2015 Volume 79 Issue 8 Pages 1685-1686
2015 Volume 79 Issue 8 Pages 1685-1686
Fibroblast growth factor 23 (FGF23) and Klotho are recently discovered hormones that have a close relationship with parathyroid hormone (PTH) in maintaining phosphorus and vitamin D metabolism.1 FGF23 is most highly expressed in osteocytes, but because FGF23 is the most soluble form of the subfamily of FGFs, it can be also found in circulating serum.2 Although this enables FGF23 to exert systemic actions, the 2 main organs of action are the kidneys and parathyroid glands (Figure).1,2 In the kidneys, FGF23 augments urinary phosphate exertion by promoting endocytosis of sodium-phosphate cotransporters (NPT2a and NPT2c) from renal proximal tubular cells.3 Additionally, FGF23 regulates vitamin D synthesis through CYP27B1 inhibition and CYP24A1 stimulation, which will lead to suppression of vitamin D activity and a decrease in the levels of phosphorus and calcium. In the parathyroid glands, FGF23 directly inhibits the synthesis and secretion of PTH.4 The importance of FGF23 has been documented in an experimental study utilizing a rat model of chronic kidney disease (CKD). Circulating FGF23 activity was lowered by using anti-FGF23 antibody and there was a significant increase in serum phosphate while the mean PTH level remained unchanged.5 These findings indicate that FGF23 is more direct and strong regulator of phosphorus than PTH, at least in CKD.
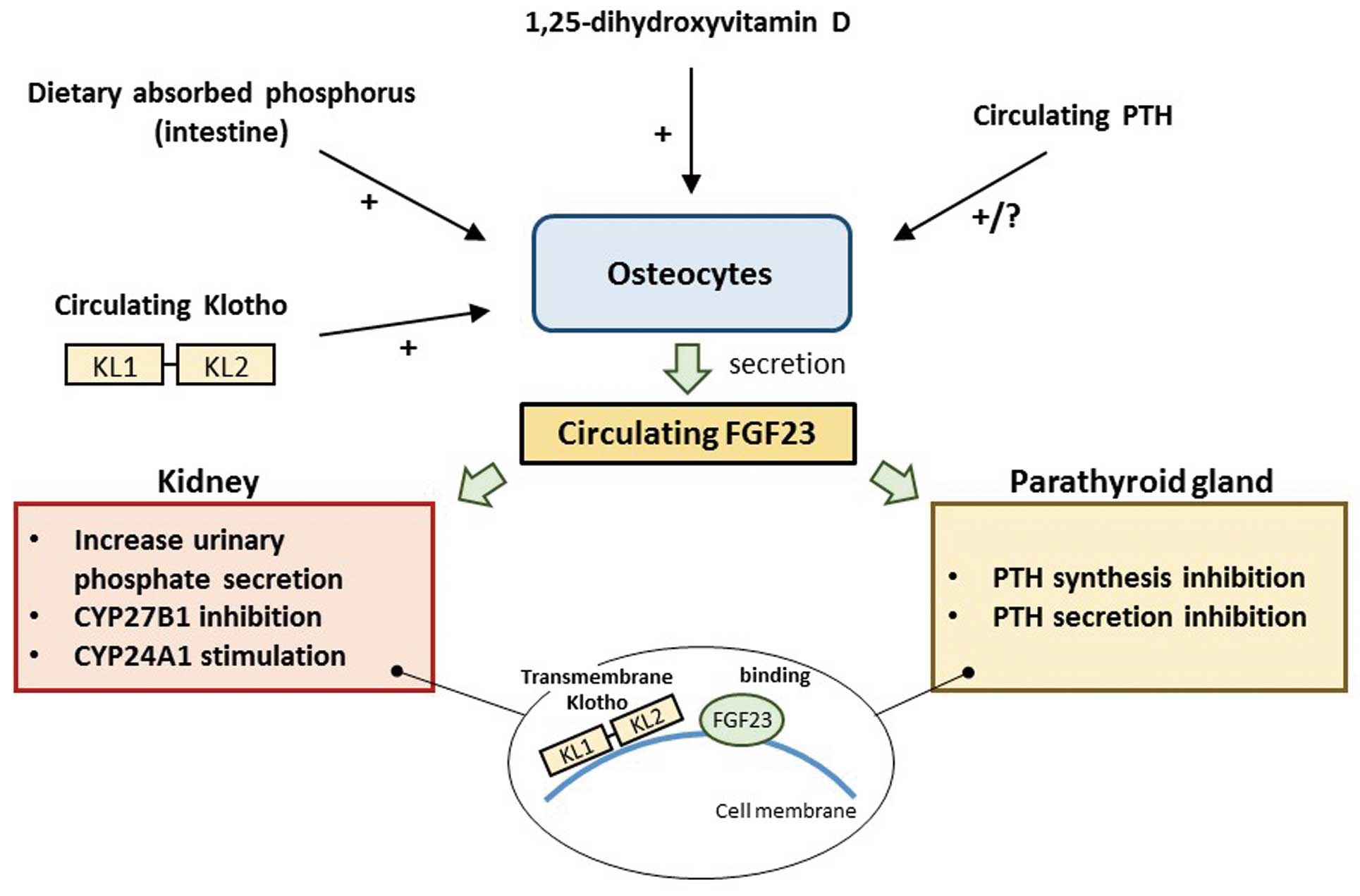
Schematic of regulation and action of fibroblast growth factor 23 (FGF23). FGF23 is mainly synthesized and secreted from osteocytes, and primarily acts in the kidneys and parathyroid glands. The phosphorus level will be decreased through augmented urinary phosphate excretion and a decrease in parathyroid hormone (PTH) level. FGF23 synthesis and secretion are regulated by various factors, such as vitamin D, absorbed phosphorus, circulating Klotho etc. Although Klotho itself has a circulating form, transmembrane Klotho works through binding with FGF23. See text for details. CYP27B1, enzyme converting vitamin D to more active metabolite; CYP24A1, enzyme regulating vitamin D catabolism; KL1 & 2, internal repeats of extracellular domain of Klotho.
Article p 1742
Klotho is a long peptide with a single transmembrane domain and an extracellular domain with 2 internal repeats (KL1 and KL2).6 Klotho has similar actions as FGF23 but transmembrane Klotho works by binding with circulating FGF23. Klotho also has a circulating form, which stimulates FGF23 synthesis and secretion in osteocytes. The circulating Klotho exhibits various systemic actions, including regulation of phosphorus and calcium metabolism.6 Although similar findings as for FGF23 have been reported from an experimental study of Klotho (ie, overexpression of Klotho leading to low levels of serum phosphate and PTH),7 the precise mechanism of interaction between FGF23 and Klotho remains unclear. They may show a balanced change in a negative-feedback pattern, but might possibly exhibit parallel changes through end-organ resistance at the level of the kidneys and parathyroid glands.
Role of FGF23 and Klotho in Cardiovascular DiseasesIn addition to their main roles in regulating phosphorus and calcium metabolism, the implication of FGF23 and Klotho in cardiovascular disease and mortality in patients with CKD has been attracting attention. Several reports have documented that higher FGF23 concentrations were independently associated with higher risk of renal disease progression, cardiovascular diseases, and death in patients with CKD.8,9 Although the mechanism remains unclear, higher FGF23 concentrations have been independently associated with higher left ventricular mass, higher incidence of left ventricular hypertrophy,10 endothelial dysfunction and arteriosclerosis with vascular calcification.11 In addition, FGF23 has been shown to directly induce hypertrophy of cardiomyocytes both in vitro and in vivo.12 These findings indicate a potential pathogenic role of FGF23 in various cardiovascular diseases. Interestingly enough, a recent report documented a paradoxical anti-calcific effect of FGF23 on human aortic smooth muscle cells, but this effect was not observed under the conditions of decreased Klotho expression.13 This may indicate a possible protective role of FGF23 and Klotho in their balanced expression, but this effect might be negated by imbalanced expression such as in patients with CKD.
Association of Circulating FGF 23 and Clinical Prevalence of AFAtrial fibrillation (AF), a most common clinical tachyarrhythmia, is known to cause atrial structural remodeling, which is characterized by atrial enlargement, cardiomyocyte degeneration, interstitial fibrosis, and endothelial dysfunction.14 Although the inflammatory process and/or stimulation through the renin-angiotensin system are understood to play important roles, various specific factors, such as oxidative stress, inflammatory cytokines etc, may possibly contribute to the construction of the arrhythmogenic substrate for AF.15 Because CKD is known to be highly associated with cardiovascular diseases, especially AF and heart failure,16 the association between the FGF subfamilies and AF is theoretically possible.
In this issue of the Journal, Miyamura et al17 report on their study of a considerable number of patients with and without AF in cohort style, and document an association between serum circulating FGF23 and AF prevalence. It was an independent predictor separate from possible related factors such as Klotho, PTH or vitamin D. This is a unique and quite interesting finding with regard to the pathogenesis of AF, especially in CKD patients. However, interestingly the association between FGF23 and AF prevalence showed a U-shaped relationship in their study. The result was that AF prevalence was lowest in fifth octile when the study population was divided into 8 groups in accordance with FGF23 levels. The precise mechanism of this U-shaped relation could not be explained, but probably indicates an indirect or multifactorial association of FGF23 to construction of the AF substrate, which should be clarified in further studies. Finally, a fundamental question still remains. If the association between FGF23 and AF prevalence exists, is it causative or just parallel? Although several simple relationships, such as underlying diseases, were denied in the multivariate analysis, this point should be clarified in future in a specifically designed study.