Abstract
Background:
Adverse effects of dietary intake of trans-fatty acids (TFA) on the incidence of coronary artery disease (CAD) are well recognized in Western countries. The risk of TFA, however, has not been well clarified in Japan. We investigated the association of serum TFA concentration with serum lipid profile, coronary risk factors, and prevalence of CAD.
Methods and Results:
A total of 902 patients, who were hospitalized at Kobe University Hospital from July 2008 to March 2012 and gave written informed consent, were enrolled in this study. Among them, 463 patients had CAD, and 318 patients had metabolic syndrome (MetS). Serum TFA, elaidic acid (trans-9-C18:1) and linolelaidic acid (trans-9, 12-C18:2), were measured on gas chromatography/mass spectrometry. Serum TFA level had a positive correlation with body mass index, waist circumference, low-density lipoprotein cholesterol, triglycerides, and apolipoprotein B48, and an inverse correlation with age and high-density lipoprotein cholesterol. Fasting serum TFA, by age quartile in the young generation with CAD and/or MetS, was higher than that in patients without CAD and/or MetS. On multivariate logistic regression, TFA was identified as a CAD risk after adjustment for classical risk factors.
Conclusions:
Serum TFA concentration was elevated in young patients with CAD and/or MetS. Diet-derived TFA may cause a serious health problem, particularly in the young generation in Japan. (Circ J 2015; 79: 2017–2025)
Trans-fatty acids (TFA) are unsaturated fatty acids with at least 1 unsaturated, non-conjugated double bond in the trans (rather than the typical cis) structure. The industrially produced TFA is formed by partial hydrogenation of vegetable oil and/or fish oil that changes the cis configuration of double bond(s) to trans, resulting in solid fat for use in margarine, fat spread and shortening, and for commercial cooking, and factory processes. Epidemiological studies in Western countries have indicated that an excessive intake of TFA is a risk factor for coronary artery disease (CAD) and cardiac sudden death.1–4
Furthermore, the consumption of TFA in the human diet has been associated with an increased risk of dyslipidemia, diabetes mellitus (DM), metabolic syndrome (MetS), allergic disease, and so on. These adverse effects of TFA have been primarily linked to its impact on lipoprotein metabolism and insulin sensitivity. In particular, TFA are known to worsen plasma lipid profile by increasing low-density lipoprotein cholesterol (LDL-C) and triglycerides (TG), as well as reducing high-density lipoprotein cholesterol (HDL-C).5–8
In addition, an excessive intake of TFA has been reported to be associated with systemic inflammation and endothelial dysfunction.9
In accordance with these experimental and observational findings, the guidelines of public organizations in Western countries (World Health Organization, Food and Agriculture Organization, The Food and Drug Administration etc) proposed an upper limit for the intake of TFA (<1% daily total energy intake).10
Editorial p 1902
In 2006, the Cabinet of Japan Food Safety Committee measured the TFA content in foods commonly distributed in Japan and estimated the average daily intake of TFA by Japanese people to be 0.7 g (0.3% of the daily total energy intake), which was much lower than that reported in Western countries.11
Also, intake of dietary lipids in Japan is considered to be somewhat different from that in Western countries, in terms of the consumption of fish. Therefore, the TFA upper intake limit that has been established in Western countries cannot be directly generalized for use in Japan and it is not appropriate to extrapolate the guidelines from Western countries directly in Japan. It is true that the dietary habit and lifestyle of the Japanese people has been rapidly westernized in recent decades, and this Western influence is particularly seen in the younger generations. It remains unknown, however, whether TFA is a risk factor for CAD in Japan.12,13
The present study was undertaken to measure the serum concentration of TFA, and to analyze the impact of serum TFA level on lipid profile and prevalence of CAD in Japan.
Methods
Serum levels of elaidic acid (trans 9-C18:1) and linolelaidic acid (trans 9, 12-C18:2) were determined using gas chromatography/mass spectrometry in patients with CAD and/or MetS. We investigated the association of the serum TFA concentration with serum lipid profiles, coronary risk factors, and prevalence of CAD. The complete Methods section is detailed in the online Supplement.
Results
Patient Baseline Characteristics
Of the total 902 patients (aged 21–91 years) enrolled in this study, 463 patients had CAD. The 439 patients without CAD (non-CAD) did not have atherosclerotic vascular diseases but had arrhythmia, valvular heart disease, or cardiomyopathy. As shown in
Table 1, there were significant differences in age, gender, prevalence of MetS, hypertension, diabetes, and dyslipidemia, between the CAD and non-CAD patients. Significantly more patients with CAD were being treated with statins compared with those without CAD for secondary prevention.14
As a result, the CAD patients had significantly lower total cholesterol and LDL-C than the non-CAD patients. In contrast, TG were higher and HDL-C lower in CAD patients than in non-CAD patients, which may reflect residual risks during statin treatment.
Table 1.
Patient Characteristics vs. Presence of CAD
| |
Without CAD (n=439) |
With CAD (n=463) |
P-value† |
| Male |
252 (57.4) |
372 (80.4) |
<0.001 |
| Age (years) |
61.2±13.1 |
68.0±10.1 |
<0.001 |
| BMI (kg/m2) |
23.5±3.9 |
24.4±3.4 |
<0.001 |
| Metabolic syndrome |
75 (17.1) |
243 (52.5) |
<0.001 |
| Hypertension |
192 (43.7) |
381 (82.3) |
<0.001 |
| DM |
58 (13.2) |
225 (48.6) |
<0.001 |
| Dyslipidemia |
165 (37.6) |
381 (82.3) |
<0.001 |
| Family history of CAD |
68 (15.5) |
130 (28.1) |
<0.001 |
| Current smoking |
86 (19.6) |
98 (21.2) |
0.557 |
| Statin therapy |
94 (21.4) |
310 (70.0) |
<0.001 |
| TC (mg/dl) |
188.2±34.9 |
167.4±34.5 |
<0.001 |
| HDL-C (mg/dl) |
56.4±16.3 |
47.4±13.3 |
<0.001 |
| LDL-C (mg/dl) |
111.1±29.6 |
98.1±29.3 |
<0.001 |
| Triglycerides (mg/dl) |
120.7±62.4 |
134.0±63.7 |
<0.001 |
| RLP-C (mg/dl) |
7.4±4.8 |
7.7±4.9 |
0.372 |
| Apo B48 (μg/ml) |
4.1±2.5 |
4.9±3.1 |
0.001 |
| FPG (mg/dl) |
95.5±18.7 |
106.7±29.2 |
<0.001 |
| Elaidic acid (μmol/L) |
13.6±5.2 |
13.5±5.5 |
0.408 |
| Linolelaidic acid (μmol/L) |
0.71±0.23 |
0.70±0.24 |
0.136 |
Data given as mean±SD or n (%). †Chi-squared test for categorical values and unpaired t-test for continuous variables. Elaidic acid and linolelaidic acid were analyzed after normalization by logarithmic transformation. Apo, apolipoprotein; BMI, body mass index; CAD, coronary artery disease; DM, diabetes mellitus; FPG, fasting plasma glucose; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; RLP-C, remnant-like particle cholesterol; TC, total cholesterol.
As shown in
Table 2, there were significant differences in age, gender, prevalence of CAD, hypertension, diabetes, and dyslipidemia, between the MetS and non-MetS patients. LDL-C in patients with MetS, however, was lower than in those without MetS, because many of the MetS patients who had CAD and/or multiple CAD risks were treated with statins.
Table 2.
Patient Characteristics vs. Presence of MetS
| |
Without MetS (n=584) |
With MetS (n=318) |
P-value† |
| Male |
363 (62.2) |
261 (82.1) |
<0.001 |
| Age (years) |
63.9±12.8 |
66.1±10.6 |
0.005 |
| BMI (kg/m2) |
22.8±3.4 |
26.1±3.2 |
<0.001 |
| Hypertension |
274 (46.9) |
299 (97.0) |
<0.001 |
| DM |
102 (17.5) |
181 (56.9) |
<0.001 |
| Dyslipidemia |
249 (42.6) |
297 (93.4) |
<0.001 |
| CAD |
220 (37.7) |
243 (76.4) |
<0.001 |
| Family history of CAD |
114 (19.5) |
84 (26.4) |
0.017 |
| Current smoking |
118 (20.2) |
66 (20.8) |
0.845 |
| Statin therapy |
178 (30.5) |
226 (71.1) |
<0.001 |
| TC (mg/dl) |
182.1±35.8 |
169.2±35.5 |
<0.001 |
| HDL-C (mg/dl) |
54.9±16.4 |
46.1±11.7 |
<0.001 |
| LDL-C (mg/dl) |
107.1±29.8 |
99.5±30.1 |
<0.001 |
| Triglycerides (mg/dl) |
117.7±60.3 |
145.4±65.0 |
<0.001 |
| RLP-C (mg/dl) |
7.1±4.7 |
8.2±5.1 |
0.002 |
| Apo B48 (μg/ml) |
4.4±2.9 |
5.0±2.9 |
0.012 |
| FPG (mg/dl) |
97.5±21.8 |
108.1±29.5 |
<0.001 |
| Elaidic acid (μmol/L) |
13.1±5.1 |
14.3±5.7 |
0.001 |
| Linolelaidic acid (μmol/L) |
0.70±0.23 |
0.73±0.24 |
0.064 |
Data given as mean±SD or n (%). †Chi-squared test for categorical values and unpaired t-test for continuous variables. Elaidic acid and linolelaidic acid were analyzed after normalization by logarithmic transformation. MetS, metabolic syndrome. Other abbreviations as in Table 1.
Among the many isoforms of industrially produced TFA, we measured elaidic acid (trans-9-C18:1) and linolelaidic acid (trans-9, 12-C18:2), because these 2 fatty acids are the most commonly contained in processed food products.8,15,16
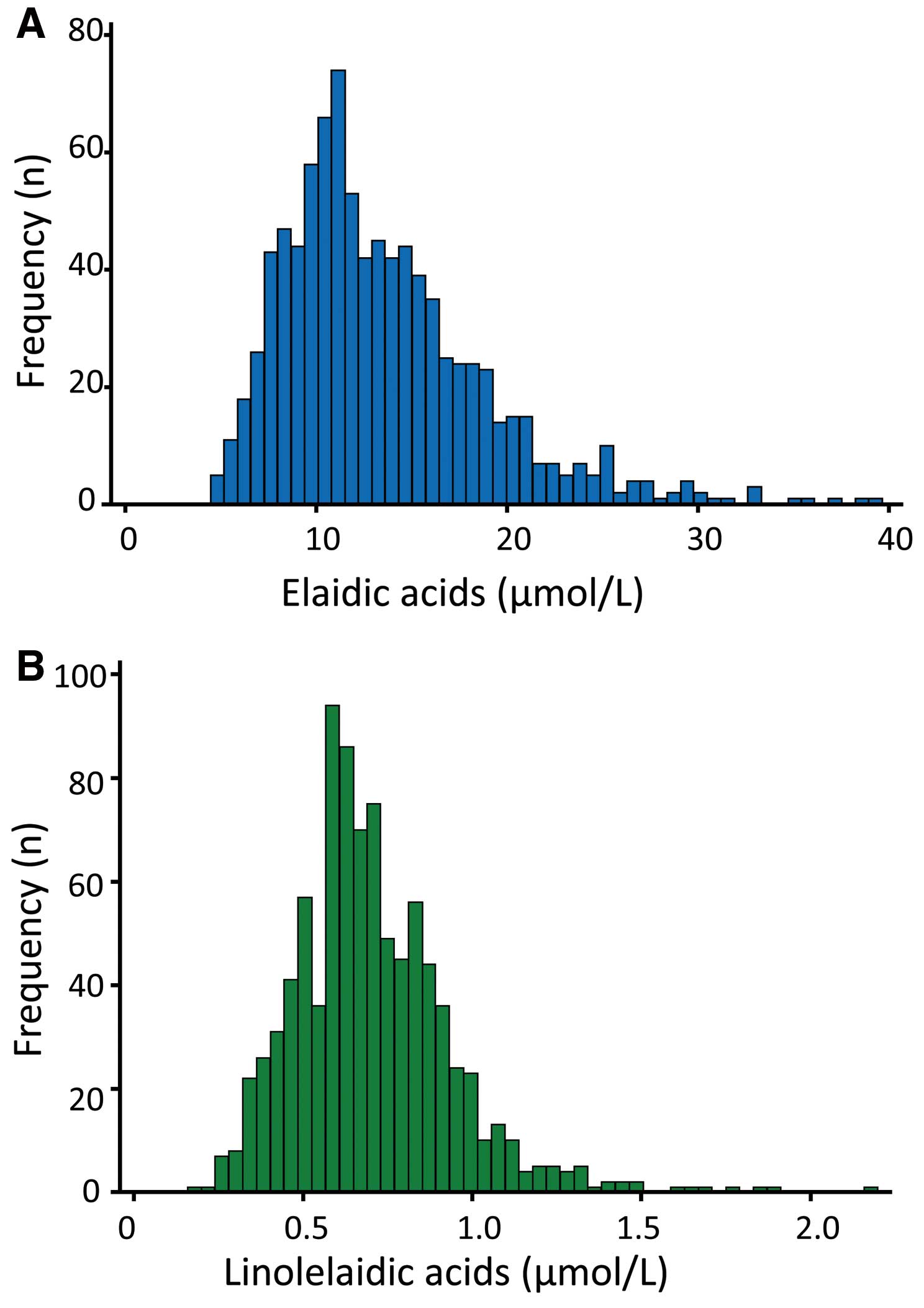
Mean (5th–95th centiles) elaidic and linolelaidic acid in all patients was 13.5 µmol/L (6.9–23.9 µmol/L) and 0.68 µmol/L (0.39–1.11 µmol/L), respectively. The distribution of serum concentration of elaidic and linolelaidic acid was skewed to the left (Figure 1), so we normalized TFA by logarithmic transformation in further statistical analysis. Serum linolelaidic acid was much lower than elaidic acid. Moreover, previous studies have reported that the C18:1 trans isomers comprise up to 50% of total TFA in industrially processed foods,15
and elaidic acid is the most widely used of the C18:1 trans isomers.8
Therefore, we focused on elaidic acid for the detailed evaluation. Serum elaidic acid had a positive correlation with body mass index, waist circumference, LDL-C, TG, remnant-like particle cholesterol (RLP-C) and apolipoprotein (apo) B48 (Figures 2B–G), and an inverse correlation with age and HDL-C (Figures 2A,H). Additionally, high elaidic acid was associated with well-characterized atherogenic factors (Table S1). There were no differences, however, in serum elaidic acid level between patients treated with and without anti-hyperlipidemic-, or anti-diabetic drugs (data not shown). This suggests that the conventional medical treatment for lifestyle-associated disease cannot reduce serum TFA level.
Next, we compared serum TFA level according to the presence of CAD or MetS. When all patients were examined, serum TFA level was similar between the non-CAD group and the CAD group (Table 1;
Figure 3A). Serum elaidic acid, however, was significantly higher in patients with MetS than in those without MetS (Table 2;
Figure 3B). This indicates that serum TFA level is regulated by lifestyle-associated metabolic disorders.
Serum TFA in CAD or MetS Patients According to Age
There was an inverse correlation between age and serum elaidic acid (r=–0.2238, P<0.001;
Figure 2A;
Table S1) or linolelaidic acid (r=–0.1755, P<0.001) in all patients. Similarly, TFA (elaidic acid) was inversely correlated with age in patients with CAD (r=–0.1484, P=0.002), those without CAD (r=–0.3187, P<0.001), with MetS (r=–0.3359, P<0.001), or without MetS (r=–0.1911, P<0.001). Because multivariate logistic regression modeling confirmed a significant association between TFA and age (data not shown), we divided the patients into 4 groups by age: first group, 21–58 years old; second group, 59–66 years old; third group, 67–74 years old; and fourth group, 75–91 years old. The patient characteristics of the quartile groups are shown in
Table 3. The older patients were found to have a higher prevalence of CAD, DM, dyslipidemia and stroke, and tended to be treated with lipid-lowering drugs. As a result, the serum levels of atherogenic lipid markers such as, LDL-C, TG, RLP-C, and apoB48, were lower in the older groups than in the younger groups. There was no association between statin use and serum TFA level in each group (data not shown).
Table 3.
Patient Characteristics According to Age Quartile
| |
Q1 (n=240)
21–58 years old |
Q2 (n=216)
59–66 years old |
Q3 (n=250)
67–74 years old |
Q4 (n=196)
75–91 years old |
P-value† |
| Male |
173 (72.1) |
161 (74.5) |
154 (61.6) |
136 (69.4) |
0.014 |
| Age (years) |
48.4±8.2 |
62.9±2.3 |
70.8±2.3 |
78.7±3.2 |
<0.001 |
| BMI (kg/m2) |
24.6±4.1 |
23.8±3.6 |
23.8±3.7 |
23.4±3.2 |
0.005 |
| MetS |
68 (28.3) |
89 (41.2) |
84 (33.6) |
77 (39.3) |
0.018 |
| Hypertension |
103 (42.9) |
152 (70.4) |
169 (67.6) |
149 (76.0) |
<0.001 |
| Diabetes |
48 (20.0) |
75 (34.7) |
88 (35.2) |
72 (36.7) |
<0.001 |
| Dyslipidemia |
113 (47.1) |
141 (65.3) |
169 (67.6) |
123 (62.8) |
<0.001 |
| CAD |
77 (32.1) |
117 (54.2) |
132 (52.8) |
137 (69.9) |
<0.001 |
| Family history of CAD |
53 (22.1) |
50 (23.2) |
53 (21.2) |
42 (21.4) |
0.961 |
| Current smoking |
74 (30.8) |
56 (25.9) |
35 (14.0) |
19 (9.7) |
<0.001 |
| Statin therapy |
70 (29.2) |
102 (47.2) |
134 (53.6) |
98 (50.0) |
<0.001 |
| TC (mg/dl) |
187.3±36.7 |
178.7±38 |
177.6±33.3 |
164.2±33.4 |
<0.001 |
| HDL-C (mg/dl) |
52.9±17.1 |
51.5±15 |
52.6±15.6 |
49.6±13.4 |
0.108 |
| LDL-C (mg/dl) |
110.4±30.4 |
105.1±32.6 |
104.5±28.3 |
96.3±27.4 |
<0.001 |
| Triglycerides (mg/dl) |
146.7±79.5 |
136.6±65.6 |
117.2±51.7 |
107.1±39.3 |
<0.001 |
| RLP-C (mg/dl) |
9.0±6.0 |
8.0±5.0 |
6.9±4.2 |
6.0±3.1 |
<0.001 |
| ApoB48 (μg/ml) |
4.7±3.4 |
4.6±2.8 |
4.5±2.8 |
4.6±2.6 |
0.932 |
| FPG (mg/dl) |
96.8±21.6 |
105.1±29.6 |
100±22.5 |
103.8±26.8 |
<0.001 |
| Elaidic acid (μmol/L) |
15.4±6.4 |
13.8±5.1 |
12.8±4.6 |
11.7±4.3 |
<0.001 |
| Linolelaidic acid (μmol/L) |
0.78±0.27 |
0.69±0.25 |
0.68±0.20 |
0.66±0.18 |
<0.001 |
Data given as mean±SD or n (%). †Chi-squared test for categorical values or 1-way ANOVA for continuous variables. Elaidic acid and linolelaidic acid were analyzed after normalization by logarithmic transformation. Q, quartile. Other abbreviations as in Tables 1,2.
We investigated the relationship between TFA and CAD in each patient group. In the first quartile and second quartile groups, serum TFA was significantly higher in the patients with CAD than in patients without CAD, but this was not true of the third and fourth quartile groups (Figure 4A). This tendency was evident in the case of MetS: in the first, second, and third age quartiles, TFA was higher in patients with MetS than in those without MetS (Figure 4B).
Table S2
presents univariate logistic regression analysis of selected classic factors17
for the risk of CAD in each group. In the first quartile and second quartile groups (21–66 years old, n=456), serum TFA was a CAD risk factor. This suggests that serum TFA concentration impacts on multiple metabolic disorders and represents a risk for CAD particularly in the young generation of Japanese people, who had seemingly been a low-risk group.
Serum TFA as a Risk Factor of CAD in the Young Generation
Because an effect of TFA appeared to be present in the young generation (Figure 4), we divided the patients into 2 groups: the young group, which consisted of the first plus second quartile groups (21–66 years old, n=456); and the old group, which consisted of the third plus fourth quartile groups (67–91 years old, n=446). We investigated the relationship between TFA and CAD in the young and old groups on multivariate logistic regression modeling (Table 4). In the young patient group, TFA was associated with CAD after adjustment for classical risks, but this was not true in the old group. This confirmed that serum TFA level is a risk factor for CAD in the younger generation.
Table 4.
Multivariate Indicators of CAD vs. Age
| Age groups |
Young group (Q1+Q2) |
Old group (Q3+Q4) |
| Factors |
OR |
95% CI |
P-value |
OR |
95% CI |
P-value |
| ln(elaidic acid) |
2.25 |
1.14 |
4.47 |
0.020 |
0.71 |
0.35 |
1.45 |
0.346 |
| Age |
1.06 |
1.03 |
1.10 |
<0.001 |
1.08 |
1.02 |
1.13 |
0.005 |
| Female |
0.35 |
0.19 |
0.64 |
0.001 |
0.37 |
0.22 |
0.60 |
<0.001 |
| BMI (kg/m2) |
1.15 |
0.68 |
1.94 |
0.612 |
1.08 |
0.51 |
2.26 |
0.845 |
| Current smoking |
1.05 |
0.98 |
1.13 |
0.138 |
0.99 |
0.92 |
1.06 |
0.762 |
| Hypertension |
3.31 |
2.01 |
5.45 |
<0.001 |
3.58 |
2.14 |
6.02 |
<0.001 |
| DM |
3.85 |
2.23 |
6.62 |
<0.001 |
3.61 |
2.12 |
6.14 |
<0.001 |
| TC (mg/dl) |
0.99 |
0.99 |
1.00 |
0.037 |
0.99 |
0.98 |
1.00 |
0.004 |
| HDL-C (mg/dl) |
0.98 |
0.97 |
1.00 |
0.064 |
0.98 |
0.96 |
1.00 |
0.037 |
Young group, 21–67 years (n=456); old group, 67–91 years (n=446). CI, confidence interval; OR, odds ratio. Other abbreviations as in Tables 1–3.
Because TG is an ester derived from glycerol and 3 fatty acids, we evaluated the role of major fatty acids and TG in CAD. As shown in
Table S3, on univariate logistic regression analysis both TFA and TG were identified as significant predictors of CAD in the young and old groups. On multivariate logistic regression analysis, TFA was a CAD risk only in the young group, while TG was a CAD risk both in the young and old groups. Interestingly, other fatty acids including palmitic, stearic, or oleic acid were not significantly correlated with CAD (Table S3), suggesting that the risk of high TG might be at least partially mediated by the diet-derived TFA in the young generation.
Discussion
Numerous epidemiological and clinical studies have demonstrated that excessive TFA intake is a risk for CAD in Western countries. From the viewpoint of public health, therefore, the amount of dietary TFA intake has been advised to be limited in many countries. Also, the food industry is required to clearly display TFA content on processed food packaging. In contrast, the traditional Japanese diet commonly consists of vegetables, soybean products, seaweed, mushrooms, fish and so on,18,19
and considered to contain a much lower volume of lipids including TFA compared with the Western diet.20
It was unclear as to whether such a low amount of TFA in foods causes a health problem in Japan.20
It should be noted, however, that the Japanese lifestyle has been westernized. In particular, the young generation in Japan tend to like the Western diet, including fast foods and processed oily foods, while the older generations still prefer the traditional Japanese diet.21
In the present study, therefore, we measured serum TFA concentration and evaluated its impact on coronary risk in Japanese subjects. We found that serum TFA concentration was associated with serum atherogenic lipid profile and the prevalence of MetS. Particularly, TFA was correlated with LDL-C and TG (Figures 2D,E). LDL-C was lower in the CAD patients than that in the non-CAD patients because they were taking LDL-lowering medication, which is referred to as “reverse causality”. Previous studies suggested that baseline LDL-C had a modest impact on CAD events during intensive lipid-lowering treatment.22–24
In contrast, serum TFA concentration was higher in young CAD patients than in non-CAD patients, and was found to be a significant risk factor for CAD in the younger generation after adjustment for classical CAD factors. Thus, the present study directly documented an impact of serum TFA concentration on CAD risk, in contrast with previous studies evaluating the risk according to estimated daily TFA intake.1–4,15
In this context, we have expanded the understanding of TFA risk from the estimated daily intake to serum concentration.
Serum TFA level is regulated by dietary TFA intake, its absorption in the intestine, and/or catabolism of exogenously derived lipids. Because humans cannot synthesize TFA, circulating TFA may be used as a tracer for intestinally derived lipids. In the present study, serum TFA level was inversely correlated with age, which may reflect the variation in the amount and composition of fatty acid intake by age. It has been reported that the older generation tends to consume fish and vegetables and avoid processed foods containing TFA, compared with the younger generation in Japan.12,13,20,25
Thus, it is necessary to evaluate the relationship between dietary TFA intake and serum TFA concentration. In contrast, serum TFA had a strong correlation with TG, RLP-C and apoB48, which represent exogenously derived lipids and act as a marker for post-prandial hyperlipidemia.26,27
Given that apoB48 exists only in chylomicron and chylomicron remnants,28
high serum TFA may be associated with the increased dietary intake as well as the delayed catabolism of chylomicron remnants. In any case, high serum TFA may imply the under-recognized adverse effect of the atherogenic intestinally derived lipids. Taken together, TFA may become an alarmingly predominant public health problem in the future in Japan.
The incidence of CAD reported in prospective studies as a result of TFA exposure has been greater than that predicted due to increased serum lipids or inflammation alone. Thus, the association between TFA consumption and cardiovascular disease events cannot be explained only by changes in lipid profile or C-reactive protein, and the mechanisms behind the adverse effects of TFA are not fully understood. It has been reported that TFA is incorporated into the cell membrane as well as circulating lipoproteins, and regulates biomolecular interaction and receptor action.29,30
Moreover, the lipotoxicity of TFA is involved in several inflammation pathways targeting multiple organs and systems,31
suggesting the direct impact of TFA on inflammation or endothelial dysfunction.32,33
It has been reported that TFA may activate pro-inflammatory toll-like receptor pathways.34
Furthermore, TFA may modulate cardiac membrane ion channel function,35
and it may have proarrhythmogenic properties.36
These direct actions of TFA may contribute to the increase of cardiovascular events.
There are several limitations in this study. First, we found that TG was also significantly associated with the prevalence of CAD in both the young and old groups (Table S3). Because TFA is a component of TG, a linear relationship between TG and elaidic acid was seen on regression modeling (Figure 2E). Therefore, the present findings are insufficient to conclude whether serum TFA level is independently involved in the genesis of CAD or merely elevated concomitantly with TG. Second, all samples were collected from hospitalized patients under fasting conditions, and we did not evaluate serum TFA concentration in young subjects in the general population, or in the post-prandial state. Because serum TFA level was inversely correlated with age, the TFA level in adolescents and young children is also of interest and should be evaluated. As seen in this study, the younger the patients, the larger the difference in serum TFA level between CAD and non-CAD patients. Therefore, we speculate that the TFA risk in adolescents or young children may be greater than in older generations. Third, the non-CAD patients in the present study were not healthy. In our preliminary study, serum elaidic acid level in healthy subjects was 11.2±4.9 µmol/L (mean±SD, n=148), which is lower than that in the present CAD or non-CAD patients (Hirata et al, unpublished data, 2013), while the impact of TFA in healthy subjects needs to be determined. Fourth, because this is a cross-sectional study, we could not determine the effect of anti-hyperlipidemic or anti-diabetic drugs on serum TFA level. Further studies, including a large-scale prospective study, are necessary to establish the strategy for risk management of TFA.
Conclusions
Serum TFA concentration was elevated in young patients with CAD and/or MetS. TFA may have yet unrecognized adverse health effects in metabolic and cardiovascular diseases, particularly in the young generations in Japan.
Acknowledgments
We thank Fujirebio (Tokyo, Japan) for measuring serum apoB48 concentration. We acknowledge the members of our laboratories for their stimulating discussions.
Disclosures
This work was supported by Grants-In-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan, and by Grants-in-Aid from the Cabinet Office Food Safety Commission of Japan.
Conflict of Interest
The authors have no conflicts of interest directly relevant to the content of this study.
Supplementary Files
Supplementary File 1
Methods
Table S1.
Serum elaidic acid and coronary risk markers
Table S2.
Univariate indicators of CAD vs. age quartile
Table S3.
Impact of typical fatty acids and TG on CAD
Please find supplementary file(s);
http://dx.doi.org/10.1253/circj.CJ-14-0750
References
- 1.
Gillman MW, Cupples LA, Gagnon D, Millen BE, Ellison RC, Castelli WP. Margarine intake and subsequent coronary heart disease in men. Epidemiology 1997; 8: 144–149.
- 2.
Willett WC, Stampfer MJ, Manson JE, Colditz GA, Speizer FE, Rosner BA, et al. Intake of trans fatty acids and risk of coronary heart disease among women. Lancet 1993; 341: 581–585.
- 3.
Ascherio A, Hennekens CH, Buring JE, Master C, Stampfer MJ, Willett WC. Trans-fatty acids intake and risk of myocardial infarction. Circulation 1994; 89: 94–101.
- 4.
Mozaffarian D, Katan MB, Ascherio A, Stampfer MJ, Willett WC. Trans fatty acids and cardiovascular disease. N Engl J Med 2006; 354: 1601–1613.
- 5.
Katan MB, Zock PL, Mensink RP. Trans fatty acids and their effects on lipoproteins in humans. Annu Rev Nutr 1995; 15: 473–493.
- 6.
Hunter JE. Dietary trans fatty acids: Review of recent human studies and food industry responses. Lipids 2006; 41: 967–992.
- 7.
Mensink RP, Zock PL, Kester AD, Katan MB. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: A meta-analysis of 60 controlled trials. Am J Clin Nutr 2003; 77: 1146–1155.
- 8.
Gebauer SK, Psota TL, Kris-Etherton PM. The diversity of health effects of individual trans fatty acid isomers. Lipids 2007; 42: 787–799.
- 9.
Mozaffarian D. Trans fatty acids: Effects on systemic inflammation and endothelial function. Atheroscler Suppl 2006; 7: 29–32.
- 10.
WHO. Diet, nutrition and the prevention of chronic diseases. World Health Organ Tech Rep Ser 2003; 916: i–viii, 1–149.
- 11.
Cabinet of Japan Food Safety Committee. Evaluative report on trans fatty acid content in foods: Total confirmation assessment in 2006. Tokyo: Cabinet of Japan Food Safety Committee, 2007 (in Japanese).
- 12.
Yamada M, Sasaki S, Murakami K, Takahashi Y, Uenishi K; Japan Dietetic Students’ Study for Nutrition and Biomarkers Group. Association of trans fatty acid intake with metabolic risk factors among free-living young Japanese women. Asia Pac J Clin Nutr 2009; 18: 359–371.
- 13.
Yamada M, Sasaki S, Murakami K, Takahashi Y, Okubo H, Hirota N, et al. Estimation of trans fatty acid intake in Japanese adults using 16-day diet records based on a food composition database developed for the Japanese population. J Epidemiol 2010; 20: 119–127.
- 14.
JCS Joint Working Group. Guidelines for secondary prevention of myocardial infarction (JCS 2011): Digest version. Circ J 2013; 77: 231–248.
- 15.
Kuhnt K, Baehr M, Rohrer C, Jahreis G. Trans fatty acid isomers and the trans-9/trans-11 index in fat containing foods. Eur J Lipid Sci Technol 2011; 113: 1281–1292.
- 16.
Nazari B, Asgary S, Azadbakht L. Fatty acid analysis of Iranian junk food, dairy, and bakery products: Special attention to trans-fats. J Res Med Sci 2012; 17: 952–957.
- 17.
D’Agostino RB Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, et al. General cardiovascular risk profile for use in primary care: The Framingham Heart Study. Circulation 2008; 117: 743–753.
- 18.
Kromhout D, Menotti A, Bloemberg B, Aravanis C, Blackburn H, Buzina R, et al. Dietary saturated and trans fatty acids and cholesterol and 25-year mortality from coronary heart disease: The Seven Countries Study. Prev Med 1995; 24: 308–315.
- 19.
Maruyama K, Iso H, Date C, Kikuchi S, Watanabe Y, Wada Y, et al. Dietary patterns and risk of cardiovascular deaths among middle-aged Japanese: JACC Study. Nutr Metab Cardiovasc Dis 2013; 23: 519–527.
- 20.
Research Program for Risk Assessment Study on Food Safety. Executive summary of effects of dietary trans fatty acids on health in Japanese, 2010. Tokyo: Food Safety Commission of Japan, 2010 (in Japanese).
- 21.
Nakamura M, Ojima S. Dietary pattern and prevention of cardiovascular disease. Jpn J Cardiovasc Dis Prev 2014; 49: 12–18 (in Japanese).
- 22.
Sbrana F, Cocci F, Papa A, Landi P, Sampietro T, Rossi G, et al. Routine laboratory tests to risk-stratify patients with chronic coronary artery disease. J Cardiol 2013; 61: 132–137.
- 23.
Tajika K, Okamatsu K, Takano M, Inami S, Yamamoto M, Murakami D, et al. Malondialdehyde-modified low-density lipoprotein is a useful marker to identify patients with vulnerable plaque. Circ J 2012; 76: 2211–2217.
- 24.
Uematsu M, Nakamura T, Sugamata W, Kitta Y, Fujioka D, Saito Y, et al. Echolucency of carotid plaque is useful for assessment of residual cardiovascular risk in patients with chronic coronary artery disease who achieve LDL-C goals on statin therapy. Circ J 2014; 78: 151–158.
- 25.
Research Program for Risk Assessment Study on Food Safety. Risk assessment report trans fatty acids in foods, 2012. Tokyo: Food Safety Commission of Japan, 2012 (in Japanese).
- 26.
Smith D, Watts GF, Dane-Stewart C, Mamo JC. Post-prandial chylomicron response may be predicted by a single measurement of plasma apolipoprotein B48 in the fasting state. Eur J Clin Invest 1999; 29: 204–209.
- 27.
Adiels M, Matikainen N, Westerbacka J, Soderlund S, Larsson T, Olofsson SO, et al. Postprandial accumulation of chylomicrons and chylomicron remnants is determined by the clearance capacity. Atherosclerosis 2012; 222: 222–228.
- 28.
Mori K, Ishida T, Yasuda T, Monguchi T, Sasaki M, Kondo K, et al. Fasting serum concentration of apolipoprotein B48 represents residual risks in patients with new-onset and chronic coronary artery disease. Clin Chim Acta 2013; 421: 51–56.
- 29.
Harris WS, Reid KJ, Sands SA, Spertus JA. Blood omega-3 and trans fatty acids in middle-aged acute coronary syndrome patients. Am J Cardiol 2007; 99: 154–158.
- 30.
Lemaitre RN, King IB, Mozaffarian D, Sotoodehnia N, Rea TD, Kuller LH, et al. Plasma phospholipid trans fatty acids, fatal ischemic heart disease, and sudden cardiac death in older adults: The cardiovascular health study. Circulation 2006; 114: 209–215.
- 31.
Estadella D, da Penha Oller do Nascimento CM, Oyama LM, Ribeiro EB, Dâmaso AR, de Piano A. Lipotoxicity: Effects of dietary saturated and trans fatty acids. Mediators Inflamm 2013; 2013: 137579.
- 32.
Lopez-Garcia E, Schulze MB, Meigs JB, Manson JE, Rifai N, Stampfer MJ, et al. Consumption of trans fatty acids is related to plasma biomarkers of inflammation and endothelial dysfunction. J Nutr 2005; 135: 562–566.
- 33.
de Roos NM, Bots ML, Katan MB. Replacement of dietary saturated fatty acids by trans fatty acids lowers serum HDL cholesterol and impairs endothelial function in healthy men and women. Arterioscler Thromb Vasc Biol 2001; 21: 1233–1237.
- 34.
Kondo K, Ishida T, Yasuda T, Nakajima H, Mori K, Tanaka N, et al. Trans-fatty acid promotes thrombus formation in mice by aggravating antithrombogenic endothelial functions via Toll-like receptors. Mol Nutr Food Res 2015; 59: 729–740.
- 35.
Katz AM. Trans-fatty acids and sudden cardiac death. Circulation 2002; 105: 669–671.
- 36.
Soares-Miranda L, Stein PK, Imamura F, Sattelmair J, Lemaitre RN, Siscovick DS, et al. Trans-fatty acid consumption and heart rate variability in 2 separate cohorts of older and younger adults. Circ Arrhythm Electrophysiol 2012; 5: 728–738.






