2015 Volume 79 Issue 8 Pages 1816-1822
2015 Volume 79 Issue 8 Pages 1816-1822
Background: High ventricular premature depolarization (VPD) burden is associated with left ventricular (LV) dysfunction that typically resolves after successful ablation. Some patients, however, have persistent LV dysfunction, even after successful radiofrequency (RF) ablation. Identifying factors associated with irreversibility of LV cardiomyopathy (CMP) may help predict clinical outcome.
Methods and Results: Patients with frequent VPD (>10%/day) who underwent successful VPD suppression were divided into 2 groups according to transthoracic echocardiography (TTE) before and after suppression: group A (n=38) had depressed LV function that normalized after VPD suppression; group B (n=19) had depressed LV function before and after suppression. Of 57 patients (43 men; mean age, 54±15 years), RF ablation was performed in 39. Clinical, electrocardiographic, and TTE parameters were compared between groups. LV end-diastolic dimension (LVEDD; group A vs. B: 54±5 mm vs. 60±10 mm, P=0.01), end-systolic dimension (group A vs. B: 42±6 mm vs. 48±11 mm, P=0.01) before VPD suppression differed significantly between groups. Pre-suppression LVEDD was ≤66 mm in all reversible-CMP patients. LVEDD >66 mm predicted irreversible CMP with 50% sensitivity, 100% specificity, 100% positive predictive value, and 81% negative predictive value.
Conclusions: LVEDD was a good predictor of irreversible LV CMP with frequent VPD, with 50% sensitivity and 100% specificity. (Circ J 2015; 79: 1816–1822)
Idiopathic ventricular premature depolarizations (VPD) are usually considered a benign condition, even when they occur frequently.1,2 Several recent studies, however, reported that a high burden of VPD is associated with left ventricular (LV) cardiomyopathy (CMP) that usually resolves after successful VPD ablation.3–10 Nevertheless, in some patients, LV function does not return to normal, even after successful VPD ablation. The mechanism(s) underlying the development of VPD-induced CMP are incompletely understood. Previous reports indicated that tachycardia-induced CMP is reversible with medical or procedural interventions, and LV diastolic dimension helps differentiate tachycardia-induced CMP from idiopathic dilated CMP.11 In patients with LV dysfunction and frequent VPD, VPD QRS duration (QRSd) is the only independent predictor for recovery of LV function after ablation. This suggests that VPD QRSd could be a marker for the severity of underlying substrate abnormality.12,13 Based on this, we hypothesized that VPD QRSd depends on ventricular myocardial conduction time and LV dimension might be correlated with VPD QRSd. In our clinical experience, some patients with frequent VPD that initially appear to be idiopathic have irreversible CMP, even after undergoing successful VPD suppression and optimal medical management for heart failure. We postulate that these patients may have pre-existing subclinical occult structural myocardial disease at baseline and this may be one of the mechanisms leading to idiopathic irreversible VPD-induced CMP. In this study, we examined echocardiographic parameters in patients with frequent VPD and LV dysfunction to determine if these parameters predicted irreversibility of LV CMP.
A total of 382 patients were diagnosed with frequent VPD at the Konkuk University Medical Center and Samsung Medical Center between January 2006 and December 2013. Among these, 83 were excluded due to episodes of sustained ventricular tachycardia (VT; n=11), coronary artery disease (n=10), non-compaction of the LV (n=1), sarcoidosis (n=3), arrhythmogenic right ventricular CMP/dysplasia (n=2), myocarditis (n=1), other large magnetic resonance imaging (MRI) abnormalities (n=3), procedural failure (n=10), or incomplete data (n=42). Among the remaining 299 patients, 235 had normal LV function. The other 64 patients had frequent VPD and LV dysfunction, but 7 were excluded due to sustained LV dysfunction with recurrence of frequent VPD during follow-up.
Inclusion criteria were as follows: frequent VPD (>10% VPD/day) with LV dysfunction (ejection fraction <50%); no known structural heart disease; successful VPD suppression at the time of final follow-up, achieved with medical therapy or radiofrequency (RF) ablation; baseline and follow-up transthoracic echocardiography (TTE) and Holter monitoring; and no episodes of sustained VT. Based on previously published experience, successful VPD suppression was defined as ≥80% reduction of 24-h VPD burden.14 VPD recurrence was defined as frequent VPD >20% of initial VPD burden on repeated follow-up on Holter monitoring.
Enrolled patients were divided into 2 groups: group A, reversible CMP, defined as normalization of LV ejection fraction (LVEF ≥50% and improvement by ≥10%) and absence of any other structural heart disease; and group B, irreversible CMP, defined as global LVEF ≤45% before ablation and <50% ≥6 months after successful VPD suppression.
A total of 57 patients met the final inclusion criteria (Figure 1). Clinical data were obtained from cardiology records: 21 (37%; group A, n=12; group B, n=9) underwent cardiac MRI and 29 (50%; group A, n=18; group B, n=11) underwent coronary angiography. All treatments for VPD suppression were performed in accordance with the institutional guidelines, and all patients provided written informed consent.

Study flowchart. LV, left ventricular; VPD, ventricular premature depolarization; VT, ventricular tachycardia.
TTE was performed before ablation using the Simpson formula to determine LVEF. For LVEF assessment, LV end-diastolic dimension (LVEDD), and LV end-systolic dimension (LVESD), the second of 2 consecutive sinus beats was used to avoid the influence of post-extrasystolic potentiation. LVEF <50% was considered abnormal. TTE with a quantitative assessment of LV function was repeated at 3–6 months after treatment.
ElectrocardiographyTwelve-lead electrocardiograms (ECG) were recorded at a sweep speed of 100 ms and analyzed offline with a Muse® Cardiology Information System, using digital calipers. Sinus QRS and VPD QRS were evaluated with respect to QRSd: sinus QRSd was defined as onset of the sinus QRS to the terminal S wave; and VPD QRSd, as onset of the VPD to the terminal S wave.
Holter MonitoringBefore treatment, Holter monitoring was performed twice per month at intervals of at least 1 week to measure mean VPD burden (% and number of VPD/day). Follow-up Holter monitoring was repeated twice at intervals of at least 1 week within 6 months after treatment (ie, RF ablation or anti-arrhythmic drug [AAD]) and thereafter at 3–6-month intervals or if VPD-related symptoms recurred.
Follow-upPatients were seen in an outpatient clinic at 3, 6, and 12–48 months after treatment. All AAD were discontinued if ablation was effective. Beta-blockers and other heart failure medications (angiotensin-converting enzyme inhibitor [ACEI]/angiotensin receptor blocker [ARB] or diuretics) were continued initially after treatment, but were discontinued if LV function and dimensions normalized. No new medications were added after successful RF ablation.
Statistical AnalysisDescriptive data are presented as mean±SD except where otherwise indicated. Between-group comparison was done using Student’s t-test. Within-group comparison was done using paired t-test. Correlations between VPD QRSd and LVEDD were assessed on bivariate correlation analysis and Pearson’s 2-tailed procedure. All analyses used SPSS version 18.0 for Windows (SPSS, Chicago, IL, USA). For all tests, P<0.05 was considered statistically significant.
We identified 57 patients (43 men; mean age, 54±15 years old) with >10% VPD/day and LV dysfunction who underwent successful VPD suppression, achieved medically in 18 patients (32%) and by RF ablation in 39 (68%). LV dysfunction was reversible in 38 patients (group A) and irreversible in 19 (group B; Table 1). RF ablation was performed in 26 group A patients (68%) and in 13 group B patients (68%). The overall acute success rate, defined as no clinical VPD during at least the first 30 min after RF ablation or during at least the first week after AAD treatment, was 100% (57/57 patients). Among patients with LV dysfunction, long-term success rate, defined as ≥80% reduction in VPD burden during routine post-treatment Holter monitoring, was 84% (46/57). The remaining 11 patients (19%) with LV dysfunction required another AAD or second RF ablation.
| Group A (n=38) | Group B (n=19) | P-value | |
|---|---|---|---|
| Demographics | |||
| Male | 30 (79) | 13 (68) | 0.51 |
| Age (years) | 55.4±14.5 | 53.3±14.2 | 0.60 |
| BMI (kg/m2) | 28.2±6.1 | 28.3±3.2 | 0.94 |
| BSA (m2) | 2.09±0.31 | 2.06±0.34 | 0.83 |
| Medical history | |||
| HTN | 14 (36) | 10 (52) | 0.27 |
| DM | 5 (14) | 2 (10) | 1.00 |
| ICD | 3 (7.8) | 10 (52) | <0.001 |
| Atrial fibrillation | 6 (16) | 3 (16) | 1.00 |
| Medication history | |||
| AAD# | 31 (81) | 13 (68) | 0.31 |
| β-blocker | 33 (86) | 13 (68) | 0.15 |
| CCB | 4 (10) | 1 (5) | 0.65 |
| ACEI | 12 (31) | 7 (36) | 0.76 |
| ARB | 8 (21) | 6 (31) | 0.51 |
| Symptom history | |||
| Asymptomatic | 17 (45) | 10 (52) | 1.0 |
| Symptom(s) | 21 (55) | 9 (48) | 1.0 |
| Palpitations | 16 (44) | 7 (37) | 0.77 |
| SOB | 1 (3) | 3 (15) | 0.10 |
| Syncope | 3 (9) | 3 (15) | 0.40 |
| Dizziness | 1 (3) | 2 (11) | 0.27 |
| Fatigue | 3 (8) | 2 (11) | 1.0 |
| Dyspnea | 22 (57) | 13 (68) | 0.32 |
| NYHA | |||
| Class I | 5 | 4 | 0.46 |
| Class II | 14 | 8 | 0.77 |
| Class III | 3 | 1 | 1.00 |
| Class IV | 0 | 0 | – |
| Symptom duration (months) | 72.3±95.9 | 46.7±54.7 | 0.46 |
| Holter monitoring | |||
| VPD burden (%) | 29.0±13.9 | 30.4±12.3 | 0.88 |
| VPD burden (n) | 34,979±16,202* | 33,549±14,639 | 0.59 |
| NSVT | 17 (47) | 9 (57) | 0.57 |
| Multifocal VPD | 9 (13) | 1 (5) | 0.65 |
| Cardiac MRI | |||
| Performed | 12 (32) | 9 (47) | 0.26 |
| Abnormal‡ | 2 (5) | 1 (5) | 1.00 |
Data given as mean±SD or n (%). *P<0.05. #Includes any class I or III AAD. ‡Defined as any area of delayed gadolinium enhancement or regional wall motion abnormality. Proportions are number of abnormal exams over number of patients who underwent MRI. AAD, anti-arrhythmic drug; ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; BMI, body mass index; BSA, body surface area; CCB, calcium channel blocker; DM, diabetes mellitus; HTN, hypertension; ICD, implanted cardiac defibrillator; LV, left ventricle; LVEDD, left ventricular end-diastolic dimension; LVEF, left ventricular ejection fraction; LVESD, left ventricular end-systolic dimension; MRI, magnetic resonance imaging; NSVT, non-sustained ventricular tachycardia; NYHA, New York Heart Association; RV, right ventricle; SOB, shortness of breath; VPD, ventricular premature depolarization.
Successful VPD suppression was performed with AAD in 12 group A patients and in 6 group B patients. Final AAD used to suppress clinical VPD included type IC drugs (flecainide in 2 patients and propafenone in 3) and a class III drug (amiodarone in 13 patients). Successful VPD suppression was achieved in group A using IC drugs in 5 patients (flecainide in 2 patients and propafenone in 3) and amiodarone in 7, and in group B using amiodarone in 6 patients. Implantable cardioverter defibrillator (ICD) was inserted in 3 patients in group A and in 10 patients in group B to prevent primary sudden cardiac death. The median time to first follow-up TTE was 3.5 months (IQR, 2.3–5.8). The first post-treatment Holter monitoring was performed at a median of 2.6 months (IQR, 1.6–3.8).
Baseline Epidemiological and Clinical CharacteristicsNo significant differences were observed between the 2 groups in age, sex, symptoms, or use of β-blockers, ACEI, or ARB (Table 1). The 2 groups had similar baseline 24-h VPD burden (group A 29.8±14.4% vs. group B 21.6±14.9%, P=0.88) and VPD number (group A 34,979±18,202 vs. group B 33,549±14,639, P=0.59). LVEDD (P=0.01), and LVESD (P=0.01) were significantly lower in group A than in group B (Table 2). Other TTE parameters were not significantly different between the groups.
| Group A (n=38) | Group B (n=19) | P-value | |
|---|---|---|---|
| LVEF (%) | 36.5±7.6 | 33.1±10.3 | 0.09 |
| LVEDD (mm) | 56±7 | 65±10 | 0.01* |
| LVESD (mm) | 42±6 | 49±11 | 0.01* |
| LA (mm) | 40±5 | 41±6 | 0.31 |
| LAVI (ml/m2) | 39±2 | 40±3 | 0.29 |
| LVSd (mm) | 9.3±0.9 | 10.1±1.1 | 0.42 |
| LVPWd (mm) | 11.3±1.5 | 10.8±0.9 | 0.38 |
Data given as mean±SD. *P<0.05. LA, left atrium; LAVI, LA volume index; LVPWd, LV posterior wall dimension; LVSd, LV septal dimension; TTE, transthoracic echocardiography. Other abbreviations as in Table 1.
Among baseline electrophysiological characteristics of patients who underwent RF ablation (Table 3), mean sinus QRSd (group A 92.1 ms vs. group B 100.4 ms, P=0.04) and VPD QRSd (group A 157.4 ms vs. group B 171.9 ms, P<0.01) were significantly different between the groups. The distribution of origin site for clinical VPD was similar for the 2 groups. When VPD site was dichotomized into septal/non-septal, outflow tract/non-outflow tract, or LV/RV sites, no significant differences were seen between the 2 groups.
| Reversible LV dysfunction (n=21)† |
Irreversible LV dysfunction (n=18)† |
P-value | |
|---|---|---|---|
| Electrocardiographic parameters | |||
| Sinus QRS (ms) | 92.1±11.8 | 100.4±15.6 | 0.04* |
| VPD QRSd (ms) | 157.4±10.5 | 171.9±17.2 | <0.01* |
| VPD site of origin | |||
| RVOT/RCC/PA | 12 (57) | 10 (55) | 0.75 |
| Other RV | 0 (0) | 0 (0) | 1.00 |
| LCC/AMC/AIV | 7 (33) | 6 (33) | 1.00 |
| Other LV | 1 (5) | 2 (11) | 0.58 |
| VPD classification | |||
| Septal (vs. non-septal) | 12 (57) | 10 (55) | 0.75 |
| Outflow tract (vs. non-outflow tract) | 19 (90) | 16 (88) | 1.00 |
| LV (vs. RV) | 8 (38) | 8 (44) | 0.20 |
Data given as mean±SD or n (%). *P<0.05. †Only patients who underwent successful VPD suppression with RFA were included. AIV, anterior interventricular vein; AMC, aorto-mitral continuity; CC, coronary cusp; LCC, left coronary cusp; PA, pulmonary artery; QRSd, QRS duration; RCC, right coronary cusp; RFA, radiofrequency ablation; RVOT, right ventricular outflow tract. Other abbreviations as in Table 1.
In all group A patients, clinical VPD were successfully suppressed with treatment. Most VPD-related symptoms resolved during follow-up. Among 38 group A patients, 21 (55%) were symptomatic and 17 (45%) asymptomatic. Among the 22 patients who complained of dyspnea, 5 had New York Heart Association (NYHA) grade I, 14 had grade II and 3 had grade III. NYHA grade dyspnea resolved in all group A patients after LV function normalized. LV function returned to normal within 6 months of successful VPD suppression in 26 of 38 group A patients (68%); between 6 months and 1 year in 5 patients (14%); and after 1 year in the remaining 7 (18%). In group A, all patients stopped AAD or heart failure management after LV dysfunction normalization. LV function was maintained as normal without amiodarone or heart failure management during clinical follow-up. ICD was used in 3 patients (7.8%) to prevent primary sudden cardiac death. ICD pacing rate was <0.1% in these patients. The last follow-up TTE was obtained at 6.5±7.9 months after treatment. LVEF or LVEDD and time at which LV dysfunction resolved were not correlated.
In all group B patients, clinical VPD were successfully suppressed with treatment. Most VPD-related symptoms resolved during follow-up in all except 1 patient. Among 19 group B patients, 9 (48%) were symptomatic and 10 (52%) asymptomatic. NYHA grade of dyspnea improved in 15 patients, and in 3 patients NYHA grade was similar to the pretreatment grade. In group B, all patients stopped AAD after successful VPD suppression, but heart failure management was continued because LV dysfunction did not normalize. ICD was implanted in 10 group B patients (52.0%) with LVEF <30% even with successful VPD suppression and proper heart failure management, to prevent primary sudden cardiac death. ICD pacing rate was <0.1% in these 10 patients. Mean follow-up period for group B patients was 42.7±17.3 months, during which further heart failure management was carried out. Mean times of last follow-up were 31.6 months for TTE and 33.5 months for Holter monitoring.
LV Dysfunction Irreversibility and LVEDDAll LVEDD were ≤66 mm in group A patients and ≤66 mm in 8 of 19 group B patients (42%; Figure 2A). On receiver operating characteristic curve analysis, LVEDD >66 mm predicted irreversible CMP after successful VPD suppression with 50% sensitivity, 100% specificity, 100% positive predictive value and 81% negative predictive value (Figure 2B).
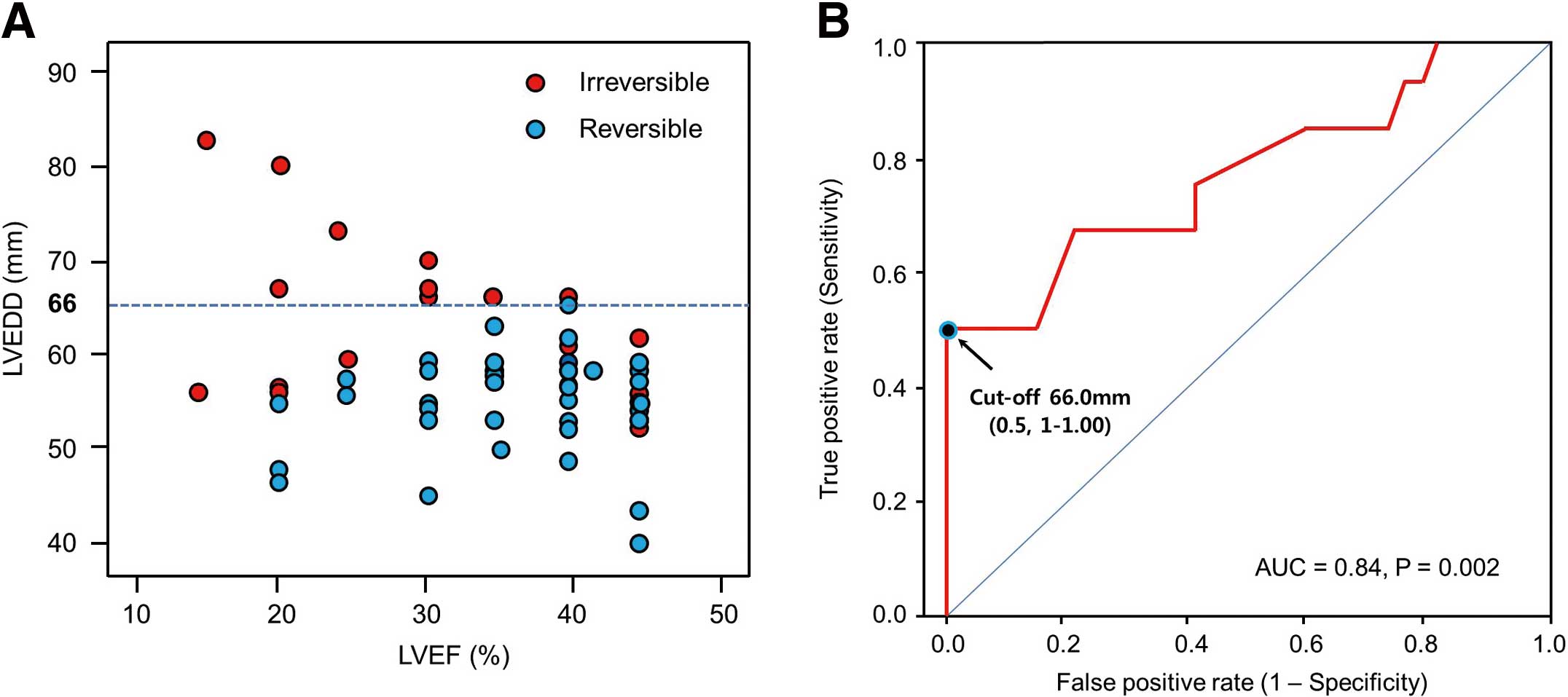
(A) Left ventricular end-diastolic dimension (LVEDD) vs. left ventricular ejection fraction (LVEF) in ( ) reversible and (
) reversible and ( ) irreversible cardiomyopathy (CMP). (B) Receiver operating characteristic curve analysis: LVEDD >66 mm predicted irreversible LV CMP with sensitivity 50% and specificity 100%. AUC, area under the curve.
) irreversible cardiomyopathy (CMP). (B) Receiver operating characteristic curve analysis: LVEDD >66 mm predicted irreversible LV CMP with sensitivity 50% and specificity 100%. AUC, area under the curve.
The relationship between VPD QRSd and LVEDD was analyzed to evaluate the association between ventricular conduction time during VPD and LVEDD during sinus rhythm (Figure 3). Bivariate correlation analysis showed a definite positive linear correlation (γ=0.64) between VPD QRSd and LVEDD (P<0.01).

Ventricular premature depolarization (VPD) QRS duration (QRSd) vs. left ventricular end-diastolic dimension (LVEDD) in ( ) reversible and (
) reversible and ( ) irreversible cardiomyopathy.
) irreversible cardiomyopathy.
AAD were used in 18 patients (AAD group) and 39 patients underwent RFCA (RFCA group) for successful VPD suppression. Amiodarone was used by 7 patients (7/38, 18%) in group A and by 6 patients (6/19, 31%) in group B. No significant difference was observed for incidence between groups A and B according to VPD suppression method (P=0.50). ECG analysis showed no significant differences between groups for sinus QRSd (AAD 95±13 ms vs. RFCA 96±22 ms, P=0.92) or VPD QRSd (AAD 165±8.7 ms vs. RFCA 164±10.1 ms, P=0.68). TTE analysis showed no significant differences between groups for LVEDD (AAD 5.9±0.7 cm vs. RFCA 5.5±0.8 cm, P=0.13), LVESD (AAD 4.7±0.8 cm vs. RFCA 4.3±0.9 cm, P=0.31), or LVEF (AAD 33±10% vs. RFCA 34±8%, P=0.69). The 2 groups had similar baseline 24-h VPD burden (AAD 29.6±14.0% vs. RFCA 31.2±12.4%, P=0.62) and VPD number (AAD 31,998±22,434 vs. RFCA 33,035±15,402, P=0.48).
Idiopathic VPD are increasingly recognized to cause LV dysfunction that is reversible with ablation treatment.3–9 Recent studies suggest that VPD frequency >24% on 24-h Holter monitoring is a risk factor for VPD-induced CMP,3,7–9 but 20–25% of patients in those studies did not reach that cut-off. At Konkuk University Medical Center, some patients diagnosed as having VPD-induced CMP had no or only partially improved LV function even after successful RF ablation. A previous study examining the longitudinal impact of VPD burden found that LV function deteriorated subclinically over 5 years in patients with ≥10–20% VPD.15 The paradigm of idiopathic VPD-induced CMP and reversible or irreversible LV dysfunction, however, cannot be explained by VPD frequency alone. Pre-existing occult structural heart disease was recently suggested as a potential mechanism of frequent VPD with LV dysfunction.16–18
In this study of patients with frequent VPD and LV dysfunction, LVEDD <66 mm predicted reversibility of LV function after successful VPD suppression with 100% sensitivity and 50% specificity. In 68% of patients with reversible CMP, LV function normalized within 6 months after VPD suppression; in 18% of patients, LV function normalized >1 year after successful suppression of clinical VPD. LVEDD >66 mm was a good predictor of irreversible CMP with 50% sensitivity and 100% specificity, 100% positive predictive value, and 81% negative predictive value. Furthermore, LV function did not normalize for at least 2 years after successful VPD suppression, even with further heart failure management.
Several studies used endomyocardial biopsy or autopsy to identify potential contributing factors to ventricular arrhythmias.17,18–21 Lemery et al described the clinical, laboratory, and electrophysiological features of patients with idiopathic VT who had no clinical evidence of heart disease.17 They detected minor structural cardiac abnormalities in >30% of these patients. Similarly, Nishikawa et al described advanced histopathological findings including myocyte hypertrophy, degeneration, interstitial fibrosis, and disarrangement of muscle bundles, in patients with idiopathic VT.19 No significant abnormalities were found on imaging in the present patients to suggest occult structural heart disease, but current imaging technology might not be sensitive enough to identify occult abnormalities that cause predisposition to overt CMP during system stress. Previous reports noted that VPD QRSd was significantly longer in patients with VPD-induced CMP than in normal controls.12,13,22 These studies identified VPD QRSd as the only independent predictor of recovery of LV function after ablation. In the current study, we also found that VPD QRSd was significantly longer in the irreversible group than the reversible group without significant difference in VPD origin site. Furthermore, VPD QRSd was significantly associated with LVEDD with a definite positive linear correlation. This suggests that LVEDD might be a good marker for irreversibility of myocardial dysfunction, although we enrolled only patients with frequent VPD and LV dysfunction. Longer VPD QRSd reflects a prolonged myocardial conduction time, which might be indirect evidence of the presence of LV dilatation. Consequently, we suggest that LVEDD might be one of the best predictors of reversibility or irreversibility of myocardial dysfunction in patients with frequent VPD and LV dysfunction.
Three patients with ICD to prevent primary sudden cardiac death had LVEF recovery to normal. In 1 patient with reversible LV dysfunction who had an ICD to prevent primary sudden cardiac death, LV dysfunction normalized 17 months after VPD suppression. The long delay suggests that this patient might not have had frequent VPD-induced CMP, but also suggests that if a patient has LV dysfunction, frequent VPD, and LVEDD <66 mm, VPD suppression and adequate heart failure management can restore LV function to normal. We noted LVEDD ≤66 mm in 8 patients in the irreversible CMP group; they might have had subclinical myocardial disease at baseline even though a few had cardiac MRI or coronary angiography. A more useful parameter to predict the reversibility or irreversibility of LV dysfunction in patients with CMP and frequent VPD is required, and the physiological and pathological basis for observed differences in outcome associated with different LV dimensions deserves further study.
Study LimitationsOne limitation of this study was that not all the patients underwent examinations necessary to evaluate underlying cardiac disease, such as cardiac MRI (37%) and coronary angiography (50%). This means that improvement in ventricular function after RF ablation may have been affected by underlying cardiac disease that could not be detected on TTE. A second limitation is that VPD recurrence might have been missed in asymptomatic VPD patients. Similar to previous studies, however, repeat Holter monitoring at regular intervals was used to identify VPD recurrence. A third limitation was the small number of study participants; further long-term studies with large samples are needed. A fourth limitation is that 24-h Holter monitor was used to determine VPD burden. A longer duration of monitoring may be preferable because of day-to-day variability in VPD frequency, especially in the presence of CMP and VPD burden >10%. Whenever feasible, ambulatory monitoring for at least 48 h is preferable. Furthermore, the VPD suppression methods differed in the 2 groups, so the treatment population consisted of a mixed group who received either RF ablation or AAD. Although the treatment modalities were not the same in the 2 groups, the distribution of treatment modality was similar between the groups. Another limitation is that patients included in the study required further treatment, which might have introduced referral bias. We were unable to track patients who were not referred for or who refused to take AAD or undergo RF ablation.
In patients with LV dysfunction and frequent VPD, LVEDD appears to be a good predictor of irreversibility of LVEF after successful suppression of VPD. LVEDD >66 mm predicted irreversible CMP with 50% sensitivity, 100% specificity, 100% positive predictive value, and 81% negative predictive value.
We wish to thank all the members of the electrophysiology laboratory at Konkuk University Medical Center for their assistance and support with the data collection.
The authors have no conflicts of interest to disclose.