Abstract
Background:
The impact of peripheral artery disease (PAD) on heart failure (HF) prognosis remains unclear.
Methods and Results:
A total of 388 consecutive decompensated HF patients were divided into 2 groups based on the presence of PAD: HF with PAD (PAD group, n=101, 26.0%) and HF without PAD (non-PAD group, n=287, 74.0%). We compared clinical features, echocardiographic parameters, cardiopulmonary exercise testing results, laboratory findings, as well as cardiac, non-cardiac, and all-cause mortality between the 2 groups. The PAD group, as compared with the non-PAD group, had (1) higher prevalence of coronary artery disease (40.6 vs. 27.5%, P=0.011) and cerebrovascular disease (34.7 vs. 18.2%, P=0.001); (2) higher tumor necrosis factor-α (1.82 vs. 1.49 pg/ml, P=0.023), C-reactive protein (0.32 vs. 0.19 mg/dl, P=0.045), and troponin T (0.039 vs. 0.021 ng/ml, P=0.019); (3) lower LVEF (42.4 vs. 48.5%, P<0.001); (4) lower peak V̇O2
(13.4 vs. 15.9 ml·kg–1·min–1, P=0.001); and (5) higher V̇E/V̇CO2
slope (38.8 vs. 33.7, P<0.001). On Kaplan-Meier analysis, cardiac, non-cardiac, and all-cause mortality were significantly higher in the PAD group than in the non-PAD group (P<0.05, respectively). On Cox proportional hazard analysis after adjusting for confounding factors, PAD was an independent predictor of cardiac and all-cause mortality (P<0.05, respectively) in HF patients.
Conclusions:
PAD was common and an independent predictor of cardiac and all-cause mortality in HF patients. (Circ J 2015; 79: 785–793)
Heart failure (HF) is a major cause of death among the elderly in many countries.1,2
Peripheral arterial disease (PAD), which is also known as lower extremity artery disease, is accompanied by systemic inflammation and is frequently associated with other cardiovascular diseases.3–8
The impact of PAD on adverse prognosis in coronary artery disease has previously been reported.9,10
Although PAD and HF share cardiovascular risk and pathophysiological features, and each has been associated with increased morbidity and mortality, little is known about the prevalence of PAD and its impact on HF.11
A few studies have been reported regarding PAD and HF, but PAD in those studies had been diagnosed based mainly on self-reported medical history, rather than objective testing in previous studies.12–15
Presence of HF masks the symptoms of PAD such as intermittent claudication, hence, presence of PAD in HF patients would be underestimated. Both the features of HF that coexist with PAD from the viewpoint of comprehensive clinical background, and the impact of accurately diagnosed PAD on prognosis in HF remain unclear.
Thus, the aims of the present study were to investigate (1) clinical features; (2) neurohumoral and inflammatory factors such as noradrenalin, renin activity, renin concentration, aldosterone, tumor necrosis factor (TNF)-α, and C-reactive protein; (3) cardiac function and myocardial damage; (4) exercise capacity, and (5) prognosis including progressive HF, cardiac, non-cardiac, and all-cause mortality in HF patients with or without PAD.
Methods
Subjects and Study Protocol
This was a prospective observational study that enrolled 388 consecutive symptomatic HF patients who had been hospitalized at Fukushima Medical University Hospital and evaluated for the presence of PAD between 2009 and 2012. The diagnosis of symptomatic HF was defined based on the Framingham criteria.16
Patients with acute coronary syndrome, dialysis, and documented cancer were excluded. PAD was diagnosed according to the PAD management guidelines, such as computed tomography, angiography, and/or ankle-brachial index (ABI).5–7
Fontaine and the Trans-Atlantic Inter-Society Consensus Document on Management of Peripheral Arterial Disease (TASC) classes were classified according to PAD guidelines.5–7
Clinical PAD was defined as the presence of symptoms such as intermittent claudication, ischemic rest pain, ulceration, or gangrene. Subclinical PAD fulfilled the aforementioned criteria5–7
and absence of said symptoms. We performed several examinations, such as general laboratory tests, echocardiography, and cardiopulmonary exercise testing, in patients who were in a relatively stable phase (before discharge) and compared parameters between the PAD and the non-PAD groups.
Hypertension was defined as the recent use of anti-hypertensive drugs, or systolic blood pressure ≥140 mmHg and/or diastolic pressure ≥90 mmHg. Diabetes was defined as the recent use of insulin or anti-diabetic drugs, fasting blood glucose ≥126 mg/dl, and/or hemoglobin A1c
≥6.5%. Dyslipidemia was defined as the recent use of cholesterol-lowering drugs, triglyceride ≥150 mg/dl, low-density lipoprotein cholesterol ≥140 mg/dl, and/or high-density lipoprotein cholesterol <40 mg/dl. Chronic kidney disease was defined as estimated glomerular filtration rate (eGFR) <60 ml·min–1·1.73 m–2.17
eGFR was measured using the Modification of Diet in Renal Disease formula.17
Anemia was defined as hemoglobin <12.0 g/dl in female subjects and <13.0 g/dl in male subjects.18
Smoking habit was defined as the smoking of any cigarettes in the last 3 months. Polyvascular disease was defined as the presence of PAD and a past history of coronary artery disease and cerebrovascular disease. Patients were followed up for progressive HF, cardiac death, non-cardiac death, and all-cause mortality. Cardiac death included death due to ventricular fibrillation or worsening HF. Progressive HF was defined as re-hospitalization due to worsening HF based on the Framingham criteria.16
Non-cardiac death included death due to respiratory failure, infection, sepsis, cancer, or digestive hemorrhage. Status and date of death were obtained from medical records. If these data were unavailable, status was ascertained by a telephone call to the patient’s referring physician. Written informed consent was obtained from all study subjects. The study protocol was approved by the ethics committee of Fukushima Medical University. The investigation conforms with the principles outlined in the Declaration of Helsinki.
ABI
ABI was blindly measured based on a standard protocol by trained technicians using a Vasera VS-1000 device (Fukuda Denshi, Tokyo, Japan) as previously reported.5–7
When using ABI, PAD was defined as ABI <0.90 or ABI ≥1.40.5–7
TNF-α and Troponin T Measurement
A blood sample was obtained from each patient at Fukushima Medical University before discharge under fasting state. High-sensitivity troponin T was measured using electrochemiluminescence immunoassay (Elecsys Troponin T hs; Roche Diagnostics, Rotkreuz, Switzerland).19
TNF-α was measured using solid-phase chemiluminescent ELISA with an immunoassay kit (Quanti Glo ELISA Human TNF-α Immunoassay; R&D Systems, Minneapolis, MN, USA).
Echocardiography
Echocardiography was performed blind by an experienced echocardiographer using standard techniques. Echocardiographic parameters investigated included interventricular septum thickness, left ventricular (LV) dimension, posterior wall thickness, LV ejection fraction (LVEF), left atrial volume, and the ratio of early transmitral flow velocity to mitral annular velocity (mitral valve E/E’), inferior vena cava diameter, peak systolic pulmonary artery pressure (SPAP), and right ventricular fractional area change (RV-FAC).20
LVEF was calculated using a modification of the Simpson method. Mitral valve E/E’ was calculated on transmitral Doppler flow and tissue Doppler. Tissue Doppler was obtained from the average of lateral and septal annulus velocities. SPAP was calculated by adding the right atrial pressure (estimated by the diameter and collapsibility of the inferior vena cava) to the systolic trans-tricuspid pressure gradient.20
RV-FAC, defined as (end diastolic area–end systolic area)/end diastolic area×100, measures right ventricular systolic function.20
All recordings were performed on ultrasound systems (ACUSON Sequoia; Siemens Medical Solutions USA, Mountain View, CA, USA).
Cardiopulmonary Exercise Testing
All subjects underwent increasing symptom-limited exercise testing using an upright cycle ergometer with a ramp protocol (Strength Ergo 8; Fukuda Denshi). Breath-by-breath oxygen consumption (V̇O2), carbon dioxide production (V̇CO2), and minute ventilation (V̇E) were measured during exercise using an AE-300S respiratory monitor (Minato Medical Science, Osaka, Japan).21,22
Peak V̇O2
was measured as an average of the last 30 s of exercise. Ventilatory response to exercise (expressed as V̇E/V̇CO2
slope) was calculated as the regression slope relating V̇E to CO2
from the start of exercise until the respiratory compensation point (the time at which ventilation is stimulated by CO2
output and end-tidal CO2
tension begins to decrease).23
Ventilatory anaerobic threshold was calculated with the V-slope method.
Statistical Analysis
Normally distributed data are presented as mean±SD, non-normally distributed data are presented as median (interquartile range), and categorical variables are expressed as numbers and percentages. Characteristics of the 2 groups were compared using the independent Student’s t-test for normally distributed data and the Mann-Whitney U-test for non-normally distributed data, whereas the chi-squared test was used for categorical variables. Data among 3 groups were compared using 1-way repeated-measures analysis of variance (ANOVA) followed by Bonferroni post-hoc test. The Kaplan-Meier method was used for determining event-free rate, and log-rank test was used for initial comparisons. Univariate and multivariate Cox proportional hazard analysis were used to analyze predictors of events with adjusting confounding factors. To prepare for potential confounding, we introduced the following factors, known to affect the risk of worsening HF, cardiac death, or all-cause mortality in HF patients: age, gender, New York Heart Association (NYHA) functional class III or IV, systolic blood pressure, heart rate, smoking habit, hypertension, diabetes, dyslipidemia, chronic kidney disease, atrial fibrillation, anemia, coronary artery disease, cerebrovascular disease, reduced LVEF (LVEF <50%), and the use of renin-angiotensin-aldosterone system inhibitors, β-blockers, diuretics, inotropic agents, statin, anti-diabetic drugs, and antiplatelet agents. P<0.05 was considered significant for all comparisons. All analysis was performed using SPSS version 21.0 (IBM, Armonk, NY, USA).
Results
The subject clinical features are summarized in
Table 1. In the hospitalized HF patients, the prevalence of PAD defined according to the PAD management guidelines was 26.0% (101/388). Out of 101 patients with PAD, there were 26 HF patients with clinical PAD and 75 HF patients with subclinical PAD. The PAD group had (1) higher age and lower ABI; (2) higher prevalence of NYHA class III or IV, reduced LVEF, smoking habit, diabetes, atrial fibrillation, chronic kidney disease, coronary artery disease, and cerebrovascular disease as well as polyvascular disease; and (3) higher use of inotropic agents, anti-diabetic drugs, and antiplatelet agents.
Table 1.
Clinical Characteristics (n=388)
| |
Non-PAD (n=287) |
PAD (n=101) |
P-value |
| Age (years) |
64.3±14.2 |
69.8±11.1 |
0.001 |
| Male gender |
182 (63.4) |
71 (70.3) |
0.207 |
| BMI (kg/cm2) |
23.7±4.1 |
22.9±3.6 |
0.079 |
| SBP (mmHg) |
132.9±35.2 |
130.0±34.8 |
0.463 |
| DBP (mmHg) |
76.8±24.5 |
73.6±24.3 |
0.140 |
| Heart rate (beats/min) |
83.0±27.1 |
87.7±27.8 |
0.140 |
| NYHA class III or IV |
107 (37.3) |
57 (56.4) |
0.001 |
| Fontaine class (I/II/III/IV) |
– |
75/13/9/4 |
– |
| TASC class (A/B/C/D/unclassified) |
– |
13/9/4/8/67 |
– |
| Clinical/Subclinical |
– |
26 (25.7)/75 (74.3) |
– |
| ABI |
1.10±0.10 |
0.76±0.15 |
<0.001 |
| ABI class (<0.7, 0.7–0.9, 0.9–1.4, unclassified) |
0/0/234/53 |
26/74/0/1 |
<0.001 |
| Reduced LVEF |
133 (46.3) |
71 (70.3) |
<0.001 |
| Smoking habit |
134 (46.7) |
61 (60.4) |
0.012 |
| Brinkman index (number/day×years) |
264.8±147.6 |
341.2±276.3 |
0.164 |
| Comorbidity |
| Hypertension |
223 (77.7) |
80 (79.2) |
0.889 |
| Diabetes |
101 (35.2) |
49 (48.5) |
0.024 |
| Dyslipidemia |
211 (73.5) |
79 (78.2) |
0.424 |
| Atrial fibrillation |
105 (36.6) |
49 (48.5) |
0.044 |
| Chronic kidney disease |
143 (49.8) |
74 (73.3) |
<0.001 |
| Anemia |
127 (44.3) |
55 (54.5) |
0.083 |
| Coronary artery disease |
79 (27.5) |
41 (40.6) |
0.011 |
| Cerebrovascular disease |
52 (18.2) |
35 (34.7) |
0.001 |
| Polyvascular disease |
0 (0) |
17 (16.8) |
<0.001 |
| Medications |
| RAS inhibitors |
218 (76.0) |
76 (75.2) |
0.893 |
| β-blockers |
234 (81.5) |
86 (85.1) |
0.450 |
| Diuretics |
171 (59.6) |
67 (66.3) |
0.238 |
| Inotropic agents |
35 (12.2) |
23 (22.8) |
0.014 |
| Anti-diabetic agents |
62 (21.6) |
35 (34.7) |
0.011 |
| Statin |
107 (37.3) |
42 (41.6) |
0.476 |
| Antiplatelet agents |
135 (47.0) |
68 (67.3) |
<0.001 |
| PAD therapy |
– |
16 (15.8) |
– |
| Peripheral percutaneous intervention |
– |
14 (13.9) |
– |
| Surgery |
– |
3 (3.0) |
– |
Data given as mean±SD, n (%) or median (IQR). ABI, ankle-brachial index; BMI, body mass index; DBP, diastolic blood pressure; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; PAD, peripheral artery disease; RAS, renin-angiotensin-aldosterone system; SBP, systolic blood pressure; TASC, Trans-Atlantic Inter-Society Consensus Document on Management of Peripheral Arterial Disease.
Comparisons of laboratory data between the 2 groups are shown in
Table 2. The PAD group had higher B-type natriuretic peptide, C-reactive protein, troponin T, and TNF-α and lower hemoglobin and eGFR. In contrast, noradrenalin, renin activity, renin concentration, and aldosterone did not differ between the 2 groups.
Table 2.
Laboratory Data (n=388)
| |
Non-PAD (n=287) |
PAD (n=101) |
P-value |
| White blood cell (103/μl) |
7.12±3.28 |
7.40±2.96 |
0.469 |
| Hemoglobin (g/dl) |
12.9±2.3 |
12.3±2.0 |
0.019 |
| B-type natriuretic peptide (pg/ml) |
269.7 (471) |
390.9 (505) |
0.031 |
| eGFR (ml·min−1·1.73 cm−2) |
59.5±25.2 |
44.7±22.6 |
<0.001 |
| C-reactive protein (mg/dl) |
0.19 (1) |
0.32 (1) |
0.045 |
| Troponin T (ng/ml) |
0.021 (0.033) |
0.039 (0.077) |
0.019 |
| Total protein (g/dl) |
7.0±0.8 |
7.0±0.6 |
0.613 |
| Albumin (g/dl) |
3.7±0.6 |
3.6±0.5 |
0.072 |
| Sodium (mmol/L) |
139.2±3.3 |
138.4±4.1 |
0.091 |
| Glucose (mg/dl) |
130.2±60.2 |
147.0±70.8 |
0.064 |
| Hemoglobin A1c (%) |
5.75±1.16 |
6.10±1.20 |
0.054 |
| Total cholesterol (mg/dl) |
180.5±45.2 |
169.4±43.6 |
0.185 |
| HDL-C (mg/dl) |
50.2±20.9 |
45.1±12.6 |
0.104 |
| LDL-C (mg/dl) |
106.0±39.7 |
102.9±35.8 |
0.591 |
| Triglyceride (mg/dl) |
118.1±67.0 |
104.3±49.4 |
0.143 |
| Plasma noradrenalin (pg/ml) |
610.0 (616) |
785.0 (787.0) |
0.355 |
| Plasma renin activity (ng·ml−1·h−1) |
1.4 (4.0) |
3.6 (6.1) |
0.823 |
| Renin concentration (pg/ml) |
11.5 (36.0) |
27.0 (41.8) |
0.845 |
| Aldosterone (pg/ml) |
86.0 (62.6) |
71.8 (53.9) |
0.955 |
| Tumor necrosis factor-α (pg/ml) |
1.49 (1.18) |
1.82 (2.16) |
0.023 |
Data given as mean±SD or median (IQR). eGFR, estimated glomerular filtration rate; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; PAD, peripheral artery disease.
Echocardiographic parameters are summarized in
Table 3. The PAD group had lower LVEF, and higher LV end-systolic dimension and mitral valve E/E’.
Table 3.
Echocardiography Data
| |
Non-PAD (n=287) |
PAD (n=101) |
P-value |
| Interventricular septum thickness (mm) |
10.9±2.4 |
10.7±2.5 |
0.446 |
| LV end-diastolic dimension (mm) |
53.1±10.6 |
54.4±10.0 |
0.318 |
| LV end-systolic dimension (mm) |
39.5±12.5 |
42.7±12.4 |
0.039 |
| Posterior wall thickness (mm) |
11.3±2.8 |
11.0±2.8 |
0.414 |
| LVEF (%) |
48.5±15.6 |
42.4±13.4 |
0.001 |
| Left atrial volume (ml) |
84.3±52.0 |
85.3±67.3 |
0.889 |
| Mitral valve E/E’ |
15.2±9.1 |
17.9±6.6 |
0.021 |
| Inferior vena cava diameter (mm) |
15.3±5.3 |
16.3±5.4 |
0.167 |
| SPAP (mmHg) |
29.0±14.1 |
28.6±14.3 |
0.829 |
| RV-FAC (%) |
41.5±11.2 |
39.8±10.9 |
0.392 |
| Tricuspid valve E/E’ |
6.2±2.4 |
5.8±2.9 |
0.594 |
Data given as mean±SD. LV, left ventricular; mitral valve E/E’, ratio of the peak transmitral velocity during early diastole to the peak mitral valve annular velocity during early diastole; RV-FAC, right ventricular fractional area change; SPAP, systolic pulmonary artery pressure; tricuspid valve E/E’, ratio of the peak transtricuspid velocity during early diastole to the peak tricuspid valve annular velocity during early diastole. Other abbreviations as in Table 1.
Comparisons of cardio pulmonary exercise testing data between the 2 groups are shown in
Table 4. Peak V̇O2, end-tidal CO2
at the respiratory compensation point, anaerobic threshold V̇O2, and ∆V̇O2/∆work rate were significantly lower, and minimum V̇E/V̇CO2
and V̇E/V̇CO2
slope were significantly higher in the PAD group than in the non-PAD group.
Table 4.
Cardiopulmonary Exercise Test (n=253)
| |
Non-PAD (n=189) |
PAD (n=64) |
P-value |
| Peak V̇O2 (ml·kg−1·min−1) |
15.9±4.6 |
13.4±4.0 |
0.001 |
| Peak SBP (mmHg) |
159.7±34.0 |
150.2±34.4 |
0.113 |
| Peak pulse pressure (mmHg) |
77.3±28.2 |
72.0±33.4 |
0.298 |
| Peak heart rate (beats/min) |
116.5±28.8 |
114.9±27.9 |
0.717 |
End-tidal CO2 at respiratory compensation point
(mmHg) |
36.0±4.7 |
33.1±5.1 |
<0.001 |
| Anaerobic threshold V̇O2 (ml·kg−1·min−1) |
11.2±2.4 |
10.1±2.1 |
0.007 |
| Minimum V̇E-V̇CO2 |
34.9±5.8 |
39.3±7.3 |
<0.001 |
| V̇E/V̇CO2 slope |
33.7±7.7 |
38.8±8.9 |
<0.001 |
| ΔV̇O2/Δwork rate (ml·min−1·Watts−1) |
8.4±3.6 |
7.2±2.2 |
0.032 |
Data given as mean±SD. ΔV̇O2/Δwork rate, rate of increase in V̇O2
to increase in work rate; peak V̇O2, peak oxygen uptake; V̇CO2, carbon dioxide production; V̇E, minute ventilation; V̇E/V̇CO2
slope, rate of increase in ventilation per unit increase in carbon dioxide, minimumV̇E-V̇CO2, and rate of minute ventilation to carbon dioxide production; V̇O2, oxygen consumption. Other abbreviations as in Table 1.
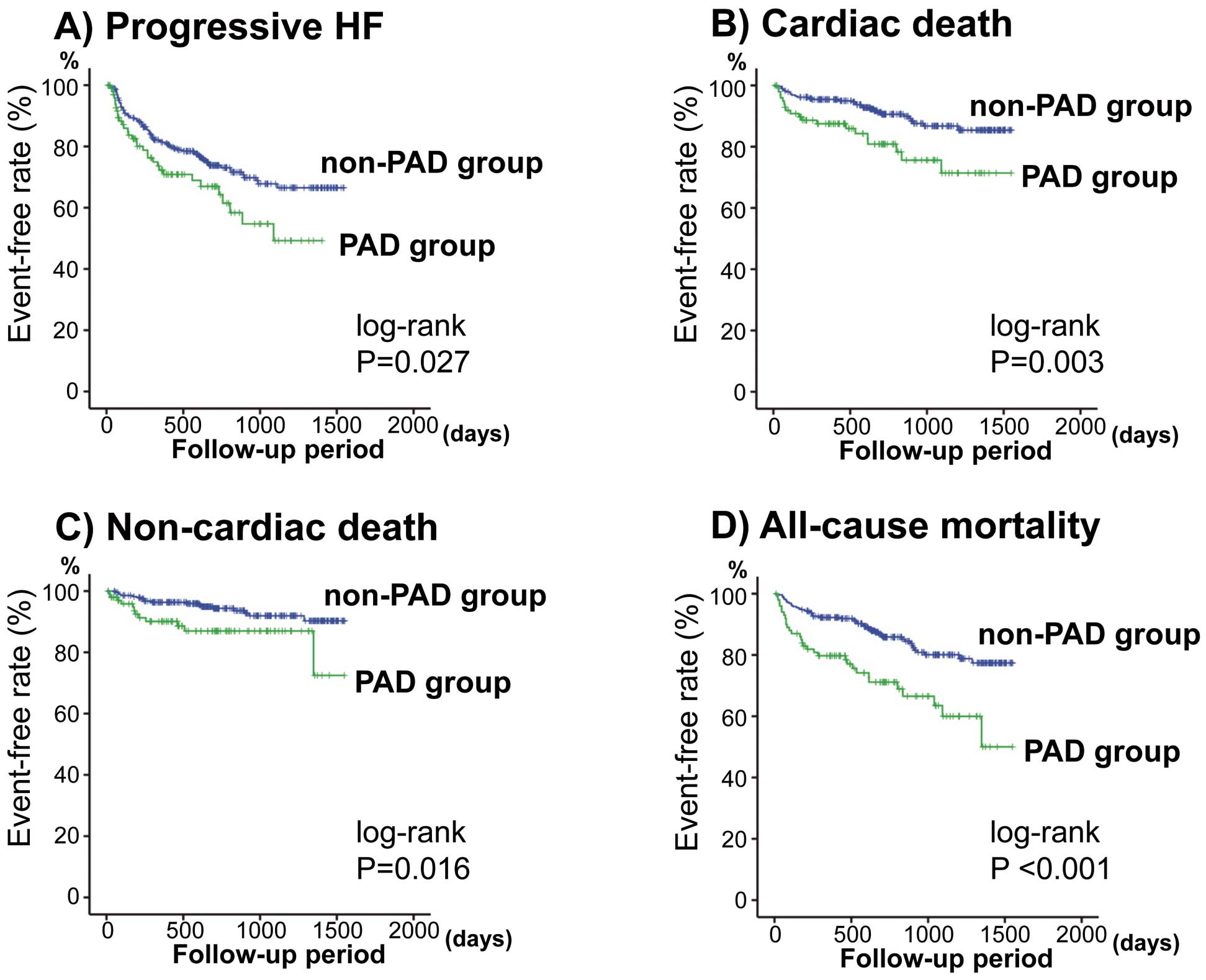
During the follow-up period (mean, 766 days), there were 107 progressive HF episodes, 48 cardiac deaths, and 29 non-cardiac deaths (cancer, n=8; respiratory failure and/or pneumonia, n=5; stroke, n=4; infection/sepsis, n=4; renal failure, n=2; aneurysm, n=2; digestive hemorrhage, n=2; and other problems n=2). In the PAD group, 32 progressive HF episodes, 19 cardiac deaths and 12 non-cardiac deaths occurred. In the non-PAD group, 75 progressive HF episodes, 29 cardiac deaths and 17 non-cardiac deaths occurred. As shown in
Figure 1, the event rates of progressive HF, cardiac, non-cardiac, and all-cause mortality were significantly higher in the PAD group than in the non-PAD group (P<0.05, respectively).
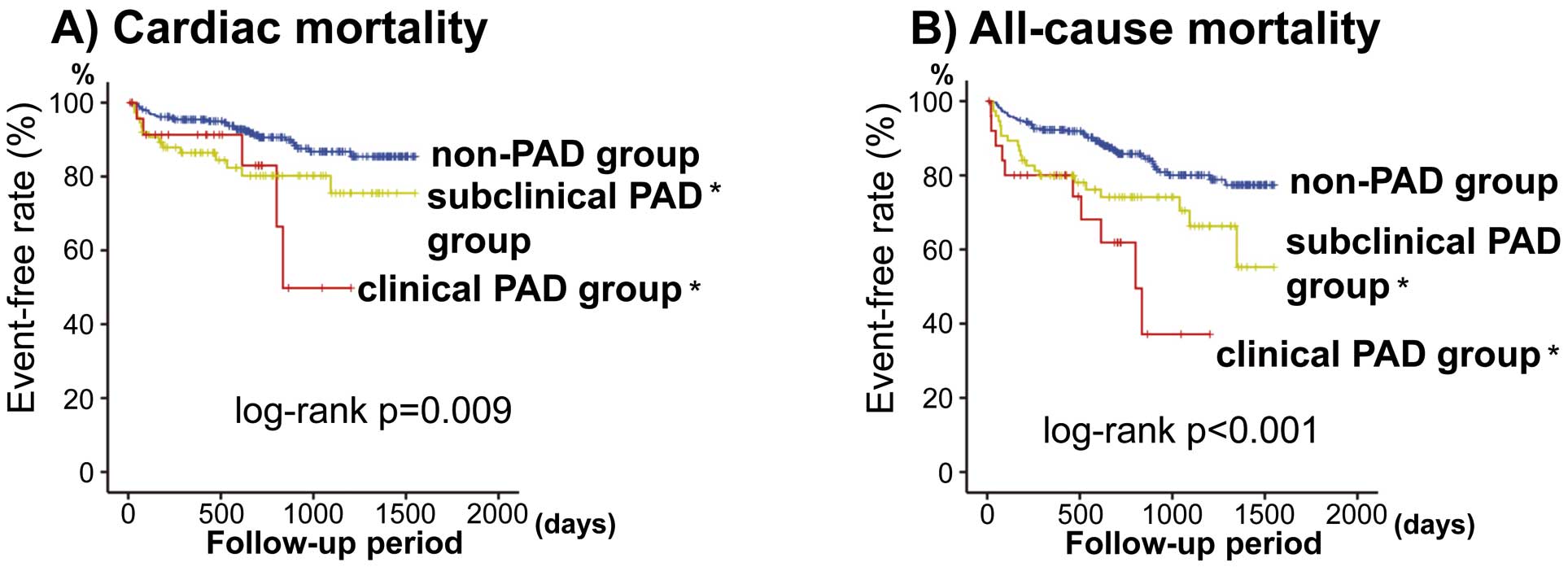
As shown in
Figure 2, we also analyzed cardiac and all-cause mortality among non-PAD (n=287), subclinical PAD (without symptom, n=75), and clinical PAD groups (with symptom, n=26). Cardiac and all-cause mortalities were significantly higher not only in the clinical PAD but also in the subclinical PAD group than in the non-PAD group. Comparisons of data among the 3 groups are given in
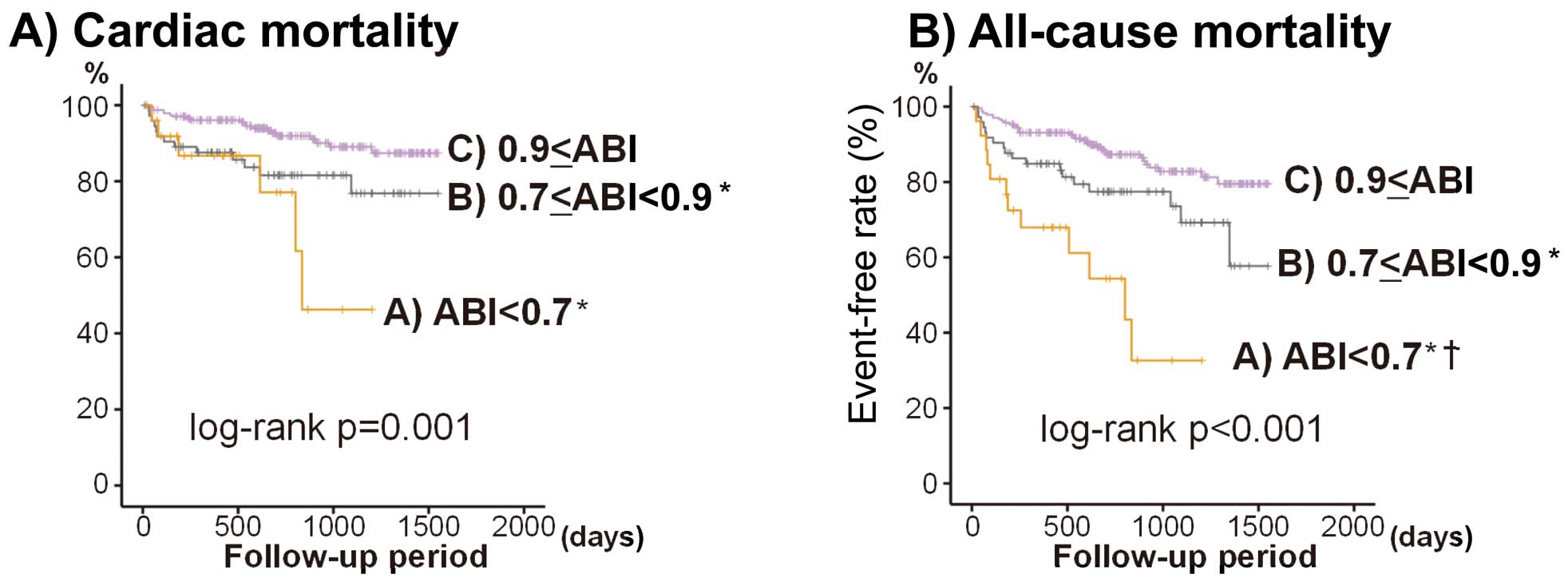
Tables S1–S4. None of the present patients had ABI ≥1.4. ABI was graded as follows: A, ABI <0.7 (n=26); B, 0.7≤ABI<0.9 (n=74); and C, 0.9≤ABI (n=234). We analyzed cardiac and all-cause mortality among ABI classes. As shown in
Figure 3, cardiac and all-cause mortalities were significantly higher in the lowest ABI group (A) and intermediate ABI group (B) than in the normal ABI group (C). In addition, we also analyzed the event rates of progressive HF, cardiac, non-cardiac, and all-cause mortality separately: (1) HF patients with ischemic HF (n=109) and non-ischemic HF (n=279); and (2) HF patients with reduced EF (<50%; n=207) and preserved EF (>50%, n=181). The event rates between the PAD group and the non-PAD group are shown in
Figures S1–S4. In ischemic HF, cardiac death and all-cause mortality were higher in the PAD group than in the non-PAD group. In HF with reduced EF, cardiac death, non-cardiac death and all-cause mortality were higher in the PAD group than in the non-PAD group (Figures S1–S4).
The Cox proportional hazard model was used to examine the prognostic value of PAD in HF patients (Table 5). On multivariate analysis, PAD was an independent predictor of cardiac and all-cause mortality (P<0.05, respectively) after adjusting for potential confounding factors (models 1–4).
Table 5.
Prognostic Value of PAD in HF
| Event |
HR |
Chi-squared |
95% CI |
P-value |
| Progressive HF |
| Unadjusted |
1.592 |
4.818 |
1.051–2.410 |
0.028 |
| Model 1 |
1.569 |
4.367 |
1.028–2.394 |
0.037 |
| Model 2 |
1.247 |
1.023 |
0.813–1.911 |
0.312 |
| Model 3 |
1.365 |
1.932 |
0.880–2.116 |
0.165 |
| Model 4 |
1.249 |
1.018 |
0.811–1.924 |
0.313 |
| Cardiac death |
| Unadjusted |
2.329 |
8.161 |
1.304–4.161 |
0.004 |
| Model 1 |
2.465 |
8.938 |
1.364–4.453 |
0.003 |
| Model 2 |
1.933 |
4.591 |
1.058–3.533 |
0.032 |
| Model 3 |
2.181 |
6.160 |
1.178–4.036 |
0.013 |
| Model 4 |
2.296 |
6.991 |
1.240–4.251 |
0.008 |
| Non-cardiac death |
| Unadjusted |
2.404 |
5.486 |
1.154–5.008 |
0.019 |
| Model 1 |
2.025 |
3.456 |
1.106–4.262 |
0.043 |
| Model 2 |
1.775 |
2.184 |
0.829–3.801 |
0.139 |
| Model 3 |
1.937 |
2.796 |
0.892–4.206 |
0.095 |
| Model 4 |
2.004 |
3.197 |
0.935–4.293 |
0.074 |
| All-cause mortality |
| Unadjusted |
2.421 |
13.228 |
1.533–3.825 |
<0.001 |
| Model 1 |
2.344 |
12.894 |
1.473–3.733 |
<0.001 |
| Model 2 |
1.940 |
7.536 |
1.209–3.114 |
0.006 |
| Model 3 |
2.165 |
9.780 |
1.334–3.514 |
0.002 |
| Model 4 |
2.225 |
10.686 |
1.377–3.594 |
0.001 |
Model 1, adjusted for gender and age. Model 2, model 1+ NYHA functional class III or IV, SBP, heart rate, and reduced LVEF. Model 3, Model 1+ presence of smoking habit, hypertension, diabetes, dyslipidemia, chronic kidney disease, atrial fibrillation, anemia, coronary artery disease, and cerebrovascular disease. Model 4, model 1+ use of RAS inhibitors, β-blockers, diuretics, inotropic agents, statin, anti-diabetic drugs, and antiplatelet agents. CI, confidence interval; HF, heart failure; HR, hazard ratio. Other abbreviations as in Table 1.
Discussion
To the best of our knowledge, the present study is the first to show the prevalence of PAD based on precise diagnosis in HF and detailed clinical features of patients with HF and PAD. The major findings of this study were that (1) the prevalence of PAD defined according to the PAD management guideline in hospitalized HF patients was 26.0% (clinical PAD, 6.7%; subclinical PAD, 19.3%); and (2) HF patients in the PAD group, as compared with the non-PAD group, had a higher prevalence of smokers, diabetes, atrial fibrillation, chronic kidney disease, coronary artery disease, and cerebrovascular disease, higher TNF-α, C-reactive protein (inflammation), and troponin T (myocardial damage), lower LVEF, lower peak V̇O2
(exercise capacity), and higher cardiac and all-cause mortality. Importantly, not only clinical but also subclinical PAD, and decreased ABI were associated with higher cardiac and all-cause mortalities.
To date, a few studies have investigated the coexistence of PAD and HF, but PAD had been diagnosed based mainly on self-reported medical history and symptoms, rather than objective testing.13–15
Jones et al analyzed the HF-ACTION trial, in which subjects underwent cardiopulmonary exercise testing and cardiac rehabilitation, and found that only 6.8% of HF (LVEF <35%) had PAD, and PAD was independently associated with increased all-cause hospitalization or all-cause mortality (HR, 1.31; 95% CI: 1.06−1.62).13
Further, Inglis et al analyzed the CORONA trial and reported that only 12.7% of HF (LVEF ≤35%) had intermittent claudication, and a higher prevalence of male gender, smoking, and diabetes, and that intermittent claudication was independently associated with increased progressive HF (HR, 1.35; 95% CI: 1.03−1.77) and all-cause mortality (HR, 1.36; 95% CI: 1.19−1.56).14
Ahmed et al carried out post-hoc analyses of the BEST trial and reported that 16.4% of HF patients had PAD based on self-reported medical history, and a history of PAD was independently associated with increased cardiovascular mortality (HR, 1.31; 95% CI: 1.04−1.63).15
From the present study, we emphasize the importance of subclinical PAD in HF. Typically, two-thirds of all PAD patients are asymptomatic.5
Not only clinical but also subclinical PAD is reportedly associated with adverse clinical outcome in patients with acute coronary syndrome.10
Lower exercise capacity brought on by HF may mask PAD symptoms such as leg fatigue and claudication and underestimate the presence of PAD. In the present study, we actively used ABI and diagnosed PAD based on PAD management guidelines, and the presence of PAD in HF was more frequent than previously reported.11,13–15
We believe that the present data indicate the importance of screening PAD (eg, ABI) in HF patients.
The presence of PAD has recently been assumed to implicate polyvascular diseases including coronary artery and cerebrovascular diseases5–7
along with systemic inflammation, oxidative stress, activated renin-angiotensin aldosterone, and sympathetic nervous system, and so on. In addition, PAD is recognized as systemic vascular dysfunction and arteriosclerosis and decreases additional exercise tolerance and activities of daily living. Endothelial dysfunction, greater arterial stiffness, and impaired exercise capacity are reportedly associated with poorer prognosis in patients with HF.21–25
PAD is associated with impaired physical function in patients with HF.26
Impaired physical function may relate to skeletal muscle abnormalities or a mixture of central and peripheral hemodynamic abnormalities.27,28
In contrast, elevated circulating TNF-α and
troponins are correlated with HF severity and adverse all-cause mortality in HF patients.19,29,30
Inflammation plays a central role in the pathogenesis of atherosclerosis. Even though troponins are specific proteins of the myocardium, recent studies have shown that troponins are occasionally elevated in non-cardiac disease such as inflammatory disease and atherosclerotic disease including sepsis,31
stroke,32
and aortic dissection.33
The present data are concordant with the previous findings that PAD concomitant with HF had features of greater inflammation,5–7,34
impaired exercise capacity,13,26
and complications with polyvascular diseases.5–7
This study adds to the current literature, and shows that PAD correlates with a more severe type of atherosclerosis even in HF.5–7,12
For treatment of patients with HF with PAD, appropriate comprehensive management including smoking cessation, diabetes control, and exercise training35
following recent guidelines5–7
is more important than usual, and may lead to improved prognosis of HF and PAD. Further investigation is needed to establish whether revascularization including peripheral percutaneous intervention36
and surgery improves the prognosis of HF and PAD.
Strengths and Limitations
The present study has several strengths and differs from previous studies12–15
in many ways. For instance, we presented comprehensive clinical features including comorbidities, inflammatory status, myocardial damage, cardiac function, exercise capacity, and detailed prognosis. Furthermore, it is noteworthy that the diagnoses of HF and PAD were accurately made by experienced cardiologists using the Framingham criteria and PAD guidelines. Hence, we have described the impact of subclinical PAD on cardiac and all-cause mortality of HF patients, and the importance of screening PAD. After adjusted for confounding risk factors, PAD was associated with high morbidity and mortality in HF.
There is a potential limitation. This was a prospective observational study including only HF patients who had undergone precise evaluation. The number of subjects was relatively small, given that this study was performed in a single institution. Therefore, further studies with a larger group are needed.
Conclusions
PAD was common and an independent predictor of cardiac and all-cause mortality in HF patients. Decreased ABI is considered as subclinical PAD and a marker of generalized atherosclerosis. The presence of PAD indicates underlying polyvascular disease, more severe type of atherosclerosis, multiple risk factors, systemic inflammation, myocardial damage, and impaired exercise capacity and is associated with poor prognosis in HF patients. These mechanisms may affect the adverse prognosis of HF with PAD. Thus, screening PAD (including subclinical PAD) and aggressive comprehensive management may improve prognosis in HF patients.
Acknowledgments
The authors thank Ms Kumiko Watanabe, Emiko Kaneda, and Yuko Niimura for their outstanding technical assistance. This study was supported in part by a grant-in-aid for Scientific Research (No. 25461061) from the Japan Society for the Promotion of Science.
Disclosures
Akiomi Yoshihisa and Satoshi Suzuki belong to an endowed department (affiliation with Fukuda Denshi, Tokyo, Japan).
Supplementary Files
Supplementary File 1
Figure S1.
Ischemic heart failure (HF; n=109).
Figure S2.
Non-ischemic heart failure (HF; n=279).
Figure S3.
Heart failure (HF) with reduced ejection fraction (EF; n=207).
Figure S4.
Heart failure (HF) with preserved ejection fraction (EF; n=181).
Table S1.
Comparisons of clinical features (n=388)
Table S2.
Laboratory data (n=388)
Table S3.
Comparisons of echocardiographic data
Table S4.
Cardiopulmonary exercise test (n=253)
Please find supplementary file(s);
http://dx.doi.org/10.1253/circj.CJ-14-1280
References
- 1.
Miura M, Sakata Y, Nochioka K, Takada T, Tadaki S, Ushigome R, et al. Prevalence, predictors and prognosis of patients with heart failure requiring nursing care. Circ J 2014; 78: 2276–2283.
- 2.
Sakata Y, Shimokawa H. Epidemiology of heart failure in Asia. Circ J 2013; 77: 2209–2217.
- 3.
Ruiz-Canela M, Martinez-Gonzalez MA. Lifestyle and dietary risk factors for peripheral artery disease. Circ J 2014; 78: 553–559.
- 4.
Lee WH, Chu CY, Hsu PC, Su HM, Lin TH, Voon WC, et al. Comparison of antiplatelet and antithrombotic therapy for secondary prevention of ischemic stroke in patients with peripheral artery disease: Population-based follow-up study in Taiwan. Circ J 2013; 77: 1046–1052.
- 5.
European Stroke Organisation,
Tendera M, Aboyans V, Bartelink ML, Baumgartner I, Clément D, Collet JP, et al. ESC Guidelines on the diagnosis and treatment of peripheral artery diseases: Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries: The Task Force on the Diagnosis and Treatment of Peripheral Artery Diseases of the European Society of Cardiology (ESC). Eur Heart J 2011; 32: 2851–2906.
- 6.
Rooke TW, Hirsch AT, Misra S, Sidawy AN, Beckman JA, Findeiss LK, et al. 2011 ACCF/AHA Focused Update of the Guideline for the Management of Patients With Peripheral Artery Disease (updating the 2005 guideline): A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2011; 58: 2020–2045.
- 7.
Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG, et al. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). Eur J Vasc Endovasc Surg 2007; 33(Suppl 1): S1–S75.
- 8.
Aronow WS, Ahmed MI, Ekundayo OJ, Allman RM, Ahmed A. A propensity-matched study of the association of peripheral arterial disease with cardiovascular outcomes in community-dwelling older adults. Am J Cardiol 2009; 103: 130–135.
- 9.
Inglis SC, Bebchuk J, Al-Suhaim SA, Case J, Pfeffer MA, Solomon SD, et al. Peripheral artery disease and outcomes after myocardial infarction: An individual-patient meta-analysis of 28,771 patients in CAPRICORN, EPEHESUS, OPTIMAAL and VALIANT. Int J Cardiol 2013; 168: 1094–1101.
- 10.
Morillas P, Quiles J, Cordero A, Guindo J, Soria F, Mazon P, et al. Impact of clinical and subclinical peripheral arterial disease in mid-term prognosis of patients with acute coronary syndrome. Am J Cardiol 2009; 104: 1494–1498.
- 11.
Hebert K, Lopez B, Michael C, Franco E, Dias A, Trahan P, et al. The prevalence of peripheral arterial disease in patients with heart failure by race and ethnicity. Congest Heart Fail 2010; 16: 118–121.
- 12.
Inglis SC, Hermis A, Shehab S, Newton PJ, Lal S, Davidson PM. Peripheral arterial disease and chronic heart failure: A dangerous mix. Heart Fail Rev 2013; 18: 457–464.
- 13.
Jones WS, Clare R, Ellis SJ, Mills JS, Fischman DL, Kraus WE, et al. Effect of peripheral arterial disease on functional and clinical outcomes in patients with heart failure (from HF-ACTION). Am J Cardiol 2011; 108: 380–384.
- 14.
Inglis SC, McMurray JJ, Bohm M, Schaufelberger M, van Veldhuisen DJ, Lindberg M, et al. Intermittent claudication as a predictor of outcome in patients with ischaemic systolic heart failure: Analysis of the Controlled Rosuvastatin Multinational Trial in Heart Failure trial (CORONA). Eur J Heart Fail 2010; 12: 698–705.
- 15.
Ahmed MI, Aronow WS, Criqui MH, Aban I, Love TE, Eichhorn EJ, et al. Effects of peripheral arterial disease on outcomes in advanced chronic systolic heart failure: A propensity-matched study. Circ Heart Fail 2010; 3: 118–124.
- 16.
McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: The Framingham study. N Engl J Med 1971; 285: 1441–1446.
- 17.
Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 2006; 145: 247–254.
- 18.
McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology: Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2012; 14: 803–869.
- 19.
Nakamura Y, Yoshihisa A, Takiguchi M, Shimizu T, Yamauchi H, Iwaya S, et al. High-sensitivity cardiac troponin T predicts non-cardiac mortality in heart failure. Circ J 2014; 78: 890–895.
- 20.
Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, et al. Guidelines for the echocardiographic assessment of the right heart in adults: A report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 2010; 23: 685–713; quiz 786–788.
- 21.
O’Neill JO, Young JB, Pothier CE, Lauer MS. Peak oxygen consumption as a predictor of death in patients with heart failure receiving beta-blockers. Circulation 2005; 111: 2313–2318.
- 22.
Arena R, Myers J, Guazzi M. Cardiopulmonary exercise testing is a core assessment for patients with heart failure. Congest Heart Fail 2011; 17: 115–119.
- 23.
Ponikowski P, Francis DP, Piepoli MF, Davies LC, Chua TP, Davos CH, et al. Enhanced ventilatory response to exercise in patients with chronic heart failure and preserved exercise tolerance: Marker of abnormal cardiorespiratory reflex control and predictor of poor prognosis. Circulation 2001; 103: 967–972.
- 24.
Katz SD, Hryniewicz K, Hriljac I, Balidemaj K, Dimayuga C, Hudaihed A, et al. Vascular endothelial dysfunction and mortality risk in patients with chronic heart failure. Circulation 2005; 111: 310–314.
- 25.
Meguro T, Nagatomo Y, Nagae A, Seki C, Kondou N, Shibata M, et al. Elevated arterial stiffness evaluated by brachial-ankle pulse wave velocity is deleterious for the prognosis of patients with heart failure. Circ J 2009; 73: 673–680.
- 26.
Edelmann F, Stahrenberg R, Gelbrich G, Durstewitz K, Angermann CE, Dungen HD, et al. Contribution of comorbidities to functional impairment is higher in heart failure with preserved than with reduced ejection fraction. Clin Res Cardiol 2011; 100: 755–764.
- 27.
Mancini DM, Walter G, Reichek N, Lenkinski R, McCully KK, Mullen JL, et al. Contribution of skeletal muscle atrophy to exercise intolerance and altered muscle metabolism in heart failure. Circulation 1992; 85: 1364–1373.
- 28.
Duscha BD, Annex BH, Green HJ, Pippen AM, Kraus WE. Deconditioning fails to explain peripheral skeletal muscle alterations in men with chronic heart failure. J Am Coll Cardiol 2002; 39: 1170–1174.
- 29.
Dunlay SM, Weston SA, Redfield MM, Killian JM, Roger VL. Tumor necrosis factor-alpha and mortality in heart failure: A community study. Circulation 2008; 118: 625–631.
- 30.
Vaz Perez A, Doehner W, von Haehling S, Schmidt H, Zimmermann AV, Volk HD, et al. The relationship between tumor necrosis factor-alpha, brain natriuretic peptide and atrial natriuretic peptide in patients with chronic heart failure. Int J Cardiol 2010; 141: 39–43.
- 31.
Mehta NJ, Khan IA, Gupta V, Jani K, Gowda RM, Smith PR. Cardiac troponin I predicts myocardial dysfunction and adverse outcome in septic shock. Int J Cardiol 2004; 95: 13–17.
- 32.
Kerr G, Ray G, Wu O, Stott DJ, Langhorne P. Elevated troponin after stroke: A systematic review. Cerebrovasc Dis 2009; 28: 220–226.
- 33.
Kelley WE, Januzzi JL, Christenson RH. Increases of cardiac troponin in conditions other than acute coronary syndrome and heart failure. Clin Chem 2009; 55: 2098–2112.
- 34.
Murabito JM, Keyes MJ, Guo CY, Keaney JF Jr, Vasan RS, D’Agostino RB Sr, et al. Cross-sectional relations of multiple inflammatory biomarkers to peripheral arterial disease: The Framingham Offspring Study. Atherosclerosis 2009; 203: 509–514.
- 35.
Januszek R, Mika P, Konik A, Petriczek T, Nowobilski R, Nizankowski R. The effect of treadmill training on endothelial function and walking abilities in patients with peripheral arterial disease. J Cardiol 2014; 64: 145–151.
- 36.
Ebisawa S, Kashima Y, Miyashita Y, Yamazaki S, Abe N, Saigusa T, et al. Impact of endovascular therapy on oxidative stress in patients with peripheral artery disease. Circ J 2014; 78: 1445–1450.