2015 Volume 79 Issue 8 Pages 1828-1834
2015 Volume 79 Issue 8 Pages 1828-1834
Background: Although amiodarone (AMD)-induced thyroid dysfunction (AITD) is an important complication of AMD therapy, little is known about AITD in adult Japanese patients with congenital heart disease (CHD).
Methods and Results: We retrospectively studied 131 adult patients with CHD who were on low-dose AMD (median, 150 mg/day). The median patient age was 28 years, and the median follow-up was 44 months. The incidence, clinical course, and risk factors of AITD, including AMD-induced thyrotoxicosis (AIT) and AMD-induced hypothyroidism (AIH), were evaluated. The total incidence of AITD was 30% (AIT: 18%, n=24; AIH: 12%, n=16). Approximately 67% of patients with AIT displayed deterioration of tachyarrhythmia, and 38% patients underwent steroid therapy. Although thyroid function and symptoms associated with AIT improved within 6 months after diagnosis in most patients with AIT (92%), 1 patient died suddenly during an acute phase of AIT. No patient with AIH exhibited deterioration of tachyarrhythmia, and 9 patients underwent thyroid hormone replacement therapy. Cox multivariate analysis identified no independent risk factor for AIT, whereas liver dysfunction (hazard ratio 2.573; 95% confidence interval 1.102–5.795) was an independent risk factor for AIH.
Conclusions: AITD commonly occurred in adult Japanese patients with CHD even though they were on a low-dose AMD regimen. Risk factors for AITD may vary according to ethnicity and diet. (Circ J 2015; 79: 1828–1834)
The development of atrial or ventricular tachyarrhythmia is a severe complication in adult patients with congenital heart disease (adult CHD), because tachyarrhythmias are often associated with adverse cardiac events such as heart failure, stroke, and sudden cardiac death.1 Amiodarone (AMD) is one of the most effective antiarrhythmic agents used to control both atrial and ventricular arrhythmias in adult CHD,2 but it has a wide range of side effects, including AMD-induced thyroid dysfunction (AITD).3 AMD is an iodine-rich agent containing 39% iodine by weight. Chronic exposure to high levels of iodine in plasma and tissue results in the development of AITD.4 AMD-induced thyrotoxicosis (AIT) is associated with an increased risk of death and adverse cardiovascular events.5,6 AMD-induced hypothyroidism (AIH) presents with nonspecific symptoms and signs such as fatigue, cold intolerance, mental sluggishness, and dry skin, similar to the symptoms of spontaneous hypothyroidism.7 In populations with CHD, female sex, complex cyanotic heart disease, and AMD dose >200 mg/day have been reported as risk factors for AITD.8 Previous Fontan-type surgery, a low body mass index (BMI), and cyanosis have been reported as risk factors for AIT in patients with CHD.8,9 The incidence of AITD differs according to iodine intake, and it might also vary among different ethnic groups and geographic areas. Little is known about AITD in Japanese adults with CHD, so the purpose of the present study was to investigate the incidence, clinical course, and risk factors of AITD, including AIT and AIH, in Japanese adults with CHD.
This was a single-center, retrospective study. We studied 131 adult patients (age ≥18 years) with CHD who had received AMD for at least 3 months. The medical records of patients at Tokyo Women’s Medical University between November 1999 and April 2012 were reviewed. The cardiac diagnoses of all participating subjects are shown in Table 1 and their baseline characteristics are summarized in Table 2. The median age of patients at the start of AMD treatment was 28 years, and the median follow-up was 44 months. The indications for AMD were atrial tachyarrhythmia (n=86, 66%) and ventricular tachycardia (n=45, 34%). The median (range) maintenance dose of AMD was 100 mg/day (50–300 mg/day). To monitor thyroid function, we measured serum levels of free thyroxine (free T4), free triiodothyronine (free T3), and thyroid-stimulating hormone (TSH) every 1–6 months using commercially available kits. The median (range) levels of TSH, free T3, and free T4 before AMD administration were 3.01 μU/ml (0.43–12.3), 2.77 pg/ml (1.05–5.57), and 1.40 ng/dl (0.84–3.39), respectively. One patient with hypothyroidism had previously received thyroid hormone replacement therapy before AMD therapy, so was excluded from the AIT group. One patient had a past history of hyperthyroidism, which was cured several years before AMD treatment. One patient was suspected of having subclinical hypothyroidism, but maintained a TSH level <20 μU/ml after AMD treatment. The plasma concentrations of AMD and its active metabolite, N-monodesethylamiodarone, were measured using high-performance liquid chromatography at the time of diagnosis of thyroid dysfunction in the AIT and AIH groups and at last follow-up in the euthyroid group.
| Total patients (n=131) | |
|---|---|
| Fontan-type surgery, n | 43 |
| Complex CHD, biventricular repair, n | 16 |
| CTGA, n | 15 |
| ASD/VSD/AVSD/MVD, biventricular repair, n | 11 |
| TOF, post cardiac repair, n | 10 |
| TGA post atrial switch operation, n | 8 |
| CoA or aortic valve disese, n | 2 |
| Not operated or palliated CHD, n | 26 |
ASD, atrial septal defect; AVSD, atrioventricular septal defect; CHD, congenital heart disease; CoA, coarctation of the aorta; CTGA, corrected transposition of great arteries; MVD, mitral valve disease; TGA, transposition of great arteries; TOF, tetralogy of Fallot; VSD, ventricular septal defect.
| Overall patients | Euthyroid group | AIT group | AIH group | |
|---|---|---|---|---|
| Baseline | ||||
| n (%) | 131 (100) | 91 (70) | 24 (18) | 16 (12) |
| Men, n (%) | 83 (63) | 60 (66) | 16 (42) | 7 (43) |
| Age at last follow-up (years) | 33 (18–55) | 31 (18–54) | 34 (18–55) | 34 (23–55) |
| Age at AMD started (years) | 28 (18–55) | 29 (18–55) | 26 (18–52) | 30 (20–54) |
| Duration of AMD therapy (months) | 44 (3–225) | 39 (3–153) | 72 (24–225) | 60 (27–168) |
| Time at onset of AIT/AIH after AMD started (months) | 39 (18–110) | 10 (3–80) | ||
| Maintenance dose of AMD (mg/day) | 100 (50–300) | 100 (50–300) | 100 (50–200) | 100 (50–200) |
| Arrhythmia | ||||
| Atrial tachyarrhythmia, n (%) | 86 (66) | 60 (65) | 17 (71) | 9 (56) |
| Ventricular tachyarrhythmia, n (%) | 45 (34) | 31 (35) | 7 (29) | 7 (44) |
| Prior Fontan surgery, n (%) | 43 (32) | 29 (32) | 8 (33) | 6 (37) |
| Cyanosis, n (%) | 42 (32) | 27 (30) | 7 (29) | 8 (50) |
| NYHA functional | ||||
| Class I–II, n (%) | 106 (81) | 74 (81) | 21 (88) | 11 (70) |
| Class III–IV, n (%) | 25 (19) | 17 (19) | 3 (12) | 5 (31) |
| BMI at initiation of AMD administration | 20 (14–28) | 20 (15–28) | 20 (16–28) | 19 (14–27) |
| Cardiac dysfunction, n (%) | 48 (37) | 34 (37) | 6 (25) | 8 (50) |
| Liver dysfunction (T-Bil ≥2 mg/dl), n (%) | 13 (10) | 6 (7) | 2 (8) | 5 (31)* |
| Follow-up data | ||||
| Plasma concentration | ||||
| AMD (μg/ml) | 0.42 (0.10–1.72) | 0.41 (0.15–1.22) | 0.43 (0.10–1.36) | 0.44 (0.21–1.72) |
| Desethylamidarone (μg/ml) | 0.41 (0.13–1.04) | 0.37 (0.20–1.04) | 0.51 (0.14–0.89) | 0.51 (0.13–0.89) |
| Cardiovascular death during follow-up, n (%) | 21 (16) | 16 (17) | 2 (8) | 3 (19) |
Values are expressed as median (range), *P<0.05 vs. euthyroid group. AIT, amiodarone-induced thyrotoxicosis; AIH, amiodarone-induced hypothyroidism; AMD, amiodarone; BMI, body mass index; CHD, congenital heart disease; NYHA, New York Heart Association; T-Bil, total bilirubin.
First, we investigated the incidence of AITD. Second, the subjects were divided into 3 groups: euthyroid, AIT, and AIT groups. We evaluated the clinical course and risk factors of AITD in Japanese adults with CHD.
AMD RegimenIn our typical treatment regimen, oral AMD was usually started at a loading dose of 400 mg/day for 3–7 days followed by a dose of 200 mg/day for several weeks. The loading or maintenance dose was adjusted on the basis of the clinical situation. The chronic maintenance dose of oral AMD usually ranged from 100 to 200 mg/day. We typically use a low-dose AMD regimen of less than 200 mg daily.
Diagnosis of AITDAITD comprises AIT and AIH. AIT was defined as the presence of a serum TSH level <0.1 μU/ml (normal range, 0.38–4.30 μU/ml), in combination with the upper limit of normal or high serum levels of free T3 (normal range, 2.4–4.00 pg/ml) and free T4 (normal range, 0.94–1.60 ng/dl). AIT was defined as type II if 2 of 3 criteria (no underlying thyroid disease, absence of goiter with negative thyroid antibodies, and low vascularity on thyroid Doppler flow) were met. If none of these criteria was met, patients were diagnosed with AIT type I. AIH was defined as a serum TSH level ≥20 μU/ml in combination with a low serum level of free T4.
Statistical AnalysisData regarding patient characteristics are expressed as the number (%) or median (range). Baseline characteristics were compared among the patients without thyroid dysfunction (the euthyroid group), AIT (the AIT group), and AIH (the AIH group) using the Mann-Whitney test, 1-way ANOVA, or the chi-squared test. Risk factors associated with AITD were analyzed using multivariate logistic regression analysis or a Cox proportional hazard model. Factors that were previously reported as risk factors for AITD such as male sex, age at the start of AMD therapy (years), maintenance dose of AMD (mg), prior Fontan surgery, cyanosis, liver dysfunction defined as a serum total bilirubin level (T-Bil) ≥2 mg/dl, New York Heart Association (NYHA) functional class ≥III, BMI at the start of AMD administration, cardiac dysfunction, plasma AMD concentration, and plasma concentration of N-monodesethylamiodarone were entered into the analysis. Cardiac dysfunction was defined as a left ventricular ejection fraction (EF) <50% or right ventricular EF <45% on echocardiography, cardiac magnetic resonance imaging, cardiac scintigraphy, or cardiac catheterization. JMP (version 11; SAS Institute, Cary, NC) was used for all analyses. A P value less than 0.05 was considered significant.
The incidence of AIT and AIH was 18% (n=24) and 12% (n=16), respectively. The incidence of AITD was thus 30% (n=40) (Table 2). Kaplan-Meier analysis showing the event-free survival curves of AIT and AIH is illustrated in Figure 1. The median time (range) to onset after the start of AMD therapy was 39 months (range, 18−110 months) for AIT and 10 months (range, 3−80 months) for AIH. All patients with AIT had type II. The proportion of patients with NYHA class ≥III in the AIT group was higher than in the AIT and euthyroid groups, although the differences were not statistically significant. The percentage of patients with liver dysfunction (T-Bil ≥2 mg/dl) in the AIH group was significantly higher than in the euthyroid group (P<0.05). Male sex, age at the start of AMD, duration of AMD treatment, maintenance dose of AMD, atrial or ventricular tachycardia, prior Fontan surgery, cyanosis, BMI, and the presence of cardiac dysfunction were not significantly different among the groups. In the AIT group, the median (range) TSH, free T3, and free T4 levels were 0.01 μU/ml (0.005–0.05), 8.02 pg/ml (2.61–19.2), and 4.82 ng/dl (2.21–7.77), respectively. The median (range) TSH, free T3, and free T4 levels in the AIH group were 27.0 μU/ml (20.2–145.7), 1.66 pg/ml (1.09–2.71), and 1.08 ng/dl (0.51–1.64), respectively.
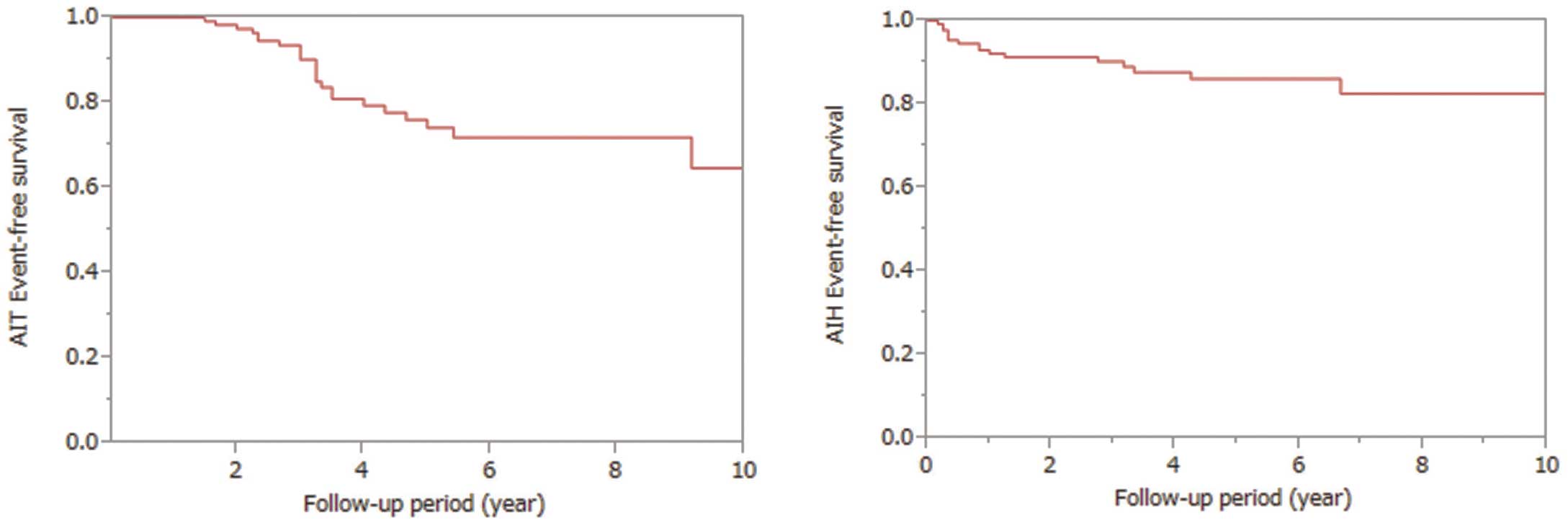
Event-free survival in the AIT (Left) and AIH (Right) groups. AIH, amiodarone-induced hypothyroidism; AIT, amiodarone-induced thyrotoxicosis.
The clinical course of patients with AIT is shown in Figure 2; 83% (n=20) of patients had symptoms associated with AIT such as palpitations and tachyarrhythmia, including 16 patients who displayed deterioration of tachyarrhythmia that led to DC shock or heart failure. The remaining 4 patients with symptoms had palpitations only, and there were 4 patients without symptoms. There were 3 patients who discontinued AMD therapy, and 21 who continued with the therapy. A β-blocker was added or the daily dose was increased in 14 patients; 5 patients were given antithyroid drugs, namely methimazole in 4 patients and propylthiouracil in 1 patient. Because antithyroid drugs for AIT type II appeared less effective, these treatments were changed to steroid therapy in all 5 patients. Overall, 9 patients received steroid therapy. For steroid therapy, prednisolone was usually started at 30 mg/day, and the dose was tapered with improvement of clinical symptoms and the thyroid hormone level. In most patients (92%, n=22) with AIT, including 15 of 16 patients who displayed transient deterioration of tachyarrhythmia (14 cases of atrial tachycardia and 1 case of ventricular tachycardia), the symptoms associated with tachyarrhythmia or thyroid function improved within 6 months after the onset of AIT. There were 2 cases of sudden cardiac death while the patients were on steroid therapy. One symptomatic patient took AMD for atrial tachycardia with a double-outlet right ventricle following a palliative procedure (Blalock-Hannon operation) and ventricular dysfunction. AMD was discontinued after the diagnosis of AIT, and the patient died in the acute phase of AIT during steroid therapy. The other patient with tetralogy of Fallot underwent intracardiac repair and died suddenly at home 4 years after the AIT episode. The total mortality rate in the AIT group was not significantly different from those in the euthyroid and AIT groups. Two patients (8%), including one on steroid therapy, displayed AIT recurrence. None of the patients required thyroidectomy.
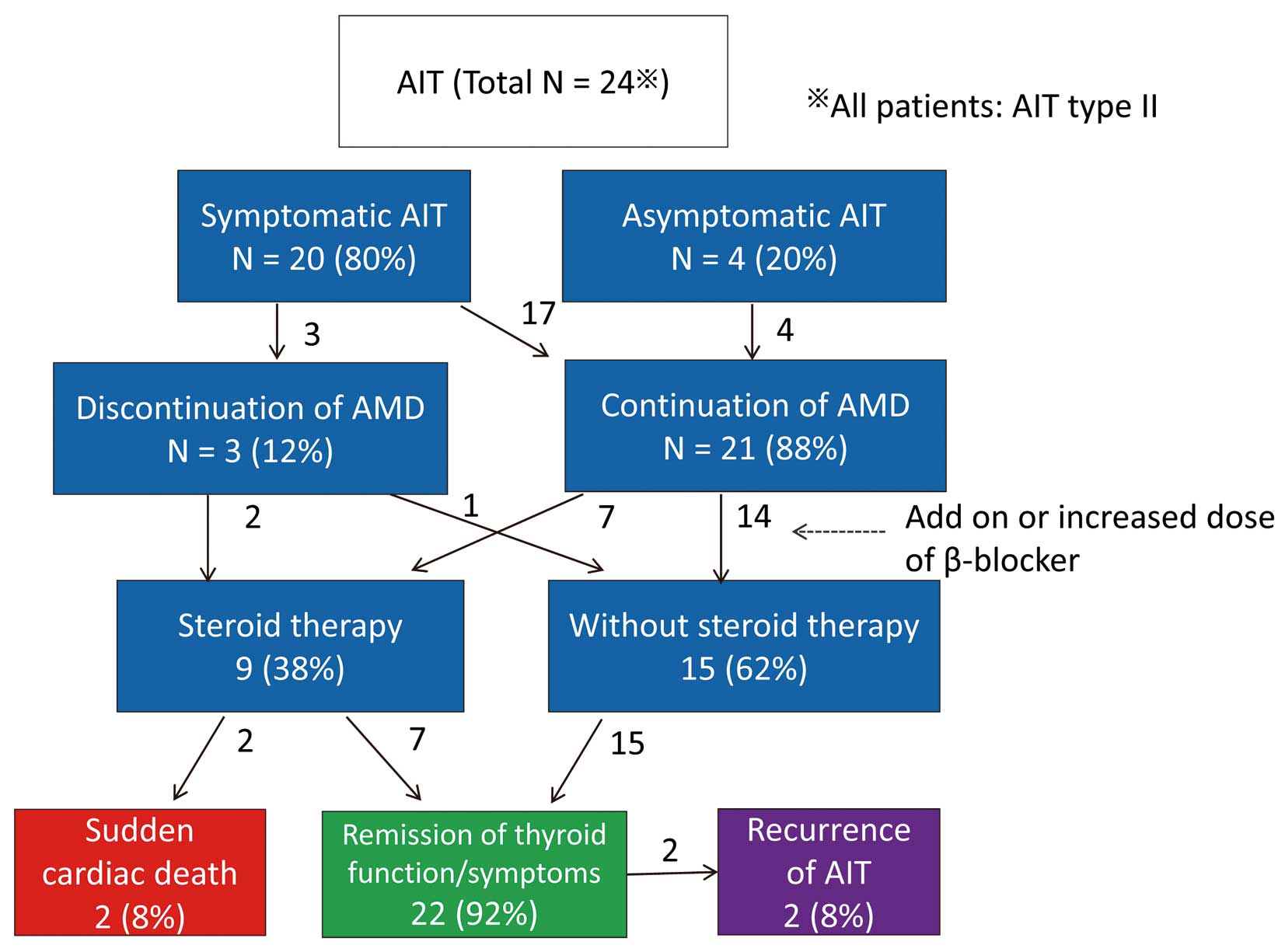
Clinical course of patients with amiodarone (AMD)-induced thyrotoxicosis (AIT).
The clinical course of patients with AIH is shown in Figure 3. Symptoms of hypothyroidism were not evident in all patients with AIH. Because 7 (44%) patients with AIH had symptomatic heart failure with NYHA class III/VI, it was difficult to distinguish whether AIH or heart failure was mainly associated with the hypothyroidism symptoms such as general fatigue and mental sluggishness. No patient with AIH displayed exacerbation of tachyarrhythmia. In total, 15 (94%) patients continued AMD therapy; 9 (56%) patients were prescribed thyroid hormone replacement therapy; 13 patients (81%) had normalized TSH levels, and 2 patients experienced AIH recurrence. Although 3 patients died of heart failure, there were no arrhythmic cardiac deaths long after a diagnosis of AIH. Mortality related to cardiovascular events in the AIH group was not significantly different from that in the AIT and euthyroid groups.
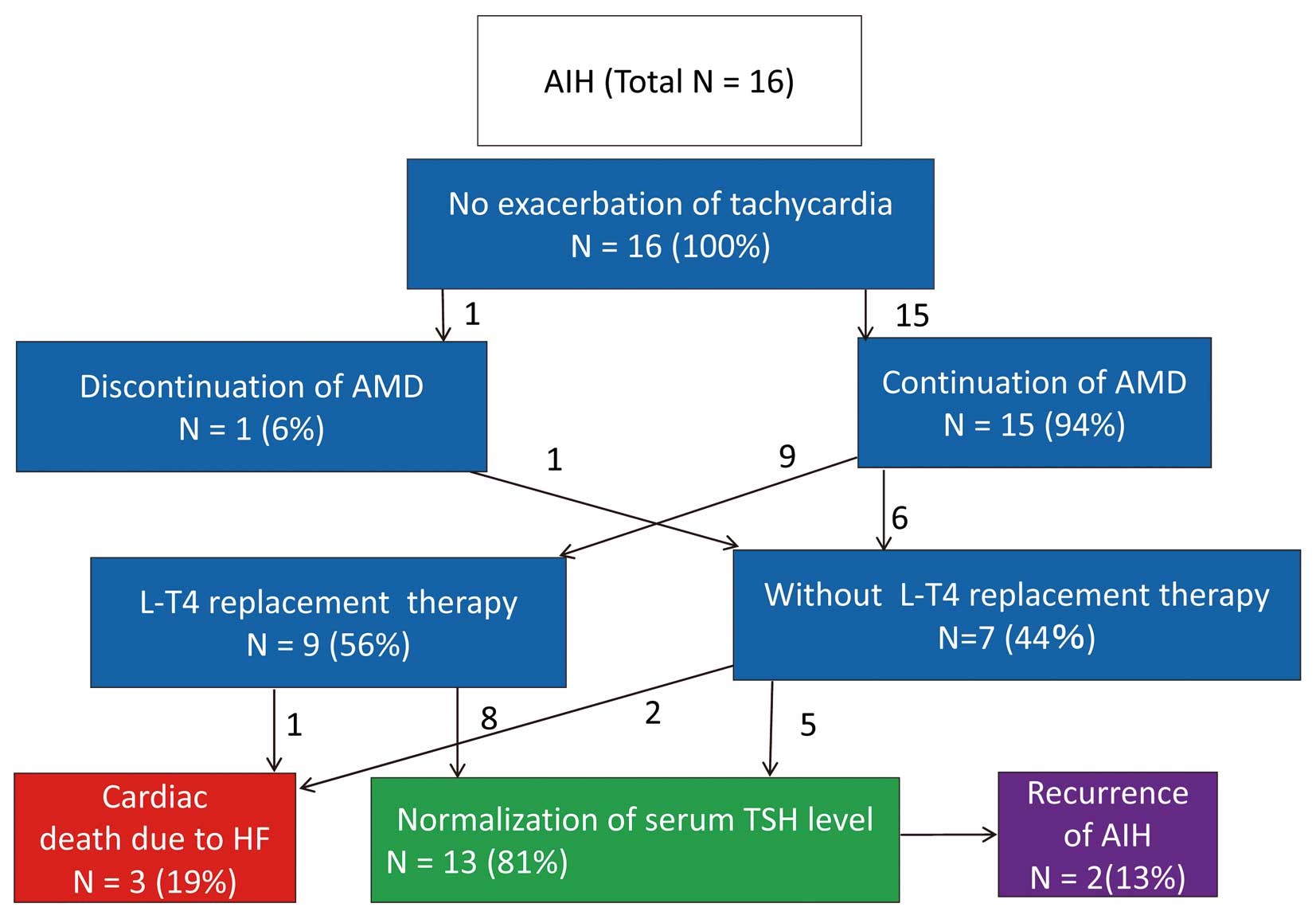
Clinical course of patients with amiodarone (AMD)-induced hypothyroidism (AIH). HF, heart failure; L-T4, l-thyroxine; TSH, thyroid-stimulating hormone.
Despite the presence of patients with a maintenance dose of AMD ≥150 mg/day, NYHA class ≥3, and cardiac dysfunction displaying a tendency to be susceptible to AIT, there was no significant independent predictor for AIT in the multivariate analysis (Table 3), whereas liver dysfunction (T-Bil ≥2 mg/dl) was a significant risk factor for AIH in the multivariate analysis (hazard ratio 2.573, 95% confidence interval 1.102–5.795) (Table 4). The plasma concentrations of AMD and N-monodesethylamiodarone displayed no significant predictive value for either AIT or AIH (Table 5).
| Risk factor | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | OR | 95% CI | P value | |
| Male | 1.042 | 0.626–1.760 | 0.874 | |||
| Age when AMD started (years) | 1.101 | 0.544–8.633 | 0.272 | |||
| Maintenance dose of AMD ≥150 mg/day | 1.527 | 0.993–2.355 | 0.053 | 1.473 | 0.955–2.281 | 0.079 |
| Prior Fontan surgery | 0.864 | 0.494–1.463 | 0.594 | |||
| Cyanosis | 1.318 | 0.754–2.263 | 0.325 | |||
| Liver dysfunction (T-Bil ≥2 g/dl) | 2.011 | 0.680–4.804 | 0.186 | |||
| NYHA functional class ≥III | 2.172 | 0.982–4.301 | 0.054 | 1.428 | 0.813–2.390 | 0.205 |
| BMI <21 at initiation of AMD administration | 0.935 | 0.608–1.436 | 0.759 | |||
| Cardiac dysfunction | 1.638 | 0.907–2.879 | 0.099 | 1.456 | 0.923–2.279 | 0.105 |
CI, confidence interval; HR, hazard ratio; OR, odds ratio. Other abbreviations as in Tables 1,2.
| Risk factor | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Male | 0.698 | 0.416–1.156 | 0.164 | |||
| Age when AMD started (years) | 0.998 | 0.975–1.022 | 0.906 | |||
| Maintenance dose of AMD ≥150 mg/day | 1.482 | 0.959–2.275 | 0.075 | 1.538 | 0.975–2.4109 | 0.060 |
| Prior Fontan surgery | 1.133 | 0.711–1.852 | 0.604 | |||
| Liver dysfunction (T-Bil ≥2 mg/dl) | 2.410 | 0.914–5.270 | 0.072 | 2.573 | 1.102–5.795 | 0.049 |
| Cyanosis | 1.018 | 0.638–1.586 | 0.936 | |||
| NYHA functional class ≥III | 1.213 | 0.703–2.000 | 0.472 | |||
| BMI >21 at initiation of AMD administration | 0.710 | 0.468–1.098 | 0.126 | |||
| Cardiac dysfunction | 0.155 | 0.993–2.420 | 0.053 | 1.486 | 0.935–2.347 | 0.092 |
Abbreviations as in Tables 1–3.
| Risk factors | Univariate analysis | ||
|---|---|---|---|
| OR | 95% CI | P value | |
| Plasma concentration of AMD and AIT | |||
| Plasma concentration of AMD* | 0.961 | 0.820–1.134 | 0.629 |
| Plasma concentration of desethylamidarone* | 0.917 | 0.823–1.156 | 0.741 |
| Plasma concentration of AMD and AIH | |||
| Plasma concentration of AMD* | 0.948 | 0.790–1.124 | 0.454 |
| Plasma concentration of desethylamidarone* | 0.948 | 0.789–1.145 | 0.574 |
*Incremental factor 0.1 μg/ml. Abbreviations as in Tables 1–3.
To our knowledge, this is the first report describing AITD in Japanese patients with CHD. In general, the overall incidence of AIT ranges from 1% to 23%, and that of AIH ranges from 1% to 32%.10 In Japanese patients without CHD, the incidence of AIT and AIH is 12.5% and 10.8%, respectively.11 In our study, the incidence of AIT and AIH in Japanese patients with CHD was 18% and 12%, respectively, which is slightly higher than in Japanese individuals without CHD. In previous reports on AITD in patients with CHD from the Royal Brompton Hospital in the UK8 and the Mayo clinic in the USA,12 the incidence of AIT was 13.6–21%, and that of AIH was 15%. The median maintenance daily dose of AMD in our study was 100 mg (range, 50–300 mg), and the mean dose was 125±58 mg, which was relatively low compared with the previous studies, in which the mean AMD dose ranged from 194 to 200 mg/day.8,12 Generally, the incidence of AIT is lower in areas of high iodine uptake than in those of low iodine uptake. The average iodine intake is approximately 1.5 mg/day in the Japanese population, vs. 0.3 mg/day in Americans. Despite iodine-rich food consumption and the use of a low-dose AMD regimen among Japanese adults with CHD, the prevalence of AITD was higher than expected.
As AMD reduces the conversion to T3 from T4 via its inhibitory effect of 5’-deiodinase, the drug increases blood levels of free T4 and decreases those of free T3. Furthermore, a direct effect of AMD on the pituitary gland decreases T3 levels in pituitary cells, resulting in increased TSH levels. Because these effects of AMD result in higher than normal serum levels of FT4 and TSH, the interpretation of precise thyroid function during AMD therapy is difficult. Fujiwara et al recommend the following new criteria for thyroid function among AMD-treated patients: 1.0 ng/dl ≤FT4 <2.4 ng/dl and 1.0 μU/ml ≤TSH <20.0 μU/ml.13 We adopted a TSH level ≥20 μU/ml as the definition of AIT to clarify the exact frequency of AITD.
Clinical Effect and Management of AITDAll participants (100%) in our study were classified as having AIT type II; thus, the proportion of patients with AIT type I was lower than in previous reports.14 The high rate of AIT type II in our study is similar to a previous study reporting that most Japanese patients with AIT had type II.15 AIT is the most important type of AITD because it is associated with adverse cardiovascular outcomes.5,6,16 AIT also increases the risk of life-threatening ventricular arrhythmia.11 In our study, there was no significant difference in total mortality in the AIT group compared with the euthyroid and AIH groups, but 1 patient died suddenly in the acute phase of AIT after steroid therapy and AMD discontinuation. Steroids represent a treatment option for AIT type II, whereas thionamides appear ineffective for AIT type II. Previous studies revealed that steroids had no effect on the time to normalization of T4 levels, and these drugs were associated with an increased event rate.5 AMD continuation does not appear to affect management outcomes or disease duration.14 Our current practice is to administer steroids only to symptomatic patients experiencing AIT while continuing AMD, because AMD discontinuation appears to increase the risk of lethal tachyarrhythmia or collapse hemodynamics. We have to remember that even supraventricular tachycardia may lead to major cardiovascular events in CHD patients with a fragile cardiovascular status. In contrast, the AIH group exhibited no increase in tachycardia episodes. Hypothyroidism is considered one of the mechanisms leading to the antiarrhythmic effects of AMD. Thyroid hormone affects cardiac contractility and peripheral vascular tone. Although AIH did not produce an arrhythmogenic effect, it may be disadvantageous for cardiac function and hemodynamics. We recommend thyroid hormone replacement therapy if patients exhibit symptoms associated with hypothyroidism or heart failure or excessively increased TSH levels.
Risk Factors for AITD and Optimal Dosage of AMD in Japanese Adults With CHDOld-fashioned Fontan-type surgery, cyanosis, and low BMI have been reported as risk factors for AIT,8,9,14 but none of these factors displayed a significant association with AIT in this study. The median BMI value in this study was 20; therefore, it follows that 49% of patients had a BMI <21, which is apparently lower than that reported previously.14
Our data showed that patients with a maintenance dose of AMD ≥150 mg/day, NYHA class ≥III, and cardiac dysfunction tended to be susceptible to AIT, based on univariate analysis, but no independent risk factor for AIT was detected on multivariate analysis. The mechanism underlying the high prevalence of AIT in patients with cardiac dysfunction and severe heart failure is unknown; however, substantial liver congestion might affect the metabolism of AMD. Elimination from the body occurs with a half-life of approximately 100 days,17 so AMD toxicity can occur after drug withdrawal.18,19 In this population, exacerbation of tachyarrhythmia induced by AIT may be fatal, and careful follow-up of thyroid function during and even upon withdrawal of AMD therapy is strongly recommended.
There are no previous reports of significant risk factors associated with AIH in patients with CHD. In the present study, liver dysfunction (T-Bil ≥2 mg/dl) was an independent risk factor for AIH in Japanese patients with CHD. The AIH group had higher proportions (32%) of patients with NYHA class ≥III and liver dysfunction (27%) than the euthyroid and AIT groups; thus, a patient with severe heart failure and compromised hemodynamics is more susceptible to AIH. Because AMD is metabolized in the liver, liver dysfunction because of liver congestion may affect the levels of circulating AMD metabolites. A daily AMD dose ≥150 mg tends to increase the risks of AIT and AIH. A lower AMD dose regimen than that used in the study may be adequate to reduce the risk of AITD in Japanese adults with CHD.
Study LimitationsThis study had some limitations, including the small sample size evaluated and its retrospective nature. Further large prospective studies in this unique population should be considered in the future.
AIT was associated with an increased risk of tachyarrhythmia, and it may have led to severe cardiac events; however, there was no exacerbation of tachyarrhythmia in the AIH group. AMD for patients with liver dysfunction is a risk factor for AIH. The incidence and risk factors for AITD may vary according to geographic region, daily iodine intake, and ethnicity. Because AITD occurred frequently in Japanese patients with CHD in the present study, despite treatment with a low-dose AMD regimen, regular blood monitoring of thyroid hormone levels is recommend for the early detection of AITD, and appropriate adjustments to the AMD regimen are necessary for Japanese adults with CHD.
The authors have no conflicts of interest to disclose.