69.7 (12.9) |
0.108 |
69.4 (11.2) |
70.0 (11.9) |
0.362 |
| Male sex, n (%) |
785 (67.5) |
1,256 (64.0) |
0.052 |
410 (65.5) |
421 (67) |
0.550 |
| BP, mean (SD), mmHg |
| Systolic |
131 (18) |
127 (19) |
<0.001 |
129 (17) |
130 (19) |
0.360 |
| Diastolic |
74 (12) |
72 (12) |
<0.001 |
73 (12) |
74 (12) |
0.652 |
| Heart rate, mean (SD), beats/min |
71 (14) |
72 (15) |
0.008 |
72 (14) |
71 (14) |
0.163 |
| Body mass index (SD), kg/m2 |
24.7 (3.6) |
23.5 (3.8) |
<0.001 |
24.2 (3.6) |
24.2 (4.1) |
0.884 |
| NYHA classification, n (%) |
|
|
<0.001 |
|
|
0.877 |
| I |
365 (30.4) |
465 (23.7) |
|
147 (23.5) |
159 (35.4) |
|
| II |
723 (62.2) |
1,286 (65.6) |
|
422 (67.4) |
409 (65.3) |
|
| III |
82 (7.1) |
196 (10) |
|
53 (8.5) |
54 (8.6) |
|
| IV |
4 (0.3) |
14 (0.7) |
|
4 (0.6) |
4 (0.6) |
|
| Current or past smoker, n (%) |
527 (45.3) |
801 (40.8) |
0.015 |
281 (44.9) |
290 (46.3) |
0.650 |
| Medical history, n (%) |
| Hospitalization for HF |
488 (42.0) |
994 (50.7) |
<0.001 |
281 (44.9) |
273 (43.6) |
0.690 |
| Hypertension |
989 (85.0) |
1,505 (76.7) |
<0.001 |
518 (82.7) |
505 (80.7) |
0.380 |
| Diabetes mellitus |
393 (33.8) |
409 (20.9) |
<0.001 |
181 (28.9) |
163 (26.0) |
0.282 |
| Dyslipidemia |
1,158 (99.6) |
1,073 (54.7) |
<0.001 |
624 (99.7) |
361 (57.7) |
<0.001 |
| Atrial fibrillation |
243 (20.9) |
848 (43.2) |
<0.001 |
200 (31.9) |
186 (29.7) |
0.426 |
| Stroke |
217 (18.7) |
338 (17.2) |
0.033 |
113 (18.1) |
119 (19.0) |
0.716 |
| Cancer |
117 (10.1) |
261 (13.3) |
0.008 |
74 (11.6) |
71 (11.3) |
0.860 |
| Previous MI |
579 (49.8) |
364 (18.6) |
<0.001 |
212 (33.9) |
211 (33.7) |
1.000 |
| Coronary artery disease |
832 (71.5) |
591 (30.8) |
<0.001 |
344 (55.0) |
307 (49.0) |
0.042 |
| Coronary artery angiography |
505 (43.4) |
360 (18.4) |
<0.001 |
209 (33.4) |
190 (30.4) |
0.374 |
| 1-vessel disease |
231 (19.9) |
208 (10.6) |
<0.001 |
110 (17.6) |
106 (16.9) |
0.764 |
| 2-vessel disease |
174 (15.0) |
103 (5.3) |
<0.001 |
72 (11.5) |
58 (9.3) |
0.391 |
| 3-vessel disease |
100 (8.6) |
49 (2.5) |
<0.001 |
27 (4.3) |
26 (4.2) |
1.000 |
| Valvular dysfunction |
212 (18.2) |
673 (34.3) |
<0.001 |
167 (26.7) |
160 (25.6) |
0.700 |
| Hypertrophic cardiomyopathy |
87 (7.5) |
272 (13.9) |
<0.001 |
67 (10.7) |
77 (12.3) |
0.425 |
| Laboratory data |
| LDL-C, mg/dl |
99 (27) |
110 (31) |
<0.001 |
101 (28) |
113 (29) |
<0.001 |
| HDL-C, mg/dl |
52 (15) |
53 (16) |
0.266 |
53 (15) |
52 (15) |
0.213 |
| Triglyceride, mg/dl |
134 (73) |
124 (82) |
0.001 |
136 (79) |
128 (84) |
0.109 |
| Hemoglobin, g/dl |
13.3 (2.1) |
13.0 (2.0) |
<0.001 |
13.2 (1.9) |
13.2 (1.9) |
0.937 |
| BNP (IQR), pg/dl |
76 (28–145) |
116 (45–197) |
<0.001 |
83 (32–155) |
102 (37–159) |
0.626 |
| Potassium, mEq/L |
4.4 (0.4) |
4.4 (0.5) |
0.036 |
4.3 (0.4) |
4.4 (0.5) |
0.829 |
| eGFR, ml·min−1·1.73 m−2 |
61 (28) |
62 (22) |
0.310 |
61 (34) |
62 (21) |
0.752 |
| CRP (IQR), mg/dl |
0.2 (0.1–0.8) |
0.3 (0.1–0.8) |
0.018 |
0.2 (0.1–0.8) |
0.3 (0.1–0.8) |
0.098 |
| LVEF, % |
66 (9) |
65 (9) |
0.223 |
65 (9) |
65 (9) |
0.851 |
| LAD, mm |
41 (8) |
43 (10) |
<0.001 |
42 (9) |
42 (9) |
0.543 |
| Medications, n (%) |
| Loop diuretics |
373 (32.1) |
892 (45.5) |
<0.001 |
246 (39.3) |
221 (35.3) |
0.161 |
| ACEI or ARB |
857 (73.7) |
1,310 (66.8) |
<0.001 |
440 (70.3) |
454 (72.5) |
0.416 |
| β-blocker |
533 (45.8) |
772 (39.4) |
<0.001 |
278 (44.4) |
268 (42.8) |
0.608 |
| Aldosterone antagonist |
174 (15.0) |
426 (21.7) |
<0.001 |
113 (18.1) |
103 (16.5) |
0.501 |
| Calcium-channel blocker |
590 (50.7) |
810 (41.3) |
<0.001 |
291 (46.5) |
304 (48.6) |
0.497 |
| Antiplatelet or anticoagulant |
1,048 (90.1) |
1,437 (73.3) |
<0.001 |
523 (83.5) |
520 (83.1) |
0.880 |
| Other lipid-lowering medicine |
43 (3.7) |
78 (4.0) |
0.774 |
28 (4.5) |
21 (3.4) |
0.382 |
| Intervention history, n (%) |
| PCI |
625 (53.7) |
360 (18.4) |
<0.001 |
217 (34.7) |
227 (36.3) |
0.595 |
| CABG |
179 (15.4) |
111 (5.7) |
<0.001 |
61 (9.7) |
47 (7.5) |
0.190 |
| ICD |
11 (0.9) |
27 (1.4) |
0.316 |
6 (1.0) |
6 (1.0) |
1.000 |
| CRTD |
1 (0.1) |
10 (0.5) |
0.063 |
0 |
2 (0.3) |
0.500 |
Values are expressed as n (%) unless otherwise indicated. The majority of variables were used for derivation of the propensity score. The following covariates were not included for derivation of the propensity score: diastolic BP; dyslipidemia; LDL-C, HDL-C, triglyceride and CRP levels; other lipid-lowering medicine, ICD, and CRTD.
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin-receptor blocker; BNP, B-type natriuretic peptide; BP, blood pressure; CABG, coronary artery bypass grafting; CRP, C-reactive protein; CRTD, cardiac resynchronization therapy defibrillator; eGFR, estimated glomerular filtration rate; HDL-C, high-density lipoprotein cholesterol; HF, heart failure; HFpEF, heart failure and preserved ejection fraction; ICD, implantable cardioverter-defibrillator; IQR, interquartile range; LAD, left atrial dimension; LDL-C, low-density lipoprotein cholesterol; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; SD, standard deviation.
During the median follow-up of 3.4 years, 440 (14.1%) patients died. In the total HFpEF cohort, crude 3-year mortality was 8.7% in patients treated with statins and 14.5% for those without statins (log-rank P<0.001) (Figure 1A). This difference in 3-year mortality remained after PS matching; 10.1% for patients with statins and 12.8% for those without statins with an adjusted hazard ratio (HR) of 0.72 (95% CI, 0.51–0.99, P=0.002) (Figure 1A, Table 2).
Table 2
shows the HRs for the outcomes in the total and matched cohorts. In the PS-adjusted, PS-stratified and IPTW analyses of the total HFpEF cohort, statin use was significantly associated with reduced all-cause death, with HRs of 0.74 (95% CI, 0.58–0.94, P=0.014), 0.67 (95% CI, 0.53–0.86, P=0.002), and 0.71 (95% CI, 0.62–0.82, P<0.001), respectively (Table 2).

Table 2.
Cox Regression Models for All-Cause Death and Hospitalization for Worsening HF by Statin Use in of Patients With HFpEF
| |
No. of events/total
(%) |
HR |
95% CI |
P value |
| All-cause death in the total cohort |
| Unadjusted |
440/3,124 (14.1) |
0.56 |
0.45–0.69 |
<0.001 |
| Adjusted with age and sex |
440/3,124 (14.1) |
0.63 |
0.50–0.78 |
<0.001 |
| Adjusted with propensity score |
440/3,124 (14.1) |
0.74 |
0.58–0.94 |
0.014 |
| Adjusted with PS-stratified Cox model |
440/3,124 (14.1) |
0.67 |
0.53–0.86 |
0.002 |
| Adjusted with IPTW |
440/3,124 (14.1) |
0.71 |
0.62–0.82 |
<0.001 |
| All-cause death in the matched cohort |
159/1,252 (12.7) |
0.72 |
0.53–0.99 |
0.002 |
| Hospitalization for worsening HF in the total cohort |
| Unadjusted |
351/3,124 (11.2) |
0.63 |
0.50–0.79 |
<0.001 |
| Adjusted with age and sex |
351/3,124 (11.2) |
0.67 |
0.53–0.85 |
0.001 |
| Adjusted with propensity score |
351/3,124 (11.2) |
0.82 |
0.70–1.19 |
0.512 |
| Adjusted with PS-stratified Cox model |
351/3,124 (11.2) |
0.87 |
0.67–1.13 |
0.300 |
| Adjusted with IPTW |
351/3,124 (11.2) |
0.94 |
0.81–1.08 |
0.388 |
| Hospitalization for worsening HF in the matched cohort |
140/1,252 (11.2) |
0.96 |
0.69–1.34 |
0.827 |
CI, confidence interval; HR, hazard ratio; IPTW, inverse probability of treatment weighted; PS, propensity score. Other abbreviations as in Table 1.
Table 3
shows the association between statin use and mode of death. Of the 440 deaths in the total HFpEF cohort, 210 were cardiovascular deaths (48%), 206 were noncardiovascular deaths (47%) and 24 were of unknown cause (5%). Among the cardiovascular deaths, HF death was most common (97 deaths, 46% of cardiovascular deaths), followed by sudden death (40 deaths, 19% of cardiovascular deaths). Of the 206 noncardiovascular deaths, cancer death was most frequent (75 deaths, 36% of noncardiovascular deaths), followed by infection death (67 deaths, 33% of noncardiovascular deaths). The ITPW analysis revealed that statin use was significantly associated with reduced incidence of sudden death (HR, 0.59; 95% CI, 0.36–0.98, P=0.041), noncardiovascular death (HR, 0.53; 95% CI, 0.43–0.66, P<0.001) and infection death (HR, 0.53; 95% CI, 0.36–0.77, P=0.001) (Table 3).
Table 3.
Adjusted Risk for Each Mode of Death in the Cohort of Patients With HFpEF
| |
Statin use in the total cohort |
Total
(n=3,124) |
Yes
(n=1,163) |
No
(n=1,961) |
Adjusted HR |
95% CI |
P value |
| All-cause death |
440 |
113 |
327 |
0.72 |
0.63–0.82 |
<0.001 |
| Cardiovascular |
210 |
64 |
146 |
1.01 |
0.83–1.22 |
0.960 |
| Heart failure |
97 |
28 |
69 |
1.16 |
0.88–1.53 |
0.288 |
| Stroke |
40 |
12 |
28 |
1.25 |
0.72–2.15 |
0.426 |
| Sudden death |
25 |
11 |
14 |
0.59 |
0.36–0.98 |
0.041 |
| MI |
11 |
3 |
8 |
0.61 |
0.27–1.38 |
0.234 |
| Other cardiovascular |
37 |
10 |
27 |
1.07 |
0.70–1.64 |
0.758 |
| Noncardiovascular |
206 |
47 |
159 |
0.53 |
0.43–0.66 |
<0.001 |
| Cancer |
75 |
22 |
53 |
0.74 |
0.53–1.03 |
0.078 |
| Infection |
67 |
14 |
53 |
0.53 |
0.36–0.77 |
0.001 |
| Renal failure |
18 |
5 |
13 |
0.73 |
0.36–1.47 |
0.371 |
| Noninfectious respiratory conditions |
5 |
1 |
4 |
0.65 |
0.07–6.28 |
0.713 |
| Gastrointestinal bleeding |
4 |
0 |
4 |
– |
– |
– |
| Other noncardiovascular |
37 |
5 |
32 |
0.27 |
0.10–0.73 |
0.009 |
| Unknown cause |
24 |
2 |
22 |
0.17 |
0.01–0.42 |
<0.001 |
Other abbreviations as in Tables 1,2.
Hospitalization for worsening HF was recorded for 351 (11.2%) patients in the total HFpEF cohort. Although the Kaplan-Meier estimates for hospitalization for worsening HF showed a significant difference between patients with and without statin use in the total cohort, the difference disappeared in the ITPW analysis (HR 0.94; 95% CI, 0.81–1.08, P=0.388). There also was no reduction in hospitalization for worsening of HF (HR 0.96; 95% CI, 0.69–1.34, P=0.827) in the matched cohort, confirming the result of the ITPW analysis (Figure 1B, Table 2).
Subgroup Analysis
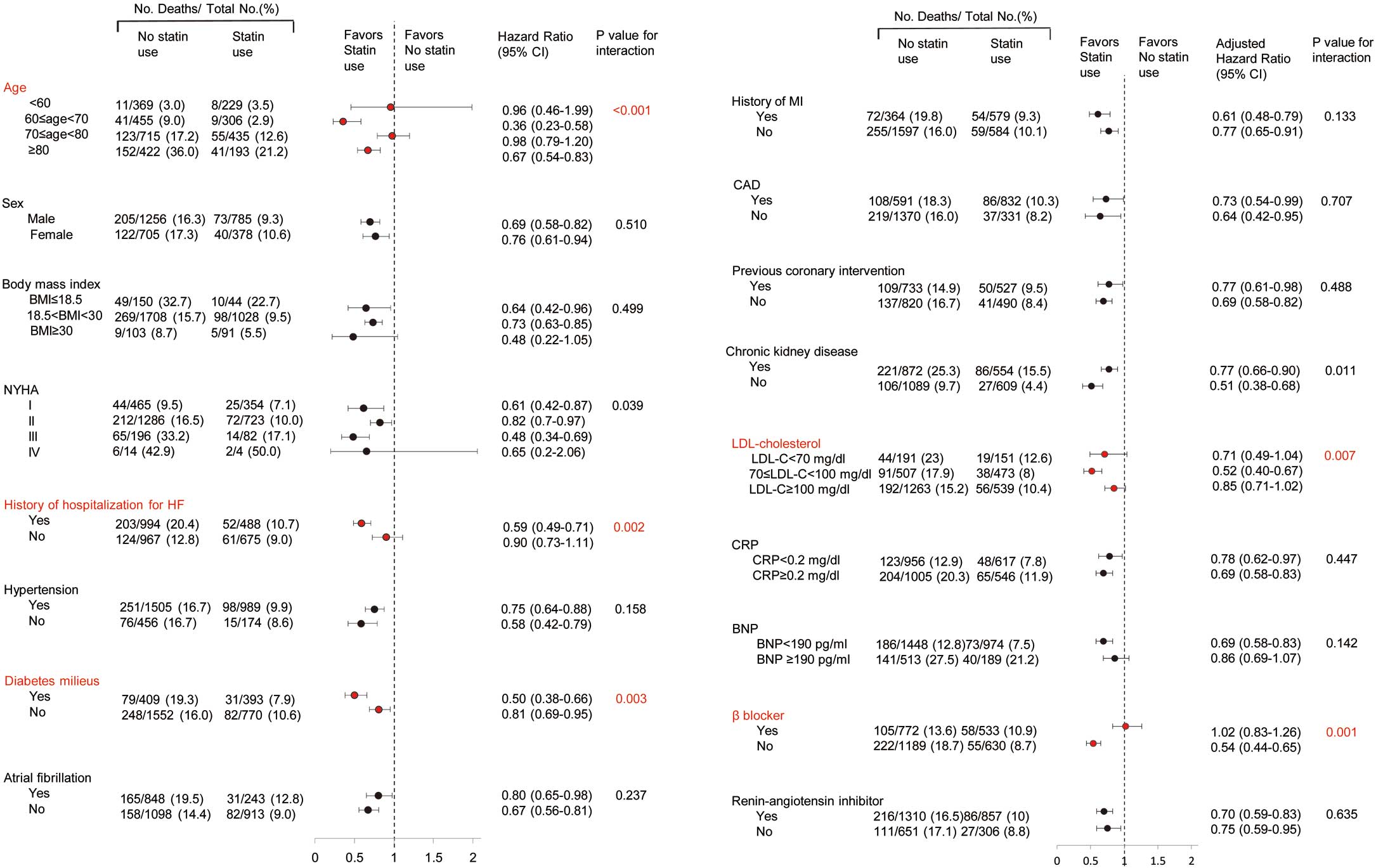
Figure 2
shows the results of subgroup analysis according to clinically relevant characteristics in the total HFpEF cohort by ITPW method. In the total HFpEF cohort, statin use significantly interacted with age, NYHA, history of hospitalization for worsening HF, diabetes mellitus, chronic kidney disease (CKD), LDL-C, and β-blocker therapy and did not interact with CAD or previous coronary intervention.
There was no interaction with C-reactive protein levels ≥0.2 mg/dl (HR, 0.69; 95% CI, 0.58–0.83) vs. <0.2 mg/dl (HR, 0.78; 95% CI, 0.62–0.97, P for interaction=0.447).
Analyses in the HFrEF Cohort
Table S3
shows the baseline characteristics of the total (n=1,420) and matched HFrEF cohorts (n=416). In the total HFrEF cohort, patients taking statins were younger, more likely to be male and had lower prevalence of hypertension and higher prevalence of prior MI as compared with those without statins, whereas baseline characteristics became comparable in the matched cohort. Although statin use was associated with reduced mortality in the total HFrEF cohort, the difference became insignificant in both the matched and total cohort with the IPTW method (HR 0.87; 95% CI, 0.73–1.04, P=0.118)
(Figure S3, Table S4).
Discussion
To the best of our knowledge, this is the first study to examine whether statin use is associated with reduced mortality in a large-scale cohort of patients with HFpEF. Using state-of-the-art PS-based analyses, the present results clearly demonstrated that statin use was associated with reduced all-cause and noncardiovascular mortality rates, specifically with reduced incidence of sudden death and infection death. The findings for HFpEF patients were further strengthened by separate analysis of the HFrEF cohort in the same registry, in which no association between statin use and mortality was confirmed, as reported in previous studies.16–18
Prognostic Effect of Statins in HFpEF Patients
The unadjusted survival curves showed a large separation between HFpEF patients with and without statin use. This beneficial association of statin use remained significant in the PS-matched, PS-adjusted, PS-stratified and ITPW models. Furthermore, statin use was significantly associated with reduced incidence of sudden death and noncardiovascular death, the latter being partly attributable a reduction in infection deaths. Because the prognosis of HFpEF patients is considerably poorer and the number of HFpEF patients has been rapidly increasing worldwide,1–3
establishment of HFpEF management is currently an urgent matter. Thus, the present results have clinical significance, providing a clue to improving the management of HFpEF patients. In addition, the clinical significance of the present study is further emphasized by the fact that all previous clinical trials of cardiovascular drugs, including ACEIs, ARBs and β-blockers, have failed to show any benefits in HFpEF patients.4–8
Mode of Death in HFpEF Patients Treated With Statins
Fukuta et al preliminarily reported that statin use was associated with lower mortality in HF patients with EF ≥50% (adjusted HR, 0.20; 95% CI, 0.06–0.62, P=0.005).28
In their study, however, they did not investigate associations between mode of death and statin treatment, possibly because of the small sample size (n=137).28
From this point of view, the present study clearly demonstrated that statin use was significantly associated with reduced all-cause and noncardiovascular mortality, specifically a reduced incidence of sudden death and infection death. Furthermore, the present study revealed that the beneficial effect of statin use was more evident in patients with a previous history of HF hospitalization, those with diabetes mellitus, those without CKD and those without β-blockers in the total HFpEF cohort. However, because the precise mechanisms for the interactions between statin use and those factors are unclear, we need to confirm these observations and to specify the characteristics of HFpEF patients who could most benefit from statin treatment in clinical practice.
Statin Use and Cardiovascular Deaths in HFpEF
In the HFpEF cohort, statin use was not associated with the incidence of overall cardiovascular deaths, which was similar to the findings in the HFrEF cohorts in previous and present studies.16–18
In the present study, however, IPTW analysis revealed that statin use was associated with reduced incidence of sudden death. Despite the large amount of data showing the beneficial effect of statin therapy on reducing mortality, information regarding efficacy with regard to sudden cardiac death is limited. In a meta-analysis, Levantesi et al reported that statin treatment was associated with a 19% significant reduction in sudden death independent of patient characteristics or changes in lipid levels in patients with CVD.29
Vrtovec et al reported that atorvastatin therapy increased heart rate variability, decreased QT variability, and shortened the QTc interval duration in patients with advanced chronic HF.30
They further demonstrated in another study that atorvastatin therapy was associated with decreased incidence of sudden cardiac death in patients with advanced HF.31
However, the effect on sudden cardiac death has never been reported in patients with HFpEF, regardless of study design or study sample size. Thus, our finding is clinically important in showing an association between stain use and reduced incidence of sudden death in HFpEF. It is conceivable that statin treatment was associated with a decrease in ventricular late potentials, resulting in a reduced incidence of fatal cardiac arrhythmia.29–31
In addition, statin treatment has been reported to reduce acute coronary events possibly related to plaque stabilization, improvement of endothelial and platelet functions, and reduction of neutrophil infiltration.32
Thus, there is a possibility that the reduced incidence of sudden death in HFpEF patients treated with statins might be attributed to fewer acute coronary events.
Noncardiovascular Deaths and Statin Use in HFpEF
Another key finding of the present study was that statin use was associated with reduced incidence of noncardiovascular deaths in the HFpEF patients. In the present study, half of the deaths in the HFpEF cohort had noncardiovascular causes, with cancer and infection as the first and second reasons, respectively. Although the beneficial effect of statin on cancer death is controversial in a broad spectrum of patients,33,34
beneficial effects of statins against infection in the general practice has been reported, including patients with vascular diseases,35
CKD,36
diabetes,37
and intensive care unit-acquired infections,38
and a meta-analysis confirmed the idea.39
Statins have also been reported to suppress the inflammatory response to endotoxin in vivo40
and decrease serum levels of tumor necrosis factor-α and interleukin-6 in patients with acute bacterial infection.41
These lines of evidence suggest that antiinfective effects of statins were involved in the reduction of infection deaths in HFpEF patients in the present study. However, there have been no studies reporting the inhibitory effects of statins on infection death in patients with HF, regardless of HFrEF or HFpEF. Thus, our finding is clinically important because it is the first to suggest the efficacy of statin use for reduction of infection deaths as well.
Consistency Analysis
In the previous randomized clinical trials, statin treatment failed to reduce mortality in HFrEF patients.16–18
In the CORONA study, rosuvastatin did not reduce the primary outcome or the number of deaths from any cause in a total of 5,011 patients aged 60 years or older with symptomatic and systolic HF of ischemic origin.16
In the GISS-HF study, rosuvastatin also did not improve clinical outcomes in 4,574 patients aged 18 years or older with symptomatic HF irrespective of cause.17
In the PEARL Study, no cardioprotective effects of pitavastatin were noted in a total of 574 Japanese HFrEF patients.18
Also, in the present HFrEF cohort, statin use was not associated with lower mortality, a consistent finding with the previous studies,16–18
confirming that our CHART-2 registry is representative of other rigorous settings. Thus, our present findings could be generalized to other HFpEF cohorts, although caution is highly warranted.
Study Limitations
First, the CHART-2 Study is an observational study in which prescription of statins was not randomized and thus the results could be affected by potential confounders. Even though we performed state-of-the art statistical analyses using PS, the influence of baseline differences in LDL-C levels and unmeasured confounding factors might not have been completely excluded. Thus, although the present study has the strength of a broad spectrum of patients in real-world practice enrolled with a minimal selection bias, caution is needed when interpreting the results. Second, comparison of the prognostic effects of statins was based on medications at enrollment, and we did not include information on dose or type of statin or on adherence to statin treatment after registration or subsequent prescription of statins during the follow-up period. These factors might have modified the actual clinical effect of statin therapy on mortality. Third, the ratio of HFpEF to HFrEF in this study of consecutive patients was approximately 2:1, which represents the high proportion of HFpEF in real-world practice in Japan, and the relatively small number of HFrEF patients in the matched cohort (n=416) might have been underpowered to detect an effect on cardiovascular events in HFrEF patients.
Conclusions
The present study demonstrates for the first time the beneficial effects of statin use in HFpEF patients. Our finding may have important clinical significance because no established pharmacological agents are yet available for HFpEF patients. Our findings need to be confirmed in future randomized clinical trials and other observational studies.
Acknowledgments
We thank all the members of the Tohoku Heart Failure Society and the staff of the Department of Evidence-based Cardiovascular Medicine for their contributions (Appendix S1). This study was supported by Grants-in-Aid from a Research Grant from the Ministry of Health, Labor, and Welfare (H.S.).
Conflicts of Interest
H.S. received lecture fees from Bayer Yakuhin, Ltd (Osaka, Japan), Daiichi Sankyo Co, Ltd (Tokyo, Japan) and Novartis Pharma K.K. (Tokyo, Japan).
The Department of Evidence-based Cardiovascular Medicine, Tohoku University Graduate School of Medicine, is supported in part by unrestricted research grants from Daiichi Sankyo Co, Ltd (Tokyo, Japan), Bayer Yakuhin, Ltd (Osaka, Japan), Kyowa Hakko Kirin Co, Ltd (Tokyo, Japan), Kowa Pharmaceutical Co, Ltd (Tokyo, Japan), Novartis Pharma K.K. (Tokyo, Japan), Dainippon Sumitomo Pharma, Co, Ltd (Osaka, Japan), and Nippon Boehringer Ingelheim Co, Ltd (Tokyo, Japan).
Supplementary Files
Supplementary File 1
Figure S1. Allocation of patients to cohorts.
Figure S2. Distribution of propensity scores (PS) in the total and matched cohorts of patients with heart failure and preserved ejection fraction (HFpEF).
Figure S3. Kaplan-Meier survival curves for all-cause death in the total and matched cohorts of patients with reduced ejection fraction (HFrEF).
Table S1. Characteristics of HFpEF patients included or excluded from PS-matched analysis
Table S2. Specific agents and doses of statins in the cohort of patients with HFpEF
Table S3. Baseline characteristics of patients with HFrEF in total and matched cohorts
Table S4. Prognostic effect of statins in the cohort of patients with HFrEF
Appendix S1. The CHART-2 Study Investigators
Please find supplementary file(s);
http://dx.doi.org/10.1253/circj.CJ-14-0865
References
- 1.
Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, et al. American Heart Association. Heart disease and stroke statistics-2013 update: A report from the American Heart Association. Circulation 2013; 127: e6–e245, doi:10.1161/CIR.0b013e31828124ad.
- 2.
Sakata Y, Shimokawa H. Epidemiology of heart failure in Asia. Circ J 2013; 77: 2209–2217.
- 3.
Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 2006; 355: 251–259.
- 4.
Cleland JG, Tendera M, Adamus J, Freemantle N, Polonski L, Taylor J; PEP-CHF Investigators. The perindopril in elderly people with chronic heart failure (PEP-CHF) study. Eur Heart J 2006; 27: 2338–2345.
- 5.
Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, et al. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: The CHARM-Preserved Trial. Lancet 2003; 362: 777–781.
- 6.
Lund LH, Benson L, Dahlström U, Edner M. Association between use of renin-angiotensin system antagonists and mortality in patients with heart failure and preserved ejection fraction. JAMA 2012; 308: 2108–2117.
- 7.
Edelmann F, Wachter R, Schmidt AG, Kraigher-Krainer E, Colantonio C, Kamke W, et al. Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: The Aldo-DHF randomized controlled trial. JAMA 2013; 309: 781–791.
- 8.
Paulus WJ, van Ballegoij JJ. Treatment of heart failure with normal ejection fraction: An inconvenient truth! J Am Coll Cardiol 2010; 55: 526–537.
- 9.
Hogg K, Swedberg K, McMurray J. Heart failure with preserved left ventricular systolic function; epidemiology, clinical characteristics, and prognosis. J Am Coll Cardiol 2004; 43: 317–327.
- 10.
Weber KT, Brilla CG. Pathological hypertrophy and cardiac interstitium: Fibrosis and renin-angiotensin-aldosterone system. Circulation 1991; 83: 1849–1865.
- 11.
Chan MM, Lam CS. How do patients with heart failure with preserved ejection fraction die? Eur J Heart Fail 2013; 15: 604–613.
- 12.
Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM Jr, Kastelein JJ, et al. Reduction in C-reactive protein and LDL cholesterol and cardiovascular event rates after initiation of rosuvastatin: A prospective study of the JUPITER trial. Lancet 2009; 373: 1175–1182.
- 13.
Gómez-Garre D, González-Rubio ML, Muñoz-Pacheco P, Caro-Vadillo A, Aragoncillo P, Fernández-Cruz A. Rosuvastatin added to standard heart failure therapy improves cardiac remodeling in heart failure rats with preserved ejection fraction. Eur J Heart Fail 2010; 12: 903–912.
- 14.
Davignon J. Beneficial cardiovascular pleiotropic effects of statins. Circulation 2004; 109: III39–III43.
- 15.
Tanaka S, Fukumoto Y, Nochioka K, Minami T, Kudo S, Shiba N, et al. Statins exert the pleiotropic effects through small GTP-binding protein dissociation stimulator upregulation with a resultant Rac1 degradation. Arterioscler Thromb Vasc Biol 2013; 33: 1591–1600.
- 16.
Kjekshus J, Apetrei E, Barrios V, Böhm M, Cleland JG, Cornel JH, et al. Rosuvastatin in older patients with systolic heart failure. N Engl J Med 2007; 357: 2248–2261.
- 17.
Gissi-HF Investigators, Tavazzi L, Maggioni AP, Marchioli R, Barlera S, Franzosi MG, Latini R, et al. Effect of rosuvastatin in patients with chronic heart failure (the GISSI-HF trial): A randomized, double-blind, placebo-controlled trial. Lancet 2008; 372: 1231–1239.
- 18.
Takano H, Mizuma H, Kuwabara Y, Sato Y, Shindo S, Kotooka N, et al. Effects of pitavastatin in Japanese patients with chronic heart failure: The Pitavastatin Heart Failure Study (PEARL Study). Circ J 2013; 77: 917–925.
- 19.
Shiba N, Nochioka K, Miura M, Kohno H, Shimokawa H; CHART-2 Investigators. Trend of westernization of etiology and clinical characteristics of heart failure patients in Japan: First report from the CHART-2 study. Circ J 2011; 75: 823–833.
- 20.
Miura M, Shiba N, Nochioka K, Takada T, Takahashi J, Kohno H, et al. Urinary albumin excretion in heart failure with preserved ejection fraction: An interim analysis of the CHART 2 study. Eur J Heart Fail 2012; 14: 367–376.
- 21.
Nochioka K, Sakata Y, Takahashi J, Miyata S, Miura M, Takada T, et al. Prognostic impact of nutritional status in asymptomatic patients with cardiac diseases: A report from the CHART-2 study. Circ J 2013; 77: 2318–2326.
- 22.
Sakata Y, Miyata S, Nochioka K, Miura M, Takada T, Tadaki S, et al. Gender differences in clinical characteristics, treatment and long-term outcome in patients with stage C/D Heart failure in Japan: Report from the CHART-2 study. Circ J 2014; 78: 428–435.
- 23.
Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, et al. 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; 62: e147–e239, doi:10.1016/j.jacc.2013.05.019.
- 24.
McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: The Framingham study. N Engl J Med 1971; 285: 1441–1446.
- 25.
Tribouilloy C, Rusinaru D, Mahjoub H, Soulière V, Lévy F, Peltier M, et al. Prognosis of heart failure with preserved ejection fraction: A 5 year prospective population-based study. Eur Heart J 2008; 29: 339–347.
- 26.
Fonarow GC, Stough WG, Abraham WT, Albert NM, Gheorghiade M, Greenberg BH, et al. Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: A report from the OPTIMIZE-HF Registry. J Am Coll Cardiol 2007; 50: 768–777.
- 27.
Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 2011; 46: 399–424.
- 28.
Fukuta H, Sane DC, Brucks S, Little WC. Statin therapy may be associated with lower mortality in patients with diastolic heart failure: A preliminary report. Circulation 2005; 112: 357–363.
- 29.
Levantesi G, Scarano M, Marfisi R, Borrelli G, Rutjes AW, Silletta MG, et al. Meta-analysis of effect of statin treatment on risk of sudden death. Am J Cardiol 2007; 100: 1644–1650.
- 30.
Vrtovec B, Okrajsek R, Golicnik A, Ferjan M, Starc V, Radovancevic B. Atorvastatin therapy increases heart rate variability, decreases QT variability, and shortens QTc interval duration in patients with advanced chronic heart failure. J Card Fail 2005; 11: 684–690.
- 31.
Vrtovec B, Okrajsek R, Golicnik A, Ferjan M, Starc V, Schlegel TT, et al. Atorvastatin therapy may reduce the incidence of sudden cardiac death in patients with advanced chronic heart failure. J Card Fail 2008; 14: 140–144.
- 32.
Kayikcioglu M, Can L, Evrengul H, Payzin S, Kultursay H. The effect of statin therapy on ventricular late potentials in acute myocardial infarction. Int J Cardiol 2003; 90: 63–72.
- 33.
Newman TB, Hulley SB. Carcinogenicity of lipid-lowering drugs. JAMA 1996; 275: 55–60.
- 34.
Deng Z, Zhang S, Yi L, Chen S. Can statins reduce risk of lung cancer, especially among elderly people?: A meta-analysis. Chin J Cancer Res 2013; 25: 679–688.
- 35.
Coleman CI, Lucek DM, Hammond J, White CM. Preoperative statins and infectious complications following cardiac surgery. Curr Med Res Opin 2007; 23: 1783–1790.
- 36.
Gupta R, Plantinga LC, Fink NE, Melamed ML, Coresh J, Fox CS, et al. Statin use and sepsis events [corrected] in patients with chronic kidney disease. JAMA 2007; 297: 1455–1464.
- 37.
van de Garde EM, Hak E, Souverein PC, Hoes AW, van den Bosch JM, Leufkens HG. Statin treatment and reduced risk of pneumonia in patients with diabetes. Thorax 2006; 61: 957–961.
- 38.
Fernandez R, De Pedro VJ, Artigas A. Statin therapy prior to ICU admission: Protection against infection or a severity marker? Intensive Care Med 2006; 32: 160–164.
- 39.
Tleyjeh IM, Kashour T, Hakim FA, Zimmerman VA, Erwin PJ, Sutton AJ, et al. Statins for the prevention and treatment of infections: A systematic review and meta-analysis. Arch Intern Med 2009; 169: 1658–1667.
- 40.
Steiner S, Speidl WS, Pleiner J, Seidinger D, Zorn G, Kaun C, et al. Simvastatin blunts endotoxin-induced tissue factor in vivo. Circulation 2005; 111: 1841–1846.
- 41.
Novack V, Eisinger M, Frenkel A, Terblanche M, Adhikari NK, Douvdevani A, et al. The effects of statin therapy on inflammatory cytokines in patients with bacterial infections: A randomized double-blind placebo controlled clinical trial. Intensive Care Med 2009; 35: 1255–1260.