2016 Volume 80 Issue 10 Pages 2149-2154
2016 Volume 80 Issue 10 Pages 2149-2154
Background: Although both β-blocker dose (BBD) and sympathetic activity efferent drive are associated with prognosis in chronic heart failure (HF), little is known about the prognostic value of the interaction between them.
Methods and Results: Potential prognostic variables including resting muscle sympathetic nerve activity (MSNA) were investigated in 133 patients with HF (ejection fraction [EF] <0.45). BBD was normalized to therapeutically equivalent doses of carvedilol. Primary cardiovascular endpoints included cardiovascular death and HF hospitalization. Predictors for outcomes were assessed on univariate, multivariate, and Kaplan-Meier analysis. EF was followed for 9 months after MSNA measurement in 102 patients. During the 1,419±824-day follow-up period, 24 patients died (sudden death, n=10; progressive HF, n=14). On multivariate Cox proportional hazard analysis, higher MSNA (P=0.037; HR, 2.01) and lower BBD (<5.0 mg/day; P=0.041; HR, 1.94) were independent predictors of cardiovascular events. Patients were divided into higher MSNA (≥64 bursts/100 beats) and lower MSNA groups. Although lower BBD remained an independent predictor in patients with higher MSNA, BBD was not statistically significant in patients with lower MSNA on univariate analysis. Additionally, there was a lower EF change in patients with lower BBD and higher MSNA.
Conclusions: Higher BBD might be necessary to avoid cardiovascular events in HF patients with central sympathetic overactivation. (Circ J 2016; 80: 2149–2154)
Beta-blockers are essential for the management of chronic heart failure (HF) with reduced ejection fraction (HFrEF).1 Previous randomized trials have demonstrated that β-blockers improve left ventricular ejection fraction (LVEF) and reduce hospitalization due to HF, and mortality rate.2–4 Carvedilol and bisoprolol additionally produce dose-related improvement in LV function and dose-related reduction in mortality and hospitalization rate in HFrEF.5,6
Sympathetic overactivation is common in chronic HF, and has been reported as a cause of HF exacerbation.7 It is well known that both plasma norepinephrine and activity in the post-ganglionic sympathetic neurons can serve as independent prognosticators of chronic HF.8,9 In contrast, β-blockers do not reduce activity in post-ganglionic sympathetic neurons during the acute phase.10
It is possible that the net effect of the sympathetic nerve activity on the myocardium could be simply determined by both the degree of central sympathetic outflow and β-blocker dose (BBD). To date, several studies using neurotransmitter imaging (meta-iodobenzylguanidine) or direct measurement of sympathetic nerve activity (muscle sympathetic nerve activity [MSNA]) have evaluated the interaction between sympathetic nerve activity and β-blockers.11–14 None of the previous studies, however, focused on the prognostic value of the interaction between the efferent drive of the sympathetic nerve activity from the central nervous system and BBD in HF. Therefore, the aim of the present study was to investigate the prognostic value of the interaction between the central sympathetic drive as evaluated using MSNA, and BBD in patients with HF.
The present study included 133 patients with stable HF (stage C; LVEF <45%). All patients were receiving treatment in accordance with the current guidelines.15 The etiology of HF was ischemic in 44 patients, and non-ischemic in 89. Patients with primary valvular heart disease, stroke, respiratory failure or pulmonary disease, severe anemia, or end-stage renal disease treated with hemodialysis were excluded. The Institutional Ethics Board of Toyama University Hospital approved the study protocol, which complied with the Declaration of Helsinki. Written informed consent was obtained from all patients before participation in the study.
Parameter MeasurementAll parameters were measured in resting patients who were awake and in a supine position as previously described.16–18 Briefly, blood pressure was serially recorded using non-invasive tonometry (Jentow 7700, Colin, Komaki, Japan). Multi-unit recordings of efferent post-ganglionic sympathetic nerve activity to skeletal muscle regions were recorded via a microelectrode inserted directly into the peroneal nerve posterior to the fibular head.16–18 The nerve signal was amplified ×100,000, passed through a band-pass filter (500–5,000 Hz), and integrated with a custom nerve-traffic analysis system (NeuropackΣ MEB-5504; Nihon Kohden, Tokyo, Japan). Blood samples were drawn from the antecubital veins and used to measure B-type natriuretic peptide (BNP) and norepinephrine. Data were analyzed in a blinded manner by 2 of the investigators (R.U. and T.A.). MSNA was expressed as the burst rate (bursts/min) and burst incidence (bursts/100 beats).
Subject CharacteristicsThe following demographic and clinical characteristics were collected from medical records: age; gender; body mass index; etiology of HF; heart rate; mean blood pressure; oxygen saturation; LVEF (calculated as the stroke volume [end-diastolic volume minus end-systolic volume] divided by the end-diastolic volume on echocardiography [Aplio SSA-770A, Toshiba, Tokyo, Japan] using the modified Simpson’s method); LV end-diastolic/systolic diameter; left atrial dimension; hemoglobin; blood urea nitrogen; serum creatinine; estimated glomerular filtration rate (eGFR) using the Modification of Diet in Renal Disease (MDRD) study equation;19 serum sodium; serum potassium; plasma renin activity; plasma aldosterone concentration; and medical treatment including the use of renin-angiotensin system inhibitors, enalapril dose, β-blockers, BBD, digitalis, loop diuretics, furosemide dose, mineralocorticoid receptor antagonist, and spironolactone and amiodarone dose. Echocardiographic data from 6 to 12 months after MSNA measurement were collected if possible. BBD at follow-up was also confirmed at the time of the end-point event or at the last visit. To evaluate the effects of differential BBD and loop diuretic dose, the doses were normalized to therapeutically equivalent doses of carvedilol and furosemide. Both bisoprolol 5 mg and azosemide 30 mg were considered to be equivalent to carvedilol 20 mg and furosemide 20 mg.19,20
Outcome AssessmentFollow-up began at the start of the MSNA measurements and ended in August 2015. The primary end-point was cardiovascular death, including pump failure death and sudden death, and hospitalization due to HF. Information on the time and cause of cardiovascular death or hospitalization due to HF was directly obtained from medical records and family doctors. Individual follow-ups were censored by death or ventricular assist device implantation. None of the patients were lost to follow up.
Statistical AnalysisData are given as mean±SD. JMP (R) 11 (SAS Institute, Cary, NC, USA) was used for statistical analysis. Between-group comparison was carried out using unpaired t-test or chi-squared test, as appropriate.
Two-way analysis of variance was applied to determine how MSNA (higher and lower MSNA groups) and BBD (lower and higher BBD groups) affected the change in LVEF, while the Bonferroni-Dunn procedure was used for multiple comparisons. Kaplan-Meier survival curve was used to determine the time-dependent cumulative cardiac event-free rates according to BBD and MSNA. These curves were analyzed using log-rank test. Statistically significant relationships between baseline explanatory variables and cardiovascular events were identified using univariate and multivariate Cox proportional hazard models. Explanatory variables with P<0.1 on univariate analysis were selected from the clinical variables. The level of statistical significance was set at P<0.05.
Table 1 summarizes the patient demographic and clinical characteristics. Approximately 90% of the patients were treated with renin-angiotensin system inhibitors. Of the 94 patients (71%) being treated with β-blockers, the majority were taking carvedilol (47%) or bisoprolol (41%), while the others were on metoprolol (12%). Mean BBD at baseline and during the follow-up period were 5.4 mg/day and 6.4 mg/day, respectively.
| All patients (n=133) | |
|---|---|
| Age (years) | 64±13 |
| Men | 77 |
| BMI (kg/m2) | 22.2±4.1 |
| Ischemic etiology | 33 |
| CRT | 5 |
| ICD | 11 |
| Atrial fibrillation | 24 |
| Heart rate (beats/min) | 67±11 |
| Mean blood pressure (mmHg) | 77±11 |
| Oxygen saturation (%) | 97±2 |
| Ejection fraction (%) | 32±7 |
| Hemoglobin (g/dl) | 13.3±2.2 |
| BUN (mg/dl) | 22.0±12.0 |
| Serum creatinine (mg/dl) | 1.0±0.4 |
| eGFR (ml/min/1.73 m2) | 58±20 |
| Na (mEq/L) | 137±4 |
| K (mEq/L) | 4.4±0.4 |
| PRA (ng/ml/h) | 9±9 |
| PAC (pg/ml) | 100±105 |
| BNP (pg/ml) | 229±210 |
| Norepinephrine (pg/ml) | 305±172 |
| MSNA burst rate (bursts/min) | 43±15 |
| MSNA burst incidence (bursts/100 beats) | 63±21 |
| Medication | |
| RAS inhibitors | 90 |
| β-blockers | 71 |
| BBD (mg/day) | 5.4±5.9 |
| Digitalis | 20 |
| Loop diuretics | 66 |
| Dose of furosemide (mg/day) | 22±28 |
| Mineralocorticoid receptor antagonists | 63 |
| Amiodarone | 28 |
Data given as mean±SD or %. BBD, β-blocker dose; BMI, body mass index; BNP, B-type natriuretic peptide; BUN, blood uremic nitrogen; CRT, cardiac resynchronization therapy; eGFR, estimated glomerular filtration rate; ICD, implantable cardioverter defibrillator; MSNA, muscle sympathetic nerve activity; PAC, plasma aldosterone concentration; PRA, plasma renin activity; RAS, renin-angiotensin system.
During the 1,419±824-day follow-up period, 24 patients (18%) died due to cardiovascular events, and 41 patients (31%) were hospitalized due to HF. Thus, out of 133 patients, 51 (38%) had cardiovascular events. On univariate analysis, MSNA burst incidence, BNP, serum sodium, plasma renin activity, LVEF, eGFR, mineralocorticoid receptor antagonist dose and BBD were significant predictors of cardiovascular events (Table 2). On multivariate Cox proportional hazard analysis, however, higher MSNA (P=0.037; HR, 2.01) and lower BBD (<5.0 mg/day; P=0.041; HR, 1.94) were independent predictors of cardiovascular events (Table 2).
| Subjects | All patients (n=133) | Higher MSNA (n=66) | Lower MSNA (n=67) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis† | Univariate analysis | Multivariate analysis† | Univariate analysis | ||||||
| Variable | P-value | HR | P-value | HR | P-value | HR | P-value | HR | P-value | HR |
| Age | 0.170 | – | 0.559 | – | 0.838 | – | ||||
| Gender | 0.137 | – | 0.874 | – | 0.056 | 0.837 | ||||
| BMI | 0.056 | 0.932 | 0.469 | – | 0.963 | – | 0.017 | – | ||
| Ischemic etiology | 0.096 | 1.622 | 0.169 | – | 0.832 | – | 0.006 | 3.922 | ||
| Atrial fibrillation | 0.147 | – | 0.971 | – | 0.545 | – | ||||
| Heart rate | 0.643 | – | 0.147 | – | 0.101 | – | ||||
| Mean blood pressure | 0.143 | – | 0.166 | – | 0.799 | – | ||||
| Oxygen saturation | 0.175 | – | 0.470 | – | 0.304 | – | ||||
| Ejection fraction | 0.001 | 0.002 | 0.051 | – | 0.003 | 0.001 | 0.055 | – | 0.154 | – |
| Hemoglobin | 0.058 | 0.886 | 0.799 | – | 0.782 | – | 0.009 | 0.739 | ||
| eGFR | 0.036 | 0.985 | 0.891 | – | 0.006 | 0.972 | 0.362 | – | 0.640 | – |
| Na | 0.002 | 0.911 | 0.873 | – | 0.045 | 0.937 | 0.749 | – | 0.118 | – |
| PRA | 0.009 | 1.036 | 0.367 | – | 0.023 | 1.047 | 0.832 | – | 0.166 | – |
| BNP | <0.0001 | 1.003 | 0.220 | – | 0.003 | 1.002 | 0.357 | – | 0.151 | – |
| Burst incidence | 0.0003 | 1.027 | 0.287 | – | 0.133 | – | ||||
| Higher MSNA group vs. lower MSNA group |
0.002 | 2.476 | 0.037 | 2.012 | ||||||
| BBD at baseline | 0.002 | 0.917 | 0.001 | 0.875 | 0.402 | – | ||||
| Lower BBD group vs. higher BBD group at baseline |
0.014 | 2.006 | 0.041 | 1.942 | 0.011 | 2.478 | 0.035 | 2.414 | 0.677 | – |
| ACEI dose | 0.695 | – | 0.593 | – | 0.682 | – | ||||
| MRA dose | 0.006 | 1.021 | 0.034 | 1.021 | 0.017 | 1.020 | 0.057 | – | 0.159 | – |
| Furosemide dose | 0.016 | 1.009 | 0.723 | 0.179 | – | 0.060 | 1.018 | |||
†Multivariate analysis was performed using factors with P<0.10, provided the univariate analysis of the “groups of β-blockers” was also significant. HR, hazard ratio; MRA, mineralocorticoid receptor antagonist. Other abbreviations as in Table 1.
To clarify the influence of sympathetic activity on the prognostic impact of BBD in chronic HF, we stratified the patients into 4 groups based on median MSNA burst incidence (64 bursts/100 beats) and median BBD (5 mg/day; Figure 1). On Kaplan-Meier analysis there was a significantly higher cardiovascular event rate in patients who had a higher MSNA burst incidence and a lower BBD (Figure 2A), as compared with lower MSNA and lower BBD (Figure 2B). On Cox regression modeling analysis of the higher MSNA, after adjusting for LVEF, BNP, serum sodium, plasma renin activity, mineralocorticoid receptor antagonist dose and eGFR, the HR of the cardiovascular events was 2.41 (95% CI: 1.06–5.74; P=0.035) in the patients with lower BBD (Table 2). In contrast, BBD was not statistically significant for lower MSNA on univariate analysis (Table 2).
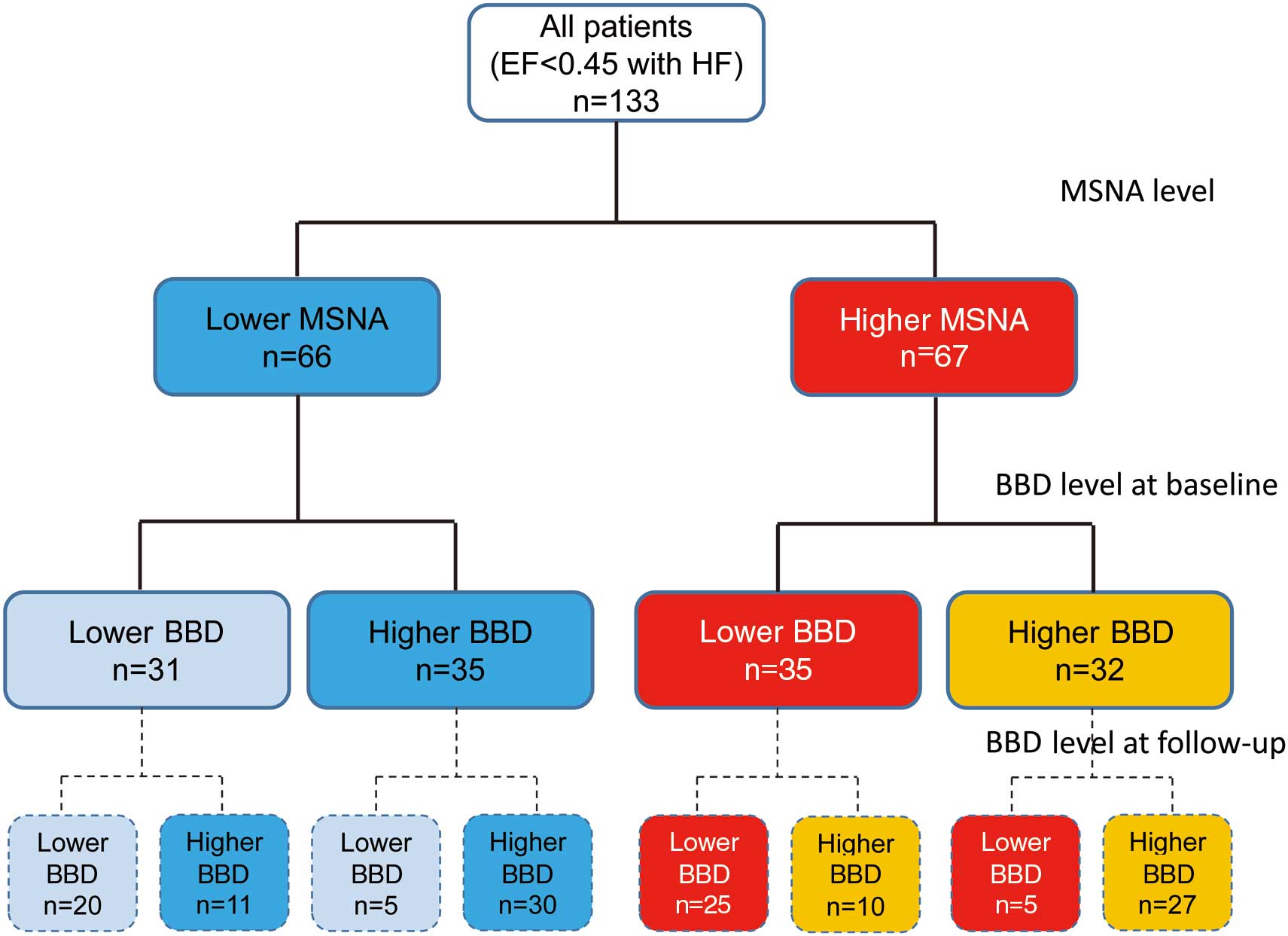
Stratification of 133 patients with heart failure (HF) into 4 groups according to median muscle sympathetic nerve activity (MSNA) burst incidence (64 bursts/100 beats) and β-blocker dose (BBD; 5 mg/day). EF, ejection fraction.

Kaplan-Meier analysis for cardiovascular death and heart failure admission vs. β-blocker dose (BBD) according to (A) lower and (B) higher muscle sympathetic nerve activity (MSNA).
Echocardiographic data from the follow-up period were confirmed in 102 of 133 patients (77%). Change in LVEF was significantly lower in patients with higher MSNA burst incidence and lower BBD, compared with lower MSNA and lower BBD (Figure 3).

Change in left ventricular ejection fraction (∆LVEF). BBD, β-blocker dose; MSNA, muscle sympathetic nerve activity.
This is the first study to show the prognostic value of the interaction between BBD and efferent drive of the sympathetic nerve activity in HF. The major findings are as follows. First, on multivariate analysis both MSNA burst incidence and BBD were independent predictors of cardiovascular events. Second, there was a difference in HR of cardiovascular events in the lower BBD group, with HR=2.41 (P=0.035) in the higher MSNA group, although on univariate analysis BBD was not significant for lower MSNA. This indicates that BBD is a powerful predictor of cardiovascular events in patients with HF, especially when accompanied by sympathetic overactivation. Thus, higher BBD might be necessary for HF patients with efferent overdrive of the sympathetic nerve activity. The dose-related impact of β-blockers in lower sympathetic nerve activity, however, remains unclear.
Effect of BBD-Sympathetic Nerve Activity Efferent Drive Interaction on OutcomeIn the experimental setting, inotrope response to direct sympathetic nerve stimulation was abolished by nerve sectioning performed distal to the electrode or by β-receptor blockade with propranolol.21 There have been no studies, however, on the effect of the interaction between BBD and the efferent drive of the sympathetic nerve activity on cardiovascular response. The transcardiac gradient of norepinephrine, which is an indirect marker of the efferent drive of cardiac sympathetic nerve activity, is significantly correlated with carvedilol dose.22 It has been previously reported that long-term carvedilol use might cause a dose-related decrease in the efferent drive of the cardiac sympathetic nerve activity.13 The present results, however, do show that the interaction between BBD and efferent drive of the sympathetic nerve activity has prognostic value in HF.
BBD and Presence of Sympathetic OveractivationThe MOCHA study prospectively examined dose-related efficacy and found that carvedilol, which produced a linear improvement in LVEF for the 12.5-mg/day, 25-mg/day, and 50-mg/day groups, led to reduction in both mortality and hospitalization rates compared with placebo.5 Subanalysis of the CIBIS II study also found a dose-related efficacy, with the probability of all-cause mortality in the bisoprolol group significantly reduced in the high (HR=0.30) and moderate (HR=0.49) dose groups compared with the low dose group after adjustment for baseline differences.6 And in a study on cardiovascular events during the past decade, there was a significant decrease compared with prior to 2006, which might be indicative of the increased BBD as a result of the recent trend of up-titration.23 In contrast, in the prospective MUCHA trial on dose-related efficacy, 20 mg/day carvedilol produced a remarkable reduction (80%) in the risk of death or cardiovascular hospitalization, with carvedilol 5 mg/day also leading to a risk reduction (71%) similar to the full 20-mg/day dose.24 The J-CHF study prospectively examined whether minimum BBD was related to survival benefits, and reported that there was no significant dose-related difference for the cardiovascular events.25
The variation in β-blocker dose dependency between the studies might be due to differences in the severity of HF. There were larger numbers of severe HF patients in the MOCHA (NYHA II, 47%; III, 52%) and CIBIS II (NYHA III, 83%; IV, 17%) studies, than in the MUCHA (NYHA II, 78%; III, 22%) and J-CHF (NYHA II, 83%; III, 17%) studies. Furthermore, sympathetic nerve activity is more enhanced in severe HF.7 In the present study, the HR of cardiovascular events in the lower BBD group was increased when there was sympathetic overactivation, but remained unchanged when there was lower MSNA. In mild-moderate HF, MSNA was presumably lower and thus, the BBD-related difference could have been unclear. In contrast, the relatively higher MSNA in severe HF might be related to the observed dose-related difference.
Clinical ImplicationsThere may be an association between the level of the efferent drive of the sympathetic nerve activity and the clinical impact of β-blockers on long-term prognosis in chronic HF. For example, in patients with higher MSNA, a relatively higher vs. lower BBD might be more beneficial. In patients with lower MSNA, long-term prognosis is not affected by BBD. Current guidelines recommend the use of β-blockers, even in early stage HF. Given, however, that it is difficult to evaluate MSNA in all patients, the use of higher BBD in all patients would be practical only in the clinical setting.
Study LimitationsThe present study had several limitations. First, 53% of the HF patients were on bisoprolol and metoprolol. Although the dose of these drugs was normalized to approximately equivalent doses of carvedilol based on efficacy, different types of β-blockers are known to have different pharmacological actions. Thus, it is possible that as compared with metoprolol, carvedilol could have additional sympathoinhibition via a β2-blocking effect on the pre-synaptic release of norepinephrine.10 To overcome this potential problem, we utilized MSNA to identify the strength of the efferent drive of the sympathetic nerve activity. Second, MSNA is associated with the activity of sympathetic nerves that innervate the blood vessels in skeletal muscles but not the heart. In a previous study, however, MSNA paralleled the cardiac sympathetic nerve activity in response to pressure changes.26 Based on these findings, MSNA can be considered to be a proxy of cardiac sympathetic nerve activity. Third, the cross-sectional design of the study meant that causality between BBD, MSNA and prognosis could not be determined. It is possible that some of the patients on higher BBD might have improved, thereby exhibiting a lower MSNA. Other patients could have been intolerant to a higher BBD and, due to poor prognosis, might have developed higher MSNA. Finally, all of the MSNA data were obtained at a single time point. Further evaluation of the time course of MSNA during up-titration of β-blockers could improve understanding of whether BBD and MSNA can interact with each other. But, even when taking the aforementioned limitations into consideration, the present findings suggest that BBD is a powerful predictor of cardiovascular events in HF patients with sympathetic overactivation, but not in those without sympathetic overactivation.
This work was supported by JSPS KAKENHI Grant Number 15K01363.
None.