2016 Volume 80 Issue 10 Pages 2109-2116
2016 Volume 80 Issue 10 Pages 2109-2116
Background: The phenotypic heterogeneity of Brugada syndrome (BrS) can lead some patients to show an additional inferolateral early repolarization pattern (ERP), or fragmented QRS (f-QRS). The aim of the study was to investigate the prevalence and clinical impact of f-QRS, ERP or combined f-QRS/ERP in high-risk patients with BrS.
Methods and Results: Patients with spontaneous or drug-induced BrS and an indication to receive an implantable cardioverter-defibrillator (ICD) were considered eligible for this study. From 1992 to 2012, a total of 176 consecutive patients with BrS underwent ICD implantation. Among them, 48 subjects (27.3%) presented with additional depolarization and/or repolarization abnormalities. f-QRS was found in 29 (16.5%), ERP in 15 (8.5%), and combined f-QRS/ERP in 4 patients (2.3%). After a mean follow-up of 95.2±51.9 months, spontaneous sustained ventricular arrhythmias were documented in 8 patients (16.7%). No significant difference was found in the rate of appropriate shocks between patients presenting with f-QRS or ERP and those without abnormalities. Patients with both f-QRS and ERP had a significantly higher rate of appropriate shocks (HR: 4.1; 95% CI: 1.1–19.7; P=0.04).
Conclusions: Fragmented QRS and ERP are common ECG findings in high-risk BrS patients, occurring in up to 27% of cases. When combined, f-QRS and ERP confer a higher risk of appropriate ICD interventions during a very long-term follow-up. (Circ J 2016; 80: 2109–2116)
Brugada syndrome (BrS) is an inheritable syndrome characterized by an increased risk of sudden death (SD) in the absence of structural heart disease.1,2 The placement of an implantable cardioverter-defibrillator (ICD) remains the only therapy with proven efficacy to prevent SD in patients with BrS at high risk for further arrhythmic events.3–5 Although the presence of spontaneous or drug-induced coved-type ST-segment elevation in the right precordial leads (V1–3) is the ECG hallmark of the syndrome, some patients show additional early repolarization signs in the inferolateral leads, or present with depolarization abnormalities.3,6–9 Early repolarization pattern (ERP) is a recognized risk factor for ventricular arrhythmias (VAs) in patients with BrS and may appear, on 12-lead ECG, as notching of the terminal portion of the QRS complex in the inferior and/or lateral leads.10,11 Similarly, abnormal fragmentation of the QRS complex (f-QRS) in the right precordial leads has been identified as an arrhythmic marker and might indicate regional conduction disturbance in the right ventricular outflow tract (RVOT) of patients with BrS.12 Although the presence of repolarization and depolarization abnormalities in patients with BrS has been previously assessed, in those studies only a small portion of patients were implanted with an ICD.7,13 Conversely, f-QRS and ERP have not been systematically investigated in high-risk patients. Moreover, a previous study on BrS patients undergoing ICD therapy showed that spontaneous type 1 ECG was not an independent predictor for further arrhythmic events.5 In addition, nearly 13% of asymptomatic patients with an ICD presented an appropriate shock during follow-up.5 Whether the preliminary results coming from previous studies suggest that these abnormalities could have a prognostic role in BrS, no data are available on the contributing role of ERP, f-QRS or combined ERP/f-QRS in determining the decision to implant an ICD in asymptomatic BrS patients considered at high risk for arrhythmic events.13 Therefore, the purpose of this study was to investigate the prevalence and the clinical impact of f-QRS, ERP or combined f-QRS/ERP in patients with spontaneous or drug-induced BrS undergoing ICD implantation.
Since 1992, all consecutive patients diagnosed with BrS have been included in a registry and followed in a prospective fashion. The Ethics Committee of the UZ Brussel-VUB approved the study protocol. A total of 524 patients with spontaneous or drug-induced BrS have been included in the registry from 1992 to 2012. Among them, 176 patients, considered to be at high-risk for further arrhythmic events, underwent ICD therapy and were included in the present study. Medical history, physical examination and baseline ECG were obtained and underlying structural cardiac abnormalities were excluded in all patients. All patients with signs of arrhythmogenic right ventricular cardiomyopathy detected by 2D transthoracic echocardiography or cardiac magnetic resonance imaging were excluded from this study.14 Patients were considered as symptomatic if they had presented with syncope and/or aborted SD. All patients with syncope, before undergoing ICD implantation, received a careful diagnostic workup in order to investigate the arrhythmic origin of the event. The syncope workup included a careful examination of the patient’s history, a 12-lead ECG, transthoracic echocardiography and Holter monitoring. Moreover, a tilt table test was performed in any case of suspected vasovagal syncope. Ajmaline (1 mg/kg) was administered intravenously over a 5-min period to unmask the diagnostic ECG pattern of BrS in cases of non-diagnostic baseline ECG. Electrophysiological study (EPS) was performed at the investigators’ preference in order to assess risk stratification.15 Genetic testing with sequence analysis of SCN5A was recommended for all patients with a diagnosis of BrS. Beginning from 2005, the indication for ICD therapy was determined using the recommendations of the second Brugada consensus conference.3 Technical aspects of ICD implantation, ICD programming and change over time of programming have been previously described.5
Patients analyzed in this study were considered at high risk of VAs if they presented at least one of the following clinical features at the time of ICD implantation or during the follow-up: aborted SD, sudden syncope, inducibility of VAs at EPS or during ajmaline challenge, family history of SD. Up to 76% of the study population patients presented with syncope or a previous episode of aborted SD. The remaining 24% of patients were asymptomatic at the time of implantation but presented inducibility of VA at EPS or during ajmaline (72%) or family history of SD with spontaneous type 1 ECG (28%). Moreover, among previously asymptomatic patients, 13% and 7% presented with sustained VA or syncope, respectively, during the follow-up.
12-Lead ECG AnalysisECGs were classified as Brugada coved-type (type 1) or saddleback (type 2) or normal. An ECG was considered diagnostic of BrS if a coved-type ST elevation ≥2 mm was documented in ≥1 lead from V1 to V3 in the presence or absence of a sodium-channel blocker agent. Abnormal fragmentation of the QRS complex (f-QRS) was defined as the presence of multiple spikes within the QRS (≥4 spikes in 1 or ≥8 spikes in all of the leads V1, V2, and V3)9 (Figure 1). Moreover, f-QRS in leads other rather than precordial ones was analyzed in any case. In patients with drug-induced BrS, the presence of f-QRS was assessed before drug administration. ECG-Holter monitoring was performed in all cases before ICD implantation, and further analyzed in order to detect occurrence of ERP during bradycardia. ERP was considered in the presence of QRS slurring (a smooth transition from the QRS segment to the ST segment) or notching (a positive J deflection of at least 1 mm inscribed on the S wave) in the inferior leads (II, III, and aVF), lateral leads (I, aVL, and V4–6), or both (Figure 2). The amplitude of the J-point elevation had to be at least 0.1 mV above the baseline level in all cases. To differentiate ERP, f-QRS and epsilon waves, the position of the slur or notch within the QRS complex was carefully evaluated. End-QRS notch or slur occurring on the final 50% of the downslope of an R-wave was considered ERP; in contrast, f-QRS complex consisting of a notch midway on the downslope of an R-wave was defined as f-QRS. Epsilon wave was defined as the presence of a reproducible low-amplitude signal between the end of the QRS complex and the onset of the T wave in the right precordial leads (V1–3).16 All baseline and follow-up 12-lead ECGs were recorded at a paper speed of 25 mm/s and amplitude of 10 mm/mV with the right precordial leads positioned at the sternal margin of the 3rd and 4th intercostal spaces. ECGs filter settings were 0.05–150 Hz. Two independent experienced electrophysiologists analyzed all ECGs; in case of disagreement a third physician was consulted.

Fragmented QRS in the right precordial leads of a Brugada syndrome patient with spontaneous type 1 ECG and syncopal episodes.
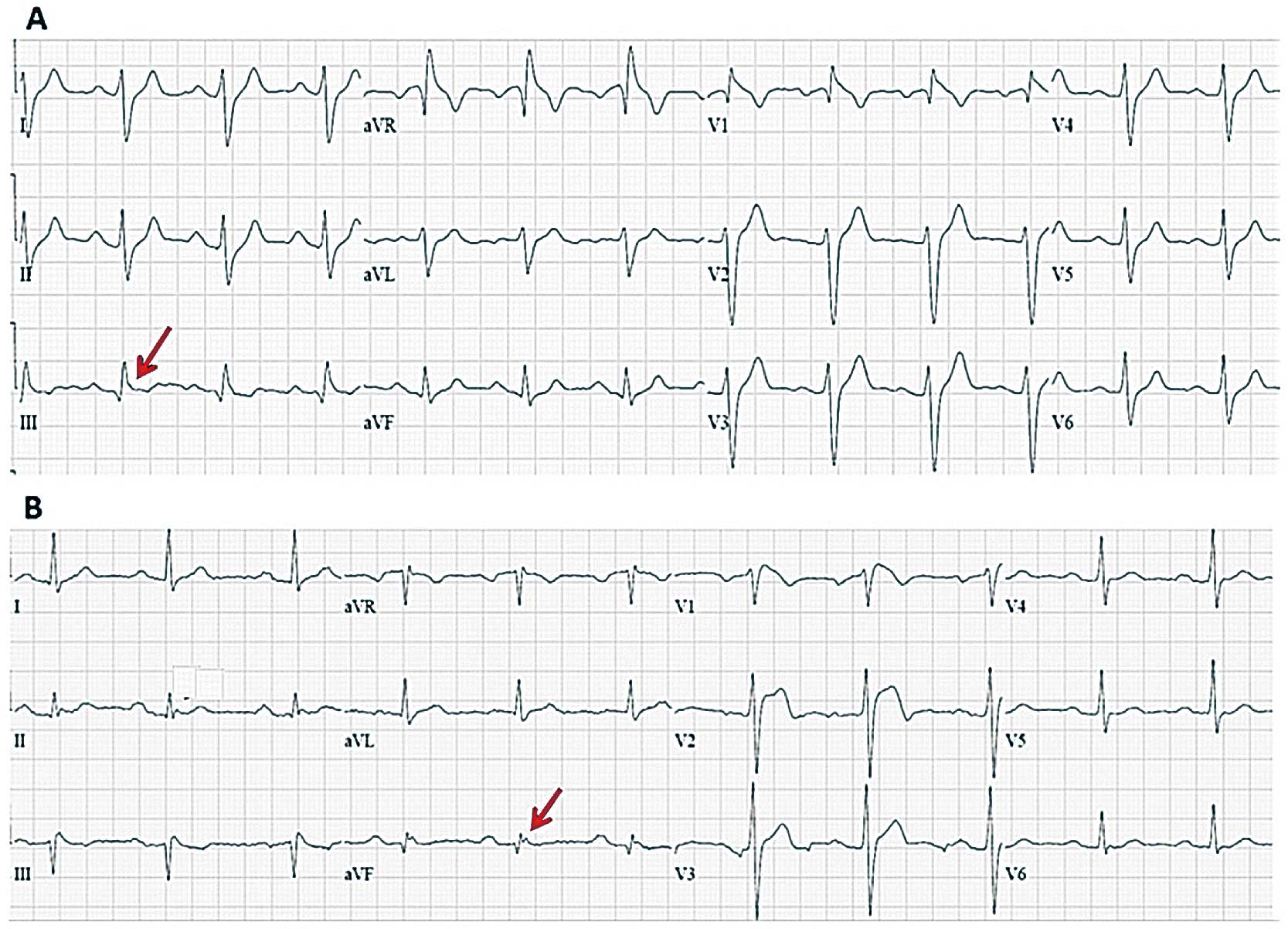
(A,B) Baseline ECGs of 2 Brugada syndrome patients with spontaneous type 1 ECG and early repolarization pattern in inferior leads (QRS slurring in patient A and J wave in patient B).
Clinical follow-up of patients consisted of physical examination and ECG performed at least every 6 months. Follow-up of the device was performed at 1 and 3 months after implantation and thereafter every 6 months. Clinical data and ECGs were regularly collected and analyzed. Only patients with a follow-up time longer than 6 months were considered eligible for this study. The definition of arrhythmic events receiving ICD therapy was predefined according to the cycle length of the arrhythmia. All available electrograms of appropriate and inappropriate shocks were analyzed independently by at least 2 investigators. Appropriate therapies were defined as shocks or antitachycardia pacing delivered for ventricular tachycardia (VT) or ventricular fibrillation (VF), and inappropriate therapies were defined as those delivered in the absence of VAs. Electrical storm was defined by 3 or more sustained episodes of VT, VF, or ICD appropriate shocks within 24 h.
Statistical AnalysisData are presented as mean±standard deviation or as absolute values and percentages where appropriate. Comparison between continuous variables was performed using the unpaired Student’s t-test or ANOVA as appropriate. The chi-squared test was used to compare categorical variables. Event-free survival was estimated by Kaplan-Meier method and compared by log-rank test. Hazard ratios (HRs) were calculated using Cox proportional hazards regression models. Cox regression analysis was used for the predictor model. Variables were selected on the basis of univariate significance and/or if they were known predictors in published research. On univariate analysis, 5 predictors (male sex, aborted SD, spontaneous Brugada type 1 ECG, VA inducibility on EPS and combined ERP/f-QRS) were significantly associated with arrhythmic events. The final model was selected by stepwise regression on the basis of likelihood ratios. A P value <0.05 was considered statistically significant. Statistical analyses were conducted using the SPSS software (SPSS v22, Chicago, IL, USA).
Of 176 consecutive patients, 48 (27.3%) presented with additional depolarization and/or repolarization abnormalities. Abnormal f-QRS complex was found in 29 (16.5%), ERP in 15 (8.5%), and both abnormalities in 4 patients (2.3%). Fragmented QRS, ERP or combined f-QRS/ERP were observed in 29% (38/133 pts) of symptomatic patients and in 23% (10/43 pts) of asymptomatic subjects undergoing ICD implantation. Baseline characteristics of the study population are shown in Table 1. No significant difference was found in the age, family history of SD or symptom status prior to ICD implantation between patients with f-QRS and/or ERP and patients without such abnormalities. Patients with f-QRS and/or ERP were more frequently male, although no statistical significance was reached (77% vs. 63.3%, P=0.08), and presented more frequently with baseline Brugada type 2 ECG as compared with patients without f-QRS/ERP (31.2% vs. 17.2%, P=0.04). A trend towards higher incidence of spontaneous Brugada type 1 ECG was also observed (35.4% vs. 19.5%, P=0.07). Moreover, the rate of probands was significantly higher in the group of patients with f-QRS/ERS (64.6% vs. 47.7%, P=0.04). Demographics, genetic profile and arrhythmic outcome according to the presence of f-QRS, ERP or both are shown in Table 2. The rates of spontaneous type 1 ECG, previous aborted SD or syncope were comparable between patients with ERP, f-QRS and combined ERP/f-QRS. Moreover, no significant differences were found in the other clinical features among patients presenting with different repolarization and/or depolarization abnormalities.
| Group I (n=48) |
Group II (n=128) |
P value | |
|---|---|---|---|
| Clinical features | |||
| Age, years | 42.7±15.7 | 40.0±17 | 0.64 |
| Male, n (%) | 37 (77.1) | 81 (63.3) | 0.08 |
| Probands, n (%) | 31 (64.6) | 61 (47.7) | 0.04 |
| Aborted SD, n (%) | 8 (16.7) | 17 (13.3) | 0.86 |
| Syncope, n (%) | 30 (62.5) | 78 (60.9) | 0.85 |
| Asymptomatic, n (%) | 10 (20.8) | 33 (25.8) | 0.46 |
| Family history of SD, n (%) | 22 (45.8) | 68 (53.1) | 0.39 |
| Previous atrial arrhythmias, n (%) | 7 (14.6) | 17 (13.3) | 0.82 |
| Previous SND, n (%) | 3 (6.2) | 6 (4.7) | 0.68 |
| Inducible at EPS, n (%)* | 18 (41.9) | 55 (44.3) | 0.85 |
| SCN5A mutation, n (%)* | 9 (27.3) | 14 (19.5) | 0.44 |
| ECG parameters | |||
| Spontaneous type 1 ECG, n (%) | 16 (33.4) | 25 (19.5) | 0.07 |
| Type 2 ECG | 15 (31.2) | 22 (17.2) | 0.04 |
| f-QRS | 29 (60.4) | – | – |
| ERP | 15 (31.2) | – | – |
| f-QRS+ERP | 4 (8.4) | – | – |
| First-degree AV block | 11 (22.9) | 29 (22.6) | 1.00 |
| RBBB | 5 (10.4) | 10 (7.8) | 0.56 |
| LAFB | 5 (10.4) | 5 (3.9) | 0.12 |
| Mean PR interval (ms) | 178.0±30.3 | 175.7±33.1 | 0.71 |
| Mean QRS complex (ms) | 108.6±20.0 | 105.4±19.5 | 0.38 |
| Mean QTc interval (ms) | 404.4±40.7 | 399.8±41.6 | 0.56 |
Group I refers to patients with f-QRS and/or ERP and Group II refers to patients without f-QRS or ERP. AV, atrioventricular; ECG, electrocardiogram; EPS, electrophysiological study; ERP, early repolarization pattern; f-QRS, abnormal fragmentation of QRS; LAFB, left anterior fascicular block; SD, sudden death; SND, sinus node dysfunction; RBBB, right bundle branch block.
| f-QRS (n=29) |
ERP (n=15) |
f-QRS+ERP (n=4) |
P value | |
|---|---|---|---|---|
| Clinical features | ||||
| Age, years | 40.7±15.9 | 46.0±10.7 | 51.0±10.7 | 0.88 |
| Male, n (%) | 23 (79.3) | 10 (66.7) | 4 (100) | 0.19 |
| Proband, n (%) | 15 (51.7) | 13 (86.7) | 3 (75.0) | 0.03 |
| Spontaneous type 1 ECG, n (%) | 10 (34.5) | 5 (33.3) | 1 (25.0) | 0.23 |
| Type 2 ECG | 9 (31.0) | 5 (33.3) | 1 (25.0) | 0.23 |
| Family history of SD, n (%) | 11 (37.9) | 9 (60) | 2 (50.0) | 0.44 |
| Aborted SD, n (%) | 4 (13.8) | 2 (13.3) | 2 (50.0) | 0.23 |
| Syncope, n (%) | 20 (68.9) | 8 (53.3) | 2 (50.0) | 0.71 |
| Previous atrial arrhythmias, n (%) | 3 (10.3) | 4 (26.7) | 0 | 0.38 |
| Previous SND, n (%) | 2 (6.9) | 1 (6.7) | 0 | 0.91 |
| Inducible at EPS, n (%)* | 10 (41.7) | 6 (40.0) | 2 (50.0) | 0.83 |
| SCN5A mutations, n (%)* | 5 (26.3) | 4 (33.4) | 0 | 0.32 |
| Outcomes | ||||
| Appropriate shocks, n (%) | 4 (13.8) | 2 (13.3) | 2 (50.0) | 0.40 |
| Inappropriate shocks, n (%) | 1 (3.4) | 4 (26.7) | 1 (25.0) | 0.18 |
| Syncope, n (%) | 0 | 1 (6.7) | 0 | 0.33 |
| Spontaneous type 1 ECG during follow-up | 13 (44.8) | 6 (40.0) | 1 (25.0) | 0.24 |
*Percentages refer to patients who underwent EPS/genetic test. Abbreviations as in Table 1.
Abnormal f-QRS Complex A total of 29 patients (23 males; mean age: 40.7±15.9 years) presented with f-QRS and 26 different families were identified.
It was present at baseline, prior to ICD implantation, in 22 patients (76%), and 7 patients presented with f-QRS at follow-up evaluations. Mean number of leads with f-QRS was 2±2.3. f-QRS was present in the right precordial leads in 21 patients (72.4%). Of them, 6 (28.5%) presented with additional f-QRS in the inferior leads. The remaining 8 patients (27.5%) presented with f-QRS in the inferior leads, exclusively. A total of 24 patients (82.7%) were symptomatic prior to ICD implantation: 4 subjects (13.8%) presented with aborted SD and 20 (68.9%) had at least 1 episode of syncope. The remaining 5 patients (17.3%) were completely asymptomatic before receiving an ICD. EPS was performed in 24 patients (82.7%). Sustained VAs were induced, during PVS, in 10 individuals (41.7%). A total of 19 genetic tests (65.5%) were obtained and 5 of them (26.3%) were positive for a mutation in the SCN5A gene.
Early Repolarization Pattern There were 15 patients (10 males; mean age: 46.0±10.7 years) with ERP, which was present in the inferior leads in 5 patients (33.3%), in the lateral leads in 6 (40%) and in the inferolateral leads in the other 4 patients (26.7%). Mean number of leads with ERP was 1.8±0.9. A total of 10 patients (66.7%) were symptomatic prior to ICD implantation: 2 subjects (13.3%) presented with aborted SD and 8 (53.3%) with syncope. EPS was performed in all patients and sustained VAs were induced in 6 individuals (40%). A total of 12 genetic tests were performed and a mutation in SCN5A was found in 4 patients (33.3%).
Combination of f-QRS and ERP A total of 4 patients presented with both f-QRS and ERP (Figure 3). Mean age was 51.0±10.7 years. All 4 were male and symptomatic before ICD implantation. Of tem, 2 patients presented with an episode of syncope and the other 2 with aborted SD; 1 patient presented with spontaneous Brugada type 1 and the other with Brugada type 2 ECG. EPS was performed in all patients and sustained VAs were induced, during PVS, in 2 subjects (50%). Two patients underwent genetic test and no SCN5A mutations were found.
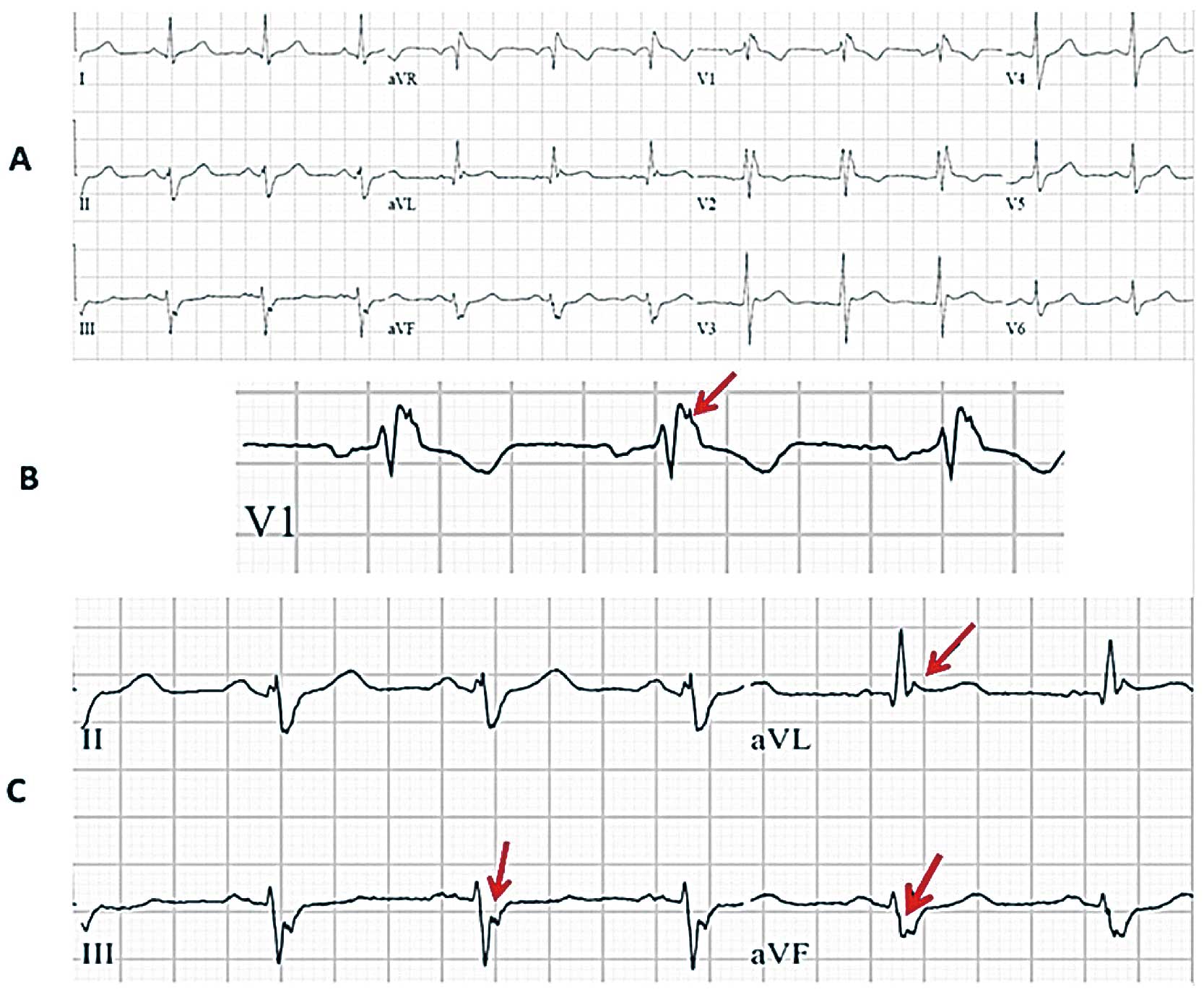
Combined fragmented QRS (f-QRS) and early repolarization pattern (ERP) in baseline 12-lead ECG of a patient with appropriate shocks (A). f-QRS can be observed in V1 (B) and in DIII-aVF (C); ERP is present in aVL (C).
After a mean follow-up of 95.2±51.9 months, spontaneous sustained VAs were documented in 8 patients (16.7%) with spontaneous or drug-induced Brugada type 1 ECG and additional f-QRS or ERP. The arrhythmic event was terminated by ICD shock in all cases. Among patients experiencing appropriate shocks, 4 (50%) presented with f-QRS, 2 (25%) with ERP and 2 (25%) with both abnormalities. Among all patients with ICDs who experienced an appropriate intervention (n=28), 6 patients were asymptomatic prior to the implantation and of them, 2 patients (2/6, 33%) had additional ERP or f-QRS on baseline 12-lead ECG. No significant difference was found in the rate of appropriate shocks between patients presenting with f-QRS or ERP and those without such abnormalities (P=0.86 and 0.94, respectively). Conversely, patients with combined f-QRS and ERP had a significantly higher rate of appropriate shocks than did patients without abnormalities (HR: 4.1; 95% CI: 1.1–19.7; P=0.04) (Figure 4).

Kaplan-Meier curve analyzing freedom from appropriate shocks according to the presence or absence of fragmented QRS (f-QRS) and/or early repolarization pattern (ERP).
Among patients with combined f-QRS/ERP and appropriate shocks, 1 had a previous episode of aborted SD and the other presented with a history of syncope. One patient (2.1%) with f-QRS died from non-cardiovascular cause. Electrical storm occurred in 1 patient (2.1%) with f-QRS; 6 patients (12.5%) had inappropriate shocks. After ICD placement, 1 patient with ERP (2.1%) experienced syncopal episodes. During the follow-up 20 patients (41.7%) presented with spontaneous Brugada type 1 ECG. Interestingly, spontaneous coved-type ECG was observed in the lateral or inferior leads in 2 patients (4.2%) with f-QRS/ERS.
To the best of our knowledge, this is the first study addressing the prevalence, and long-term follow-up of f-QRS and ERP appearing in high-risk patients with BrS treated with ICD therapy. We found that prevalence of f-QRS and/or ERP was 27.3%. Notably, the rate of f-QRS or ERP in asymptomatic BrS patients undergoing ICD implantation was not negligible (23%). Moreover, among asymptomatic patients experiencing appropriate shock during the follow-up, 33% presented f-QRS or ERP. These findings underline the importance of a careful evaluation of the baseline ECG in asymptomatic BrS patients in order to identify additional repolarization or depolarization abnormalities that could improve risk stratification, particularly when these combined abnormalities are found in the same patient’s ECG. Our study expands previous knowledge on the frequency of ajmaline-induced coved-type ECG in the inferolateral leads, which was previously reported by our group in up to 4.6% of patients with BrS.7 Conversely, presence of spontaneous type 1 ECG in the inferolateral leads has been considered an exceptional finding in BrS. In this study, we report for the first time the occurrence of spontaneous type 1 ECG in the lateral leads in 4.2% of BrS high-risk patients presenting with repolarization and depolarization abnormalities.
Fragmented QRS in BrSFragmentation of the QRS complex has been shown to be a marker of local myocardial conduction abnormalities and a predictor of cardiac events in patients with coronary artery disease, non-ischemic cardiomyopathy and in patients with primary electrical disorders.17–19
Priori et al reported f-QRS in 8% of patients presenting with spontaneous or drug-induced Brugada type 1 ECG in the absence of previous documented VAs.12 Although the most powerful predictor of events in this study was the presence of syncope and spontaneous type 1 ECG, f-QRS was also identified as significant predictor of life-threatening arrhythmias and was considered useful to identify candidates for prophylactic ICD therapy. In another study by Morita et al, f-QRS was identified in 43% of patients with spontaneous BrS and was an indicator of susceptibility to arrhythmias in an experimental model of BrS.9 In fact, f-QRS results from functional delay in epicardial activation and provides the substrate for VAs. Apiyasawat et al found f-QRS to be an independent predictor for appropriate ICD interventions in patients undergoing ICD therapy for various indications.20 In their study, f-QRS was present in 19% of patients with BrS having undergone ICD therapy, and similarly, in our study 16.5% of BrS patients undergoing ICD therapy presented with f-QRS, but the presence of this abnormality was not associated with a higher rate of ICD appropriate interventions as compared with the patients without f-QRS.
Unlike in myocardial infarction, f-QRS has been described only in the right precordial leads in BrS, indicating a regional conduction disturbance in the RVOT of these patients.9 In our study, f-QRS was localized in the inferior or inferior and precordial leads in 12.5% of patients with ICD. The preferential occurrence of f-QRS in the right precordial leads suggests a localized conduction abnormality within the RVOT area. In a series of patients with BrS and recurrent VAs, epicardial mapping showed abnormal low voltage and fractionated late potentials (LPs) in the anterior part of the RVOT and ablation over this area was shown to prevent further VAs.21 Presence of f-QRS in other leads might underline a wider arrhythmogenic substrate with additional areas of conduction slowing. Abnormal delayed depolarization in other cardiac portions might contribute to the appearance of f-QRS in leads other than precordial ones.
Early Repolarization Pattern in BrSERP is a relatively common finding in patients with BrS. Our group previously reported the presence of inferolateral ERP in 11% of patients with BrS and an association with a more severe phenotype.7 This subgroup was more likely symptomatic (59% of patients) and presented more frequently with spontaneous type 1 ECG (38% of patients).4 However, no follow-up data were provided. In the present study, ERP was observed in 8.5% of high-risk patients and no significant association with an increased risk of further arrhythmic events was found. Interestingly, our present data regarding the phenotype of high-risk patients with ERP are consistent with those previously reported: 66% of patients were symptomatic and 33% presented with spontaneous type 1 ECG. A multicenter study previously observed inferolateral ERP in 12% of patients with BrS and no significant association with a worse outcome.8 However, in contrast to our results, Kaneko et al showed that patients with BrS and electrical storms have a higher prevalence of ERP.11 Moreover, ERP was observed by Kawata et al in 63% of patients with BrS and documented VF and a worse outcome was observed in these patients when ERP was persistent.10
To date, there are no validated techniques to provoke ERP, although 12-lead Holter monitoring might be useful to detect evidence of ERP during bradycardia.22 Preliminary results have suggested a diagnostic value of the Valsalva maneuver in identifying concealed ERP cases among family members of ERP syndrome patients.23 In our study, ECG-Holter monitoring was performed in any case after an established diagnosis of BrS in order to detect occurrence of ERP during bradycardia, but no other provocative tests were performed. In the specific setting of drug-induced BrS, the prevalence of ERP is even more difficult to assess. In fact, for BrS patients with spontaneous ERP, ajmaline might induce the Brugada type 1 ECG but can attenuate the inferolateral ER abnormalities. On the other hand, it has been reported that up to 4% of BrS patients with a normal baseline ECG can display ERP at the end of ajmaline challenge.7 In this current study, ajmaline challenge unmasked BrS in up to 79% of patients and none of those without ERP at baseline showed its appearance during ajmaline challenge.
For several years ERP has been considered a benign finding with a high prevalence in the general population. Recently, this view has been challenged by different studies. Tikkanen et al observed ERP in 5.8% of community-based general population middle-aged subjects.24 Interestingly, individuals with an ERP >0.2 mV in the inferior leads presented a significantly increased risk of death from cardiac causes and had a higher risk of fatal arrhythmic events. Moreover, Haïssaguerre et al provided evidence of an increased prevalence of ERP in patients with history of idiopathic VF.25 Based on these findings, BrS and idiopathic VF with ERP have been grouped under the heading of J-wave syndromes and are considered as 2 distinct primary electrical disorders sharing common pathophysiological and clinical similarities.26 Antzelevitch and Yan provided evidence, in experimental studies, of an outward shift in the repolarizing current caused by a decrease in sodium or calcium channel currents or an increase in outward potassium currents, which creates a notch in the action potential of the epicardium, resulting in a transmural voltage gradient. Accentuation of such condition in the RVOT gives rise to the BrS coved-type ECG in the right precordial leads; whereas a J-point elevation, a distinct J-wave or an end-QRS slur can manifest in the inferior and/or lateral leads, when the inferolateral ventricle is affected.26 Patients with BrS and additional repolarization abnormalities, such as ERP in the inferolateral leads, might have a larger anatomic arrhythmic substrate involved, which might explain the ECG abnormalities.
Although there is general agreement about f-QRS as a useful sign of depolarization abnormalities, the underlying mechanisms of ERP are still matter of debate.27 Some investigators, in fact, consider this ECG finding as a sign of delayed depolarization of the inferolateral area rather than early repolarization. Although the question is still open, the rare observation of LPs together with the disappearance of the junctional changes during exercise and infusion of isoproterenol seems to favor the diagnosis of early repolarization more than late depolarization.27
Combination of f-QRS and ERPThe combination of f-QRS and ERP is considered a rare finding in BrS. To the best of our knowledge, only a previous Japanese study reported the prevalence of such combination.13 Tokioka et al found the presence of both abnormalities in 3.6% (9/246) of patients with BrS. In that study f-QRS and ERP were found as independent predictors of further arrhythmic events. Similarly, in our study, the prevalence of combined f-QRS and ERP was observed in 2.3% of high-risk patients (4/176). As shown in Figure 4, and different from the Japanese study, these abnormalities, if considered isolated, did not show any prognostic value in predicting further arrhythmic events in the high-risk population. However, f-QRS and ERP conferred, when combined, a higher risk of experiencing ICD appropriate interventions at very long-term follow-up. The inability of f-QRS or ERP alone to predict arrhythmic events might be explained by the presence of other more powerful prognostic determinants in high-risk patients such as the presence of spontaneous type 1 ECG or symptom status. On the other hand, the combination of such ECG abnormalities might conspire to enhance the arrhythmic risk by affecting both the depolarization and repolarization phases of the cell’s action potential in a very select population of high-risk patients. Based on these findings, particular attention should be paid when assessing the arrhythmic risk of asymptomatic patients presenting with type 1 ECG and combined ERP and f-QRS. To date, there are still many controversies regarding the prognostic role of ES in asymptomatic patients. Although large studies agree that EPS inducibility is greatest among BrS patients, to date there is no consensus on the value of EPS in predicting outcome.22 However, 2 recent meta-analyses have showed that in BrS, inducible VAs at programmed ventricular stimulation are associated with future arrhythmic events, independent of the symptom status of patients.28,29
Study LimitationsThis was a single-center experience conducted in a population of patients with heterogeneous clinical characteristics. The number of high-risk patients presenting with combined ERP/f-QRS was small. The nature of this analysis, conducted on patients having undergone ICD implantations over the past 20 years, was retrospective. ERP is a dynamic finding; thus, the prevalence of such an abnormality might be underestimated. Although, evidence of LPs on signal-averaged ECG (SAECG) has been shown to be useful in identifying high-risk BrS patients, in this study SAECG was not routinely performed. Thus, no correlation between LPs in SAECG and f-QRS on standard ECG can be drawn. Moreover intracardiac mapping was not systematically performed to detect local conduction delay. EPS with PVS as well as genetic test was not performed in all patients. Furthermore, in patients with a lifelong risk of arrhythmias, a mean follow-up time of 8 years might be considered short.
Additional repolarization or depolarization abnormalities are common findings in high-risk spontaneous or drug-induced BrS, occurring in 27% of patients undergoing ICD implantation. Interestingly, f-QRS or ERP was observed in 33% of asymptomatic patients with an ICD subsequently experiencing appropriate shocks during the follow-up. When combined, f-QRS and ERP confer a higher risk of appropriate ICD interventions during very long-term follow-up.
P.B.: consultant to Biotronik; speakers fees from Medtronic, Biotronik. A.A.: Sorin Group, Medtronic, Biotronik, Resmed, DC Devices, EBR Systems, Biosense Webster, Biologics Delivery Systems Group, Brystol-Myers Squibb, Leadxx. G.-B.C.: Medtronic AF Solution. C.d.A.: Medtronic AF Solution.