2015 Volume 79 Issue 8 Pages 1699-1705
2015 Volume 79 Issue 8 Pages 1699-1705
Background: To validate the criteria for endovascular aneurysm repair (EVAR) or open repair of abdominal aortic aneurysm (AAA) at Nagoya University Hospital, the results of both treatments were retrospectively compared.
Methods and Results: Patient selection for EVAR was primarily based on suitable anatomy, minimum age 75 years, and significant comorbidity. From June 2007 to April 2014, 426 patients were treated via EVAR (EVAR group) and 346 patients were treated with open surgery (OS group). The mortality rates of the EVAR and OS groups were not significantly different (0.2% vs. 1.1%; P=0.33). Patient age, operation time, amount of bleeding, and duration of hospital stay were significantly lower in the EVAR group compared with the OS group. The incidence of comorbidity was higher in the EVAR group compared with the OS group. The incidence of early postoperative complications was significantly higher in the OS group, whereas the incidence of late complications for both groups was similar. The cumulative aneurysm-related survival rates were similar (98.9% vs. 98.5%; P=0.767). The cumulative survival rates and reintervention-free rates at 5 years were lower for the EVAR group (76% vs. 89%, P=0.019; 81% vs. 89%, P=0.046).
Conclusions: Patient selection practices and criteria for EVAR and open repair at Nagoya University Hospital are generally acceptable. (Circ J 2015; 79: 1699–1705)
Endovascular aneurysm repair (EVAR) affects outcome of abdominal aortic aneurysm (AAA) treatment. An AAA may rupture, with frequent fatal consequences. In large-scale randomized trials, EVAR has reduced short-term mortality compared with open repair.1,2 Elderly patients may be treated due to the low perioperative mortality rate and acceptable mid-term outcome.3,4 EVAR for large infrarenal AAA in anatomically suited high-risk surgical patients is safe and provides lasting protection against AAA-related mortality.5 Several publications regarding long-term results for EVAR are available.1,2,6,7 In these trials no differences were seen in the long-term rates of mortality or aneurysm-related mortality between EVAR and open repair.7,8 EVAR is more cost-effective than open repair.3,7,8 EVAR is associated with more device-related complications; therefore, patients treated with EVAR will likely require reintervention.8 These findings, however, were obtained from a prospective controlled study and the indication for EVAR and open repair in the treatment of AAA was not adequately verified on a real clinical basis.
Because the indications for EVAR pertain to high-risk and anatomically suitable patients, open repair is suggested for anatomically complicated patients. Although the treatment of more patients with open repair in the era of EVAR has become technically difficult, the results have not been adequately investigated. To validate the indication for EVAR and open repair procedures for AAA, we retrospectively compared the initial results with the incidence of postoperative complications, and the mid- and long-term results between EVAR and open surgery (OS).
Between June 2007 and April 2014, 782 AAA were treated via elective surgery at Nagoya University Hospital; the cases were identified using a collected database. All data were analyzed via a retrospective observational study by 3 vascular surgeons in accordance with the Nagoya University Ethics Committee standards. All patients provided informed consent. Maximum shortest AAA diameter >5.0 cm on computed tomography (CT) was considered an indication for surgery.9 Suprarenal AAA, infectious AAA, and cases involving rupture were not included in the study group. Five cases of juxta-renal AAA that were treated via fenestrated EVAR or the chimney technique were excluded from this study. One patient with occluded iliac artery was treated with aorto-uni-iliac stent graft without femoro-femoral bypass. A total of 426 patients were treated via EVAR (EVAR group) and 348 were managed with OS (OS group).
The selection criteria for EVAR included suitable anatomy, minimum age 75 years, significant comorbidity, high surgical risk and prior history of major abdominal surgery. The criteria for OS repair were maximum age 74 years, low surgical risk, or unsuitable anatomy for EVAR, particularly with a neck length <10 mm or a neck in which the entire circumference was spanned by calcifications or thrombi. EVAR was performed in 95 patients with neck angulation >60° and in 15 patients with neck length 10–14 mm. Twenty-one patients underwent bilateral hypogastric embolization due to an unsuitable landing zone of common iliac arteries. A total of 131 patients (30.6%) received EVAR outside of the indications for use.
The stent grafts implanted in the EVAR group were as follows: Zenith (Cook, Bloomington, IN, USA) in 147 patients, Excluder (WL Gore, Flagstaff, AZ, USA) in 167 patients, Talent (Medtronic, Santa Rosa, CA, USA) in 5 patients, Endurant (Medtronic) in 79 patients, Powerlink (Endologix, Irvine, CA, USA) in 17 patients, and other clinical trial devices in 10 patients. The devices were selected due to their specific characteristics and surgeon preference. The implanted prostheses in the OS group were as follows: Gelsoft (Vascutek, Scotland, UK) in 65 patients, Hemashield (Boston Scientific, New York, NY, USA) in 192 patients, J graft shield (Japan Lifeline, Tokyo Japan) in 28 patients, Triplex (Terumo, Tokyo, Japan) in 57 patients, and other clinical trial prostheses in 6 patients.
Coronary artery disease was defined as previously diagnosed myocardial infarction or ongoing angina. Cerebrovascular disease included all forms of stroke, including transient ischemic attack. Lung disease included chronic obstructive pulmonary disease, which was defined as forced expiratory volume in 1 s (FEV1) <60% (this value was calculated by dividing FEV1 by the forced vital capacity [FVC]), and a prior history of asthma. Heart disease included heart failure prior to surgery, any form of cardiac valve disease, and arrhythmia that required treatment. Renal disease included treatment via hemodialysis for chronic renal failure and abnormal serum creatinine (>2.0 mg/dl). Malignancy included any form of malignant disease, irrespective of whether the disease was in remission. Preoperative serum blood urea nitrogen (BUN) and creatinine were compared to evaluate preoperative renal function. Ejection fraction (EF) was measured on echocardiography to determine cardiac function and vital capacity/predicted vital capacity (%VC), and FEV1/FVC (FEV1%) was determined via spirometry as a measure of respiratory function. These were compared between the groups. Aneurysm diameter was defined using the maximum minor axis diameter on axial CT for fusiform aneurysms and the maximum major axis diameter for saccular aneurysms.10
The technical success of EVAR was defined as successful stent graft deployment without either a type I or type III endoleak. Patients were monitored as outpatients for 3–130 months (mean, 26.5 months) after surgery.
Operative variables, such as operation time, amount of bleeding, and hospital stay duration, were compared. Early postoperative complications were defined as any type of postoperative morbidity that required surgical or medical treatment during hospital stay. Late postoperative complications were defined as any complications that were diagnosed after discharge. Early postoperative complications, such as pneumonia, heart failure, renal failure, arrhythmia, wound infection, postoperative bleeding, delirium, cerebrovascular disease, graft infection, graft limb occlusion, ischemic enteritis, or intestinal obstruction, were noted in both groups; other complications were also described. Operative death was defined as any death within 30 days of surgery in both groups; all types of endoleaks were encountered in the EVAR group.
EVAR patients were primarily monitored in the outpatient clinic, and abdominal CT with contrast was performed at 3, 6, and 12 months after surgery and each year thereafter, depending on the patients’ health. Follow-up for the OS group entailed physical examination and abdominal CT, if available. The incidence of late postoperative complications was determined via medical records.
Statistical AnalysisData were analyzed using SPSS for Windows (version 13.0, Chicago, IL, USA). Patient demographics, co-morbidities, perioperative events and outcomes were compared between the OS group and the EVAR group. Categorical variables were analyzed using the chi-squared or Fisher exact tests; continuous variables were analyzed using Student’s t-test for parametric data. Kaplan-Meier analysis was performed to study long-term survival rates, reintervention-free rates, and aneurysm-related survival rates. P<0.05 was considered statistically significant.
Eighty-three patients (23.9%) from the OS group underwent suprarenal aortic clamping. To treat AAA with involvement of the common iliac arteries or the short common iliac arteries, the hypogastric arteries of the EVAR group were embolized using embolic coils. A total of 116 patients (27.2%) underwent unilateral hypogastric preoperative or intraoperative embolization. Twenty-one patients (4.9%) underwent bilateral hypogastric embolization at separate times during the preoperative period. Twelve patients (2.8%) underwent unilateral hypogastric pre- or intraoperative embolization with reconstruction of the contralateral hypogastric artery. No patients underwent embolization of the hypogastric arteries in the OS group.
Mean patient age in the EVAR group was significantly higher than in the OS group (77.9 vs. 70.7 years old, P<0.001). No statistically significant differences between the EVAR group and the OS group were observed regarding sex, aneurysm diameter, and the presence of hypertension, hyperlipidemia, diabetes mellitus, coronary artery disease, heart disease, or renal disease. The proportion of patients in the EVAR group with cerebrovascular disease, lung disease, and malignancy was significantly higher than that in the OS group (16.4% vs. 9.5%, P=0.016; 13.8% vs. 8.0%, P=0.015; 25.6% vs. 14.9%, P<0.001, respectively; Table 1). No significant differences in serum BUN, creatinine, or EF were observed, but there were significant differences in %VC and FEV1% between the groups (103.7% vs. 108.3%, P=0.002; 68.7% vs. 71.1%, P=0.013; Table 2). The follow-up rate for patients in the EVAR and OS groups who were seen at least once as outpatients was 94.1% and 97.1%, respectively.
| EVAR group (n=426) | OS group (n=348) | P-value | |
|---|---|---|---|
| Age (years) | 77.9±6.2 | 70.7±7.3 | <0.001* |
| Sex (M/F) | 72/354 | 52/296 | 0.485 |
| Aneurysm diameter (mm) | 53.2±1.5 | 53.4±9.3 | 0.800 |
| Hypertension | 303 (71.1) | 260 (74.7) | 0.515 |
| Hyperlipidemia | 174 (40.8) | 156 (44.8) | 0.137 |
| Diabetes mellitus | 47 (11.0) | 52 (44.8) | 0.234 |
| CAD | 141 (33.1) | 113 (32.5) | 0.370 |
| CVD | 70 (16.4) | 33 (9.5) | 0.016* |
| Lung disease | 59 (13.8) | 28 (8.0) | 0.015* |
| Heart disease | 31 (33.1) | 25 (7.2) | 0.140 |
| Renal disease | 17 (3.9) | 18 (5.2) | 0.490 |
| Malignancy | 109 (25.6) | 52 (14.9) | <0.001* |
Data given as mean±SD or n (%). *Statistically significant difference. CAD, coronary artery disease; CVD, cerebrovascular disease; EVAR, endovascular aneurysm repair; OS, open surgery.
| EVAR group (n=426) | OS group (n=348) | P-value | |
|---|---|---|---|
| BUN (mg/dl) | 19.4±7.0 | 18.7±7.2 | 0.15 |
| Cr (mg/dl) | 1.10±0.98 | 1.07±0.83 | 0.64 |
| EF (%) | 63.8±10.8 | 64.2±11.9 | 0.62 |
| %VC (%) | 103.7±21.2 | 108.3±18.3 | 0.002* |
| FEV1% (%) | 68.7±14.2 | 71.1±11.5 | 0.013* |
| Operation time (min) | 156.4±55.5 | 240.5±66.6 | <0.001* |
| Bleeding (g) | 209.0±224.5 | 2,075.3±1,823.2 | <0.001* |
| Postoperative hospital stay (days) | 7.8±7.3 | 15.9±18.9 | <0.001* |
| Mortality | 1 (0.2) | 3 (1.1) | 0.33 |
Data given as mean±SD or n (%). *Statistically significant difference. BUN, blood urea nitrogen; Cr, creatinine; EF, ejection fraction; FEV1%, forced expiratory volume in 1 s/forced vital capacity; %VC, vital capacity/predicted vital capacity. Other abbreviations as in Table 1.
Operation time, amount of bleeding, and duration of postoperative hospital stay were significantly lower in the EVAR group compared with the OS group (156.4 min vs. 240.5 min, P<0.001; 209.0 g vs. 2,075.3 g, P<0.001; 7.9 days vs. 16.0 days, P<0.001; Table 2).
Several types of postoperative complication occurred in both groups. Compared with the EVAR group, the OS group had a significantly higher rate of early postoperative complications, such as arrhythmia, renal insufficiency, delirium, ischemic enteritis, and intestinal obstruction (1.2% vs. 3.7%, P=0.029; 0.9% vs. 3.1%, P=0.035; 2.1% vs. 6.2%, P<0.001; 0.2% vs. 3.1%, P=0.003; 0% vs. 1.5%, P<0.001, respectively). One patient with renal insufficiency in the OS group required permanent hemodialysis. Three patients with ischemic enteritis underwent intestinal resection due to necrosis or stricture of their intestines. Each case of graft infection was treated via open surgical graft replacement using a new bifurcated prosthesis. No significant differences between the 2 groups were observed in the incidences of pneumonia, heart failure, wound infection, postoperative bleeding, cerebrovascular disease, graft infection, or graft limb occlusion (Table 3).
| EVAR group (n=426) | OS group (n=348) | P-value | |
|---|---|---|---|
| Pneumonia | 7 (1.6) | 10 (2.9) | 0.32 |
| Heart failure | 6 (1.4) | 4 (1.2) | 1.0 |
| Arrhythmia | 5 (1.2) | 13 (3.7) | 0.029* |
| Renal failure | 4 (0.9) | 11 (3.1) | 0.035* |
| Hemodialysis | 0 (0) | 1 (0.3) | 0.45 |
| Liver dysfunction | 0 (0) | 7 (2.1) | 0.04* |
| Wound infection | 2 (0.5) | 5 (1.5) | 0.25 |
| Post-procedure bleeding | 5 (1.2) | 6 (1.8) | 0.59 |
| Delirium | 9 (2.1) | 27 (6.2) | <0.001* |
| CVD | 2 (0.5) | 1 (0.3) | 1.00 |
| Graft infection | 1 (0.2) | 1 (0.3) | 1.0 |
| Graft limb occlusion | 2 (0.5) | 2 (0.6) | 0.872 |
| Ischemic enteritis | 1 (0.2) | 10 (3.1) | 0.003* |
| Resection of intestine | 0 (0) | 3 (0.9) | 0.051 |
| Intestinal obstruction | 0 (0) | 10 (1.5) | <0.001* |
| GI bleeding | 0 (0) | 2 (0.3) | 0.125 |
| Limb ischemia | 1 (0.2) | 4 (1.2) | 0.182 |
Data given as n (%). *Statistically significant difference. GI, gastrointestinal. Other abbreviations as in Table 1.
Postoperative CT during admission indicated 6 type I endoleaks and 2 type III endoleaks: 6 endoleaks were treated via insertion of additional stent grafts, but 2 type I endoleaks remained untreated at discharge; therefore, the total technical success rate of EVAR at discharge was 99.5%. One patient in the EVAR group underwent open conversion because the delivery system could not be retrieved. The mortality rate in the EVAR group was 0.2% (1/426), whereas the mortality rate in the OS group was 1.1% (4/348): these rates were not significantly different. The cause of death in the EVAR group was sudden death due to cardiac insufficiency, whereas the primary causes of death in the OS group were renal failure after a thrombectomy; pseudoaneurysm rupture at the proximal anastomosis; multi-organ failure due to intestinal necrosis; and subarachnoid hemorrhage.
The rates of incisional hernia and pseudoaneurysm were significantly higher in the OS group compared with the EVAR group (0.2% vs. 10.3%, P<0.001; 0% vs. 1%, P=0.042; EVAR vs. OS, respectively). Graft infection and graft occlusion developed in 1 patient (0.2%) and 4 patients (0.9%), respectively, in the EVAR group, and in 3 patients (0.9%) and 3 patients (0.9%), respectively, in the OS group; this difference was not statistically significant (Table 4).
| EVAR group (n=426) | OS group (n=348) | P-value | |
|---|---|---|---|
| Incisional hernia | 1 (0.2) | 36 (10.3) | <0.001* |
| Pseudoaneurysm | 0 (0) | 4 (1.1) | 0.042* |
| Graft infection | 1 (0.2) | 3 (0.9) | 0.334 |
| Graft occlusion | 4 (0.9) | 3 (0.9) | 0.62 |
| Endoleak (type I, III) | 19 (4.5) | ||
| Endoleak type II with sac enlargement† | 12 (2.8) | ||
| Sac enlargement‡ | 5 (1.1) | ||
| Total | 40 | 46 | 0.10 |
Data given as n (%). *Statistically significant difference. †Endoleak with sac enlargement >5 mm. ‡Reason not specified. Aabbreviations as in Table 1.
Late postoperative complications in the EVAR group were primarily due to stent graft problems. Additional proximal stent grafts were inserted in 5 patients with type Ia endoleak. One patient with type Ia endoleak underwent replacement of the endograft with a bifurcated graft (Table 5). A type II endoleak was detected in 36% (94/261) of the patients at 12 months, 40% (70/175) of the patients at 24 months, 41% (52/127) of the patients at 36 months, 39% (26/67) of the patients at 48 months, and 41% (12/39) of the patients at 60 months, all of whom were followed on contrast CT. Ten of 12 patients whose aneurysm sac diameter enlarged by >5 mm compared with the preoperative measurement underwent transarterial embolization (Table 5). The aneurysm sac in 4 patients was embolized using embolic coils and n-butyl-2-cyanoacrylate (NBCA) via the inferior mesenteric artery (IMA); the aneurysm sac in 4 patients was embolized via the iliolumbar artery, which branches off from the hypogastric artery. The iliolumbar artery in 2 patients was embolized using coils because the catheter could not reach the aneurysm sac via the iliolumbar artery. Sac shrinkage was observed in 127 patients (44.4%) at 12 months and in 52% of patients (108/207) at 24 months on CT.
| Type of complication | Additional procedures | No. patients |
|---|---|---|
| Type Ia endoleak | Additional proximal stent graft | 5 |
| Bifurcated graft replacement | 1 | |
| Type Ib endoleak | Additional distal stent graft | 3 |
| Additional distal stent graft+IIA embolization | 6 | |
| Type II endoleak | Transarterial embolization | 10 |
| Type III endoleak | Redo EVAR | 1 |
| Additional stent graft | 3 | |
| Infection | Bifurcated graft replacement | 1 |
| Removal of coil | 1 | |
| Limb occlusion | Femoro-femoral bypass | 2 |
| Thrombectomy+bare stent | 2 |
EVAR, endovascular aneurysm repair.
Open repair was performed in 3 EVAR patients. One patient developed a stent graft infection and subsequently underwent removal of the infected stent graft, which was replaced with a new prosthetic bifurcated graft. The second patient underwent stent graft replacement with a new prosthesis due to a type I endoleak, and the third patient underwent removal of an infected embolized coil at the hypogastric artery (Table 5). Two patients suffered ruptured aneurysms, and 1 patient survived after developing a type Ib endoleak, which was successfully treated via hypogastric embolization and extension of the distal limb to the external iliac artery. Another case of aneurysm rupture was treated via placement of aortic cuffs on the body of a stent graft due to suspicion of graft material tear; the affected patient did not survive. On autopsy a graft tear from the sewing site to the location of the stent was seen.
The cumulative survival rate in the OS group at 5 years was 89%, whereas that in the EVAR group was 76%; this difference was statistically significant (Figure 1). The cumulative reintervention-free rates at 5 years were 89% and 75%; this difference was statistically significant (Figure 2). The cumulative aneurysm-related survival rates were 98.8% and 98.5%; this difference was not statistically significant (Figure 3).
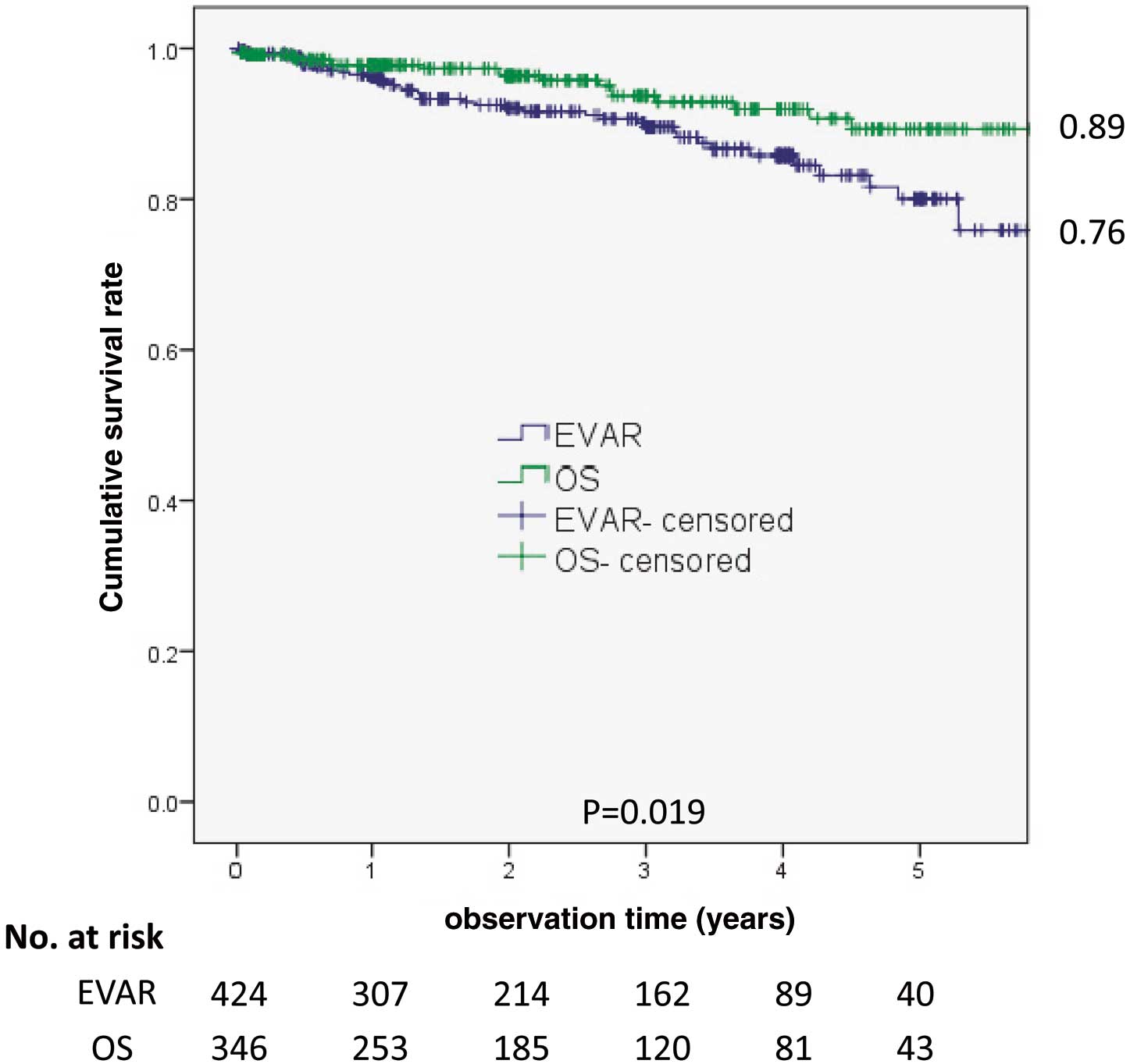
Cumulative survival rates. The cumulative survival rate was significantly lower for the endovascular aneurysm repair (EVAR) group. OS, open surgery.

Cumulative aneurysm-related survival rate. No significant differences between the 2 groups were observed. EVAR, endovascular aneurysm repair; OS, open surgery.

Cumulative reintervention-free rate. The cumulative reintervention-free rate was significantly higher for the open surgery (OS) group. EVAR, endovascular aneurysm repair.
The present retrospective analysis demonstrated better outcomes for EVAR compared with OS in terms of early postoperative morbidity and mortality, but EVAR patients were older and had more complications. The early postoperative complications and duration of postoperative hospital stay for the EVAR group were lower than that for the OS group. Complications in the EVAR group were primarily related to stent grafts and were subsequently treated via endovascular intervention without difficulty. Complications noted in the OS group, however, included numerous severe conditions that required complicated treatment, including OS procedures. The usefulness of EVAR was verified in elderly and high-risk patients with AAA.
Ten patients (3.1%) in the OS group developed ischemic enteritis; 3 of these patients underwent bowel resection due to intestinal necrosis or stricture. One patient did not survive as a result of multiple organ failure after surgery. Conversely, only 1 case (0.3%) of ischemic colitis was observed in the EVAR group, which was similar to examples noted in previous publications. The US database study showed that ischemic colitis occurred after open elective repair (1.9%) more frequently than after EVAR (0.5%; both P <0.001).11 The incidence of ischemic colitis after elective open AAA repair ranges between 1% and 6% and is associated with increased mortality.12,13 Although replanting the IMA to prevent ischemic colitis has been discussed for many years, no definite conclusions about its role have been reached.14 Our policy to reconstruct the IMA is based on the fact that blood flow was not detected in the marginal artery of the sigmoid mesocolon after revascularization. The present relatively higher incidence of severe ischemic enteritis indicates that our policy to reconstruct the IMA did not facilitate prevention of this disease.
Delirium is a common postoperative complication, particularly in the setting of high-risk surgery, and is associated with age, lifestyle, and mental health.15 The incidence of delirium was higher in the OS group than in the EVAR group, regardless of the former’s lower average age. This finding supports the greater degree of invasiveness of OS, as demonstrated by the longer operation time and higher blood loss.
Incisional hernia, usually a rare, late, postoperative complication, did not occur in the OS group. The cumulative incidence of hernia repair after aortic reconstructive surgery was 10.4% after 6 years. Body mass index and AAA repair are each significantly associated with incisional hernia repair.16 Systemic connective tissue defects may affect the structural integrity of the aortic and abdominal walls.17
Late complications in the EVAR group were primarily related to an endoleak and graft occlusion. Our experiences regarding type III endoleak, including graft fabric tears, are published elsewhere.18 Five type Ia endoleaks were successfully treated via endovascular procedures, and 1 patient was treated via replacement of the endograft with a bifurcated graft in an open procedure. Type II endoleak is reportedly more common, the incidence of which varies between 10% and 44%, which is consistent with the present results.16 Currently, no definite criteria regarding type II endoleak management exist. Treatment should be reserved for persistent endoleak, which causes sac enlargement >5 mm compared with the preoperative diameter. We attempted embolization of type II endoleak in 10 patients using a percutaneous transarterial approach. Complete sac embolization was achieved in 5 patients, 4 patients required 2 attempts, and 1 patient required 3 embolizations. Endoleaks persisted in 5 of 10 patients. We used only embolic metal coils but the embolizations were incomplete in the setting of sac thrombosis. We use liquid embolic substances and embolic coils and are assisted by skillful radiologists but the technique remains complicated and challenging.
Thirty-day operative mortality rates after AAA repair were decreased in the EVAR group compared with the OS group.1,2,19,20 In the EVAR era, AAA patients with more complicated anatomy have been treated via OS because suprarenal aortic clamping is required in >20% of patients. The mortality rate was only 1.1% in the OS group, which appears to be superior to the mortality rates noted in previous publications.21 Numerous early postoperative complications occurred in the OS group, but the majority of these complications were manageable and curable.
Differences in long-term all-cause mortality were not observed, but previous studies have reported that aneurysm-related mortality rate were significantly lower in the EVAR group after 6 years.19 Stather et al undertook a large meta-analysis and reported that EVAR and open repair were equivalent with respect to all-cause mortality at 2 years, 4 years, and longer periods after surgery and with respect to late AAA-related mortality.22 In the present study, the cumulative survival rate was lower in the EVAR group compared with the OS group, which can be explained by the older age of the EVAR group. No differences in aneurysm-related survival rate between the 2 groups were observed, which indicates that EVAR is as tolerable as OS with regard to mid- and long-term follow-up. Additional interventions, however, are sometimes necessary to maintain aneurysm stability. Although the OS group was associated with numerous types of morbidity, the mortality rate was low, suggesting that the OS group’s combination of younger patients and older complicated patients yields excellent initial and long-term results.
Although stent grafts have facilitated the treatment of complicated anatomical conditions via endovascular procedures, open repair for AAA remains an important treatment strategy. Long-term surveillance and additional interventions are mandatory among specific types of EVAR patients. Periodic examinations, which are performed primarily via CT, are expensive and expose patients to radiation. In addition, long-term results beyond 10 years remain unknown. Therefore, we believe that open repair remains the gold standard for young patients and low-risk patients.
This study has limitations because it was a retrospective and non-randomized controlled study that may have been affected by biases related to comparisons of the results of each procedure. The EVAR procedures included our initial experiences with the procedure. These experiences may have resulted in increased morbidity, which decreased as the learning curve for the procedure became less steep. Our patient selection practices and criteria for EVAR and open repair were generally acceptable in this setting.
None.