Abstract
Background:
MicroRNAs (miRNAs) are key players in cardiovascular development and disease. However, not only miRNAs of a cardiac origin have a critical role in heart function. Recent studies have demonstrated that miR-122-5p, a hepatic miRNA, increases in the bloodstream during ischemic cardiogenic shock and it is upregulated in the infarcted myocardium. The aim of the present study was to determine the potential of circulating miR-122-5p as a biomarker for early prognostic stratification of ST-segment elevation acute myocardial infarction (STEMI) patients.
Methods and Results:
One hundred and forty-two consecutive STEMI patients treated with primary angioplasty were included in the study. Serum levels of miR-1-3p, -122-5p, -133a-3p, -133b, -208b-3p and -499a-5p were measured at the time of cardiac catheterization by quantitative polymerase chain reaction and related to in-hospital and long-term outcome. During a follow up of 20.8 months, 9 patients died, 6 had recurrence of myocardial infarction, and 26 patients suffered an adverse cardiovascular event. Event-free survival was significantly worse in patients with a higher miR-122-5p/133b ratio (3rd tertile distribution, above 1.42 Log(10)), having almost a 9-fold higher risk of death or myocardial infarction and a 4-fold higher risk of adverse cardiovascular events.
Conclusions:
This study showed that the miR-122-5p/133b ratio is a new prognostic biomarker for the early identification of STEMI patients at a higher risk of developing major adverse events after undergoing primary percutaneous coronary intervention. (Circ J 2016; 80: 2183–2191)
Myocardial infarction (MI) is a leading cause of death in the industrialized world and despite all the improvements achieved in diagnosis and therapeutics, it is still associated with high morbidity and mortality. Risk stratification of MI patients has been the subject of intense research. Several biomarkers have been identified and the search for new ones with more accurate profiles is very intense.
MicroRNAs (miRNAs) are small non-coding RNAs involved in the post-transcriptional regulation of gene expression.1
Increasing evidence supports the involvement of miRNAs in many pathological cardiovascular conditions including heart failure, cardiomyopathy, left ventricular (LV) hypertrophy and neoangiogenesis.2–4
Some of these miRNAs are actively secreted by cells and can be detected in all body fluids.5
The ability of quantifying circulating miRNAs allowed to explore their diagnostic and prognostic value.6
Because many miRNAs are expressed in a tissue-specific manner, initial studies evaluated whether acute MI induces the release of cardiac-enriched miRNAs into the bloodstream. After MI, several studies described an early increase of specific cardiac-enriched circulating miRNAs (miR-1-3p, miR-133a-3p, miR-133b, miR-208a-3p, miR-208b-3p and miR-499a-5p), even before the rise in troponin.7–13
However, these studies failed in relating the levels of those miRNAs with the clinical outcome after MI.14–17
Therefore, the prognostic value of other than prototypic cardiac miRNAs started to be investigated with some promising results.18–20
MiR-122-5p is the most abundantly expressed miRNA in the liver and plays a role in cholesterol biosynthetic pathways and fatty acid metabolism.21
MiR-122-5p was found to be overexpressed in the liver of hyperlipidemic animals22
and increased in the bloodstream of patients with hyperlipidemia, being positively correlated with total cholesterol, triglycerides and low-density lipoprotein cholesterol.23
In a porcine model of cardiogenic shock, Andersson et al24
described a significant increase in the circulating levels of miR-122-5p, the magnitude of which was superior to the cardiac-enriched miRNAs. Interestingly, this increase did not seem to be explained by liver ischemia and was attenuated by therapeutic hypothermia. Gao et al23
found that circulating miR-122-5p is associated with the presence and severity of coronary artery disease, even after adjustment for other cardiovascular risk factors. Experimental studies documented an overexpression of miR-122-5p in the infarcted myocardium25
as well as in the cardiac tissue of the Pax-8 gene knockout mice, which is characterized by presenting ventricular septal defects and LV dilatation.26
It was also demonstrated that miR-122-5p has a pro-apoptotic effect in H9C2 myocytes.26
More recently, overexpression of miR-122-5p was confirmed in the cardiac tissue of patients who died from acute MI.27
Therefore, we hypothesized that circulating miR-122-5p could be important in assessing the severity of MI. As miRNAs act as regulatory networks that have to be viewed together, we compared the circulating levels of miR-122-5p with those of the cardiac-enriched miRNAs, and we assessed the usefulness of this combination in a miRNA signature for prognostic purposes in patients with ST-segment elevation acute MI (STEMI).
Methods
Study Design and Patient Selection
A prospective observational single-center study of consecutive adult STEMI patients who underwent primary percutaneous coronary intervention between June 2008 and June 2009 was performed. For inclusion in this study, patients were required to have simultaneously: (1) an age of 18 years and over; (2) continuous chest pain upon presentation lasting more than 30 min; and (3) ST-segment elevation ≥0.2 mV in at least 2 contiguous precordial leads or ≥0.1 mV in at least 2 contiguous limb leads or presumably new left bundle branch block on admission electrocardiogram. Patients with an absence of a troponin I serum level increase during the initial 24 h of hospitalization were excluded from the study. A total of 142 patients satisfying the described criteria were enrolled in the study. An additional group of 18 non-STEMI controls was also used as a reference for the serum miRNA levels.
Patients were interviewed at 12 months and a structured questionnaire for the description of events was applied. Long-term prognosis was evaluated by the occurrence, at any time after STEMI, of: (1) death (all-cause mortality); (2) death or MI; and (3) any adverse cardiovascular event, defined as death, MI, unstable angina, stroke or hospitalization due to acute heart failure.
The study’s protocol complies with the 1964 Declaration of Helsinki and was approved by the local ethics committee. All the recruited patients gave their informed consent prior to their inclusion in the study.
Quantification of Classical Biomarkers
For biomarkers’ quantification, a blood sample was drawn from the femoral arterial sheath before any interventional procedure. Serum was separated by centrifugation at 3,000 rpm for 15 min at 4℃ and aliquots were stored at –80℃ until used. We used standard laboratory protocols for the determination of the serum concentrations of myocardial necrosis biomarkers (creatine kinase MB mass and creatine kinase), N-terminal prohormone of brain natriuretic peptide (NT-proBNP), renal function parameters (creatinine, urea, uric acid and glomerular filtration rate by the abbreviated Modification of Diet in Renal Disease formula), liver biomarkers (alanine transaminase, aspartate transaminase, γ-glutamyltransferase and bilirubin), inflammatory biomarkers (white blood cell and neutrophil counts, C-reactive protein and interleukin-6) and haemoglobin-A1C. Troponin I was measured using a 3-site sandwich high sensitivity immunoassay adapted in a Centaur XP system (Siemens HealthCare), with a functional sensitivity of 0.017 µg/L. Time progression of troponin I confirming MI was required in all patients.
Transthoracic echocardiography was performed in all the patients 24–72 h after angioplasty (GE Vivid 7; GE Healthcare). The LV ejection fraction (LVEF) was calculated in blinded post-processing using the biplane Simpson’s method.
Quantification of Circulating Serum miRNAs by qRT-PCR
In all patients, circulating levels of miR-1-3p, miR-122-5p, miR-133a-3p, miR-133b, miR-208b-3p and miR-499a-5p were quantified by qRT-PCR in serum samples taken at the time of cardiac catheterization. In a random subset of 40 patients, miRNA levels were also measured at subsequent time points (8, 16, 24, 48 and 72 h after angioplasty). Total RNA was isolated from 300 μl serum by Trizol®
reagent extraction and subsequent RNA purification using the Qiagen’s RNAeasy columns as described elsewhere.11
For normalization purposes, an internal locked nucleic acid (LNA) synthetic spike-in control, UniSp6 (Exiqon), was added to the serum before RNA isolation. Isolated RNA was used as a template for the synthesis of cDNA with the Universal miRCURY LNATM
cDNA synthesis kit, following the instructions from the manufacturer (Exiqon). Quantification of selected miRNAs was performed by using qPCR with specific LNA primers from the same manufacturer (Exiqon). Expression levels of selected miRNAs were quantified by using the Delta-Ct method, normalized by comparison with the spike-in RNA control (see
Supplementary Methods
for full protocol details on miRNA extraction, cDNA synthesis and qPCR quantification).
Statistical Analysis
The serum miRNA levels quantified, as described above, were log-transformed before being used as continuous variables. Normally distributed variables were expressed as the mean±standard deviation and compared using a Student’s t-test or an ANOVA. Non-normally distributed variables were reported as median and interquartile range (IQR) and compared using the Mann-Whitney U-test or the Kruskal-Wallis test. Categorical variables were described as percentages of the cohort and compared by using Fisher’s exact test. Principal component analysis was used to identify the most representative of cardiac-enriched miRNAs, to be used as a comparator for miR-122-5p. The minimum number of components needed to represent their correlated variances was identified by the Scree plot representation and the communalities extraction, and Eigenvalues were determined to identify the miRNA that conveyed the best quality to the model. The Kaiser-Meyer-Olkin measure of sampling adequacy and P value of the Bartlett’s test of sphericity were used as quality measures of the model. Short- and long-term prognostic impact of baseline clinical and laboratory characteristics were evaluated by univariate and multivariate Cox proportional hazard analyses. To avoid overfitting, only variables with a P value below 0.25 were inputted in the multivariate analyses, which were conducted by applying a stepwise forward conditional technique (P-value cut-off for entry=0.05 and for removal=0.10). The Kaplan-Meier method was used to calculate the cumulative incidence of adverse events, and log-rank statistics were used to compare them according to the tertile distribution of the miR-122-5p/133b ratio and LVEF. All analyses were 2-sided and a P-value <0.05 was considered statistically significant. Statistical analysis was performed by using SPSS 20.0 (SPSS Inc).
Results
Analysis of Selected Circulating miRNAs and Clinical Parameters in STEMI Patients
A total of 142 STEMI patients were enrolled in the study, with a mean age of 62±12 years (range: 37–91 years), and clinical characteristics summarized in
Table 1. At the time of admission before angioplasty, blood pressure was 140±30/83±18 mmHg and 93.7% of patients had no signs of heart failure. Median IQR onset of symptoms to cardiac catheterization time was 4.6 (3.0–9.1) h. A random subset of 40 patients was used to characterize the time-course evolution of circulating miRNA levels within 72 h after coronary angioplasty, which was then compared to those measured in a group of 18 healthy individuals (Figure 1). We used qPCR to evaluate the circulating levels of the hepatic miR-122-5p, the muscle-enriched miR-1-3p, miR-133a-3p and miR-133b, and the cardiac specific miR-208b-3p and miR-499a-5p. The circulating levels of the cardiac- and muscle-enriched miRNAs peaked at 8 h and decreased progressively afterwards (Figures 1A,C–F), showing a very similar pattern to that observed for troponin I (Figure 1G) and creatine kinase MB (Figure 1H). However, the liver-specific miR-122-5p presented higher levels at the time of cardiac catheterization, which decreased during the initial 24 h and then rose again (Figure 1B).

Table 1.
Population Characteristics
| |
STEMI patients (n=142) |
| Male gender, frequency (%) |
107 (75.4) |
| Age (years, mean±SD) |
62±12 |
| Cardiovascular risk factors, frequency (%) |
| Hypertension |
84 (59.2) |
| Hypercholesterolemia |
75 (52.8) |
| Diabetes |
33 (23.2) |
| Smoking |
62 (43.7) |
| Former smoker (without consumption in the last year) |
8 (5.6) |
| Familial history of coronary artery disease before the age of 50 years |
22 (15.5) |
| Past history of cardiovascular disease, frequency (%) |
| MI |
17 (12.0) |
| Percutaneous coronary intervention |
17 (12.0) |
| Coronary artery bypass graft surgery |
4 (2.8) |
| Stroke |
9 (6.3) |
| Peripheral artery disease |
2 (1.4) |
| Angiographic and procedural characteristics, frequency (%) |
| No. of vessels with significant lesions |
| 1 vessel |
79 (55.6) |
| 2 vessels |
43 (30.3) |
| 3 vessels or left main coronary artery |
20 (14.1) |
| Culprit coronary artery |
| Anterior descending territory (or diagonal branches) |
61 (43.0) |
| Right coronary artery |
55 (38.7) |
| Circumflex artery (or marginal obtuse branches) |
25 (17.6) |
| Left main coronary artery |
2 (1.4) |
| Coronary bypass |
2 (1.4) |
MI, myocardial infarction; STEMI, ST-segment elevation acute myocardial infarction.
Due to the observed increased variability in the serum miRNA levels at the time of cardiac catheterization, we extended the miRNA quantification to the whole population study at this time point (n=142) (Figure 2). All the quantified circulating miRNAs showed a significant increase with respect to the healthy control group; the liver-specific miR-122-5p being the most abundant (Figure 2B). In terms of clinical parameters, no association was detected between circulating miR-122-5p levels and gender, ethnicity, hemodynamic parameters, hepatic aminotransferases or myocardial necrosis biomarkers within the studied population (Tables S1,S2). No correlation was observed between miR-122-5p and interleukin-6 levels at the time of hospital admission (Figure S1). However, patients with higher levels of miR-122-5p at the time of the cardiac catheterization (ie, Log(10) >–4.36, 3rd tertile) exhibited higher inflammatory parameters during the initial 72 h, with higher maximum white blood cell count (13.06 [10.78–15.80] vs. 11.90 [9.62–13.84], P=0.018), maximum neutrophil count (9.82 [8.25–12.89] vs. 8.95 [7.20–11.02], P=0.029) and maximum C-reactive protein (6.25 [2.80–11.60] vs. 3.05 [1.69–7.19], P=0.012) (Figures S2–S4). No relationship was observed between miRNA levels and the pharmacological treatment or the time between drug administration and blood sample collection (Table S3). The only P2Y12
inhibitor that was used in this population was clopidogrel and it was administered before the cardiac catheterization in almost all patients (97.2%). Of note, 54 patients (39.4%) received heparin prior to blood sample collection (bolus, 5000 IU; median time: 33 min), with no apparent modifications in their serum miRNA levels in comparison to the remaining patient population (Table S3).

Circulating levels of the several cardiac-enriched miRNAs were highly correlated with each other (Figure 2G), and were inversely associated with systolic blood pressure and positively correlated with the simultaneous concentrations of troponin I and creatine kinase MB mass (Table S1). Of note, their levels were not associated with the peak concentrations of the myocardial necrosis biomarkers, NT-proBNP or LVEF.
Circulating miR-122-5p/133b Ratio at the Time of Angioplasty Is a Prognostic Predictor of Adverse Cardiovascular Events in STEMI
As both miR-122-5p and cardiac-enriched miRNAs circulating levels were elevated at the time of the urgent cardiac intervention (when compared with those of healthy individuals), we hypothesized that the out-of-proportion increase of miR-122-5p evaluated by its relative increase in comparison to circulating cardiac-specific or muscle-enriched miRNAs could be a relevant physiological parameter. To analyse this hypothesis, we performed a principal component analysis, reducing the variance of the cardiac-enriched miRNA levels to the minimum number of variables. At the time of the coronary catheterization, miR-133b was the most representative among the analysed circulating miRNAs, being responsible for 83% of the correlated variance (Kaiser-Meyer-Olkin measure of sampling adequacy=0.88; P value of the Bartlett’s test of sphericity <0.001) (Figure 2H). Therefore, the miR-122-5p/133b ratio was used to assess the relative increase of miR-122-5p in subsequent analyses.
During hospitalization, 11 patients (7.7%) progressed to cardiogenic shock and 3 patients died (2.1%). Patients who progressed to cardiogenic shock or died had higher serum levels of creatinine, lower LVEF, higher circulating levels of miR-122-5p, and a higher miR-122-5p/133b ratio (Table 2). No significant differences of the muscle or cardiac-enriched miRNA levels were found in relation to the hemodynamic stability, LVEF or in-hospital outcome. The remaining 128 patients were subjected to a long-term follow up after hospitalization. During a median (IQR) follow up of 20.8 (17.7–23.5) months, 9 (6.3%) patients died (7 due to cardiac causes) and 6 (4.2%) suffered myocardial re-infarction. A total of 26 (18.6%) patients had adverse cardiovascular events (a composite of death, MI, unstable angina, stroke or hospitalization due to acute heart failure). Considering the 3 prognostic endpoints (all-cause mortality, death or MI, any adverse cardiovascular event), patients who evolved unfavourably had higher miR-122-5p/133b ratios at the time of the urgent cardiac catheterization, were more likely to present with left main or multivessel coronary disease, were older and had a lower LVEF. However, the only independent prognostic predictors were the miR-122-5p/133b ratio and LVEF (Table 3;
Tables S4–S6). Both parameters were found to be moderately accurate for event prediction within 2 years after STEMI (Figure S5). None of the muscle and cardiac circulating miRNAs has prognostic significance by itself.
Table 2.
Association of Baseline Clinical and Laboratory Characteristics With the Risk of Adverse In-Hospital Outcome (Death or Cardiogenic Shock)
| Variable |
Death or
cardiogenic
shock (n=12) |
Favourable
in-hospital
outcome (n=130) |
P value |
Univariate logistic
regression analysis |
| OR |
95% CI |
| Age, years (mean±SD) |
65±11 |
61±12 |
NS (0.29) |
1.02 |
0.97~1.08 |
| Hypertension, frequency (%) |
7 (58.3) |
77 (59.2) |
NS (0.95) |
0.96 |
0.29~3.20 |
| Diabetes, frequency (%) |
4 (33.3) |
29 (22.3) |
NS (0.33) |
1.81 |
0.50~6.64 |
| Smoking, frequency (%) |
5 (41.7) |
57 (43.8) |
NS (0.92) |
0.92 |
0.27~3.23 |
| Past history of MI, frequency (%) |
0 |
17 (13.1) |
NS (0.17) |
1.46 |
0.14~13.60 |
3 vessels or left main coronary artery disease,
frequency (%) |
2 (16.7) |
18 (13.8) |
NS (0.61) |
1.35 |
0.27~6.74 |
| Anterior MI, frequency (%) |
7 (58.3) |
56 (43.1) |
NS (0.63) |
0.93 |
0.28~3.09 |
| Troponin I*, μg/L (median (IQR)) |
0.95 (0.18~5.27) |
0.30 (0.06~3.26) |
NS (0.35) |
0.98 |
0.94~1.03 |
| NT-proBNP*, Log(10) pg/ml (median (IQR)) |
448 (12~1,145) |
202 (56~701) |
NS (0.18) |
1.25 |
0.89~1.75 |
| Creatinine*, mg/dl (median (IQR)) |
1.0 (0.90~1.29) |
0.90 (0.70~1.0) |
0.025 |
1.31 |
0.69~2.49 |
| Aspartate transaminase, IU/L (median (IQR)) |
54 (31~129) |
32 (22~67) |
NS (0.16) |
1.00 |
0.99~1.00 |
| Alanine transaminase, IU/L (median (IQR)) |
29 (19~72) |
28 (20~41) |
NS (0.47) |
1.01 |
0.99~1.02 |
| eGFR*, ml/min/1.73 m2 (median (IQR)) |
87 (56~89) |
93 (76~121) |
NS (0.13) |
0.99 |
0.96~1.01 |
| Circulating miR-122-5p*, Log(10) (median (IQR)) |
−3.7 (−4.7~−2.88) |
−5.1 (−6.7~−4.1) |
0.022 |
1.54 |
1.03~2.31 |
| miR-122-5p/133b ratio*, Log(10), median (IQR) |
1.99 (0.85~2.51) |
0.86 (0.0~1.80) |
0.025 |
1.52 |
1.10~2.08 |
| LVEF†, % (median (IQR)) |
35 (29~54) |
54 (45~60) |
0.012 |
0.91 |
0.85~0.97 |
CI, confidence interval; eGFR, estimated glomerular filtration rate; IQR, interquartile range; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NS, not significant; NT-proBNP, N-terminal prohormone of brain natriuretic peptide; OR, odds ratio; SD, standard deviation. *Quantification at the time of the urgent cardiac catheterization. †Quantification at 24~72 h after the coronary angioplasty.
Table 3.
Association of Baseline Clinical and Laboratory Characteristics With Long-Term Prognosis as Evaluated by All-Cause Mortality, Death or MI, and Any Adverse Cardiovascular Event
| Variable |
Multivariate cox regression analysis |
| Hazard ratio (95% CI) |
P value |
| Age, years |
1.14 (0.95~1.36) |
NS (0.84) |
| NT-proBNP*, Log(10), pg/ml |
1.68 (0.67~4.20) |
NS (0.94) |
| miR-122-5p/133b ratio*, Log(10) |
1.49 (1.09~2.03) |
0.012 |
| LVEF†, % |
0.92 (0.85~0.995) |
0.038 |
*Quantification at the time of the urgent cardiac catheterization. †Quantification at 24~72 h after the coronary angioplasty. Accuracy of the multivariate Cox regression analysis model for prediction of all-cause mortality: AUC=0.81 (95% CI 0.64~0.99); P=0.010. Statistical power: 45.0% (α error: 5%). Abbreviations as in Table 2.
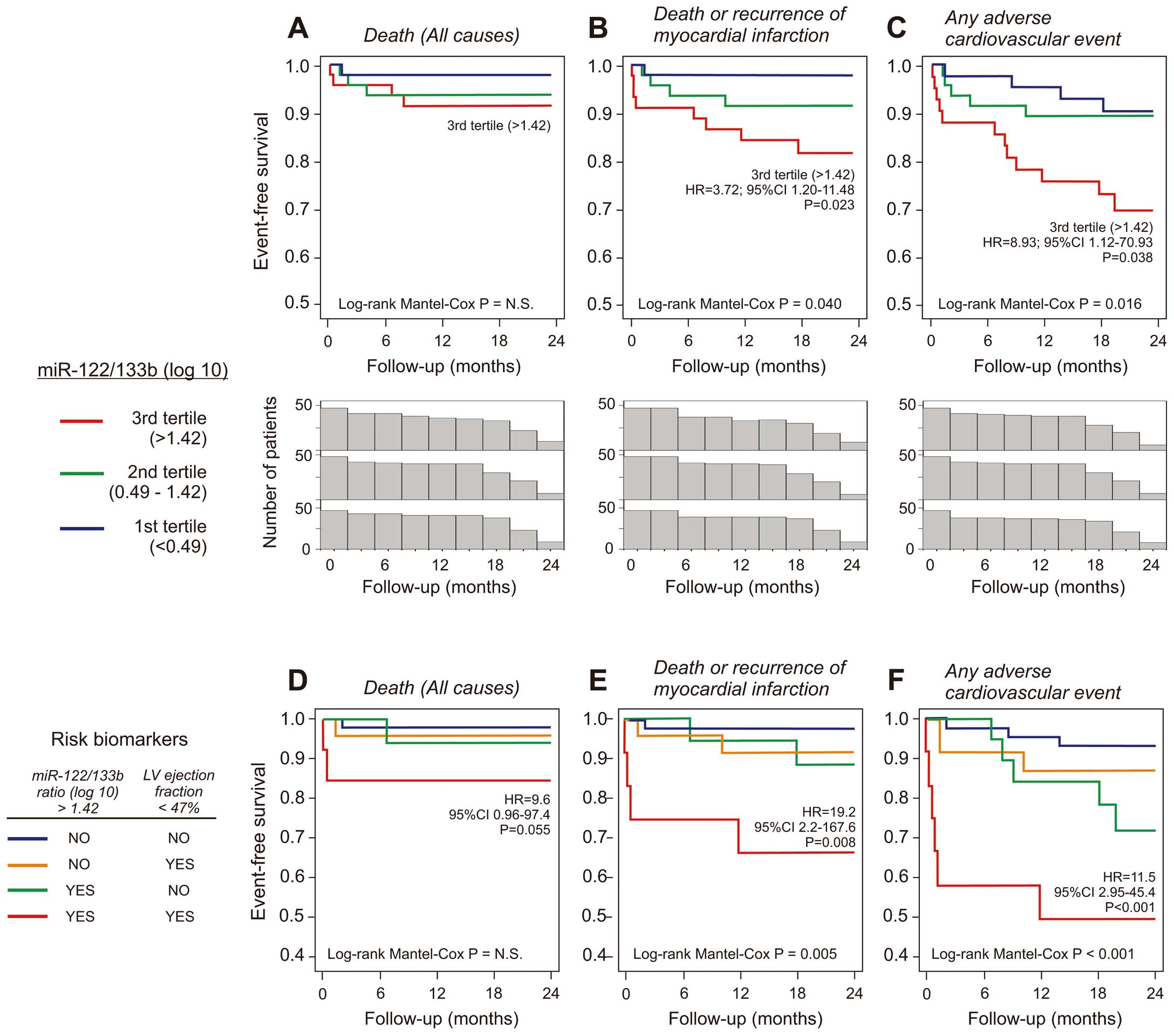
The prognostic impact of the miR-122-5p/miR-133b ratio was not influenced by the time from the onset of symptoms to cardiac catheterization, pharmacological treatment pattern, time between drug administration and blood sample collection, or Thrombolysis in Myocardial Infarction (TIMI) score at hospital admission (Table S7). We also performed a univariate regression analysis for the evaluation of the prognostic value of the out-of-proportion of miR-122-5p in relationship to all the analysed cardiac and muscle miRNAs; the miR-122/133b ratio gave the best prognostic power in all the analysed endpoints (Table S8). At the moment of angioplasty, patients with a high miR-122-5p/133b ratio (3rd tertile distribution, above 1.42 Log(10)) were at a significantly increased risk of adverse prognosis, with almost a 9-fold higher risk of death or MI and 4-fold higher risk of adverse cardiovascular events (Figures 3A–C). Combining the miR-122-5p/133b levels with the LVEF, the highest risk of adverse endpoints was observed in patients with an increased miR-122-5p/133b ratio and reduced LVEF (Figures 3D–F).
Discussion
Cells are able to actively secrete miRNAs in response to different physiological conditions. The levels of specific miRNAs in biofluids can be related with pathological processes, allowing their use as disease biomarkers. The use of miRNAs as biomarkers is empowered by their intrinsic stability in biofluids, and the sensitivity and specificity of the available methods for their quantification. Probably the most fertile medical area in the use of circulating miRNAs as biomarkers is oncology, where the prognostic value of diverse miRNAs has been established for several types of cancer,5
and their molecular expression signatures exploited to infer clinical management decisions.28
In this study, we demonstrated for the first time that the relative increase of serum miR-122-5p, as expressed by the miR-122-5p/133b ratio, is a relevant prognostic predictor after STEMI.
Among the various circulating miRNAs investigated for MI detection and prognosis, the most attractive are the muscular miR-1-3p, miR-133a-3p and miR-133b, and the cardiac-specific miR-208b-3p and miR-499a-5p. Previous studies reported that these circulating miRNAs peak within 8 h after infarction, following the troponin I time-course.7–12
In our study, the serum levels of the selected muscular and cardiac miRNAs also showed a time-course evolution that resembles the troponin I profile, being in agreement with previously available data.16,29
In contrast with other studies, we did not observe significant relationships between cardiac-enriched miRNA serum levels and population characteristics, such as gender, age, obesity, hypertension or smoking habits.30–32
Recent studies suggested that systemic heparin administration might result in derangements of microRNA measurements in a time-dependent manner.33
In our cohort of patients, 39.4% of the individuals were treated with heparin prior to blood sample collection, during the emergency medical services transport, or at the emergency room. We did not detect any relationship between circulating miRNA levels and the pharmacological treatment, or the time between drug administration and blood sample collection.
The available data reporting the predictive value of circulating miRNAs in patients with coronary artery disease is still very limited. Initial studies were focused on cardiac-enriched circulating miRNAs, such as miR-133a-3p, which was found to be associated with myocardial salvage, infarct size and microvascular obstruction.15
Nevertheless, evidence accumulated so far is very consistent in excluding an independent association between circulating levels of cardiac-enriched miRNAs and clinical outcome.15,30,34
Therefore, the prognostic value of circulating miRNAs other than prototypic cardiac miRNAs started to be investigated with promising results.35
A prospective study involving 820 participants observed that the risk of future MI was associated with baseline levels of miR-126, miR-197 and miR-223.20
Devaux et al14
also observed that patients who developed adverse LV remodelling after STEMI presented 2-fold lower levels of miR-150 at hospital discharge. Recent retrospective studies reported higher levels of circulating miR-155 and miR-380 in patients who died within a year after MI,19
and significant increases of the p53-responsive miR-192, miR-194 and miR-34a in those who developed heart failure.36
The basis for our working hypothesis was the observation made by Andersson et al24
whereby circulating miR-122-5p increased more than cardiac-enriched miRNAs in an porcine model of severe MI with cardiogenic shock. Interestingly, this increase did not seem to be explained by liver ischemia and was attenuated by therapeutic hypothermia. MiR-122-5p is mostly expressed in the liver, participating in the regulation of cholesterol metabolism.21
MiR-122-5p was also found to be upregulated in the infarcted myocardium,25,27
and experimental studies suggested that it can have pro-apoptotic effects in cardiomyocytes.26
miRNAs act within complex regulatory networks where their regulatory actions are frequently redundant, coordinated and complementary. During progression to experimental cardiogenic shock, both circulating miR-122-5p and the cardiac-enriched miRNAs increase,24
but the magnitude of the augmentation is different and therefore their relative ratios are expected to profoundly change. Therefore, we hypothesized that circulating miR-122-5p and the cardiac-enriched miRNAs should be evaluated together and that the clinical relevance could result from the magnitude of the relative increase more than the absolute value of each of the miRNAs levels. The strategy of using ratios of circulating miRNAs has also been recently applied for the detection and characterization of esophageal adenocarcinomas, demonstrating its intrinsic discrimination power.37
Our data showed that circulating miR-122-5p levels are associated with a short-term outcome and its relative increase was strongly associated with long-term morbidity and mortality. We also observed a consistent association between miR-122-5p and inflammatory biomarkers. Understanding the miR-122-5p as an inflammatory biomarker instead of an exclusively liver-specific miRNA might be an attractive hypothesis to explain its prognostic impact and to understand the physiological cross-talk between heart and liver. It would be plausible that liver congestion or ischemia result in the release of miR-122-5p to the systemic circulation. However, we noted that the increase of the miR-122-5p circulating levels at the time of the coronary catheterization did not seem to be explained by these mechanisms. Indeed, miR-122-5p circulating levels were not correlated with the laboratorial markers of hepatic injury, namely aspartate aminotransferase or alanine aminotransferase. In addition, we compared the miR-122-5p levels at the time of the coronary catheterization with the maximum concentration of the hepatic biomarkers between hospital admission and 72 h after the angioplasty (a more accurate marker of hepatic ischemia), but no significant correlation was detected. Moreover, hepatic aminotransferases at hospital admission were also not associated with the clinical outcome. We speculate that factors predisposing to an adverse outcome are also related to higher titles of circulating miR-122-5p at STEMI presentation, but our study did not clarify its origin in this setting. In healthy individuals, circulating miR-122-5p was detected as exclusively present in the non-vesicular form.38
It has been also proposed that circulating protein-bound miRNAs are by-products of dead cells, and it seems intuitive that liver ischemia might be implicated in miR-122-5p release. However, like Andersson et al,24
we did not observe any association between miR-122-5p and liver necrosis biomarkers. Therefore, it remains possible that protein carrier pathways participate in the specific release of these complexes from hepatocytes. Moreover, it is noteworthy that miR-122-5p progressively decreased during MI progression, which could justify why previous small sized studies, with different times of blood sample collection and patients’ characteristics, reported conflicting trends regarding miR-122-5p levels at MI presentation.10,11,13
In summary, we showed that the relative increase of circulating miR-122-5p, as reflected by a high miR-122-5p/133b ratio, is a predictive biomarker to identify patients at higher risk of an adverse prognosis after STEMI. The power of early prognostic stratification with this biomarker is increased when evaluated in association with LVEF.
Study Limitations
In this study, we only evaluated the prognostic impact of the circulating miRNAs at the time of cardiac catheterization. It remains possible that later time points may provide additional information on clinical outcome. Another possible limitation with the present study is related to the assessment of the impact of STEMI by using LVEF and not cardiac magnetic resonance imaging, and also the low number of events during follow up that limited the statistical power of prognostic analysis. Nevertheless, our results suggest that the miR-122-5p/-133b ratio is a relevant prognostic discriminator to predict adverse outcomes in STEMI.
Acknowledgments
This work was supported, in part, by the Programme for Advanced Medical Education (Fundação Calouste Gulbenkian, Ministry of Health and Foundation for Science and Technology), the João Porto Research Grant from the Portuguese Society of Cardiology (to N.C.-D.) and the Santa Maria University Hospital – Sanofi Aventis Grant for Clinical Research (to N.C.-D.). M.C.C. was supported by a Foundation for Science and Technology postdoctoral fellowship (Ref. SFRH/BPD/65121/2009).
Conflicts of Interest
On behalf of all authors, the corresponding authors declare no conflicts of interest.
Supplementary Files
Supplementary File 1
Methods
Table S1.
Association of the circulating levels of miRNAs with clinical and laboratorial parameters
Table S2.
Correlation analysis of the circulating miR-122-5p levels at the time of the coronary catheterization with the concentration of hepatic biomarkers (aspartate and alanine aminotransferases)
Table S3.
Comparison of the circulating levels of miRNAs with respect to pharmacological treatment and the time between drug administration and blood sample collection at urgent cardiac catheterization
Table S4.
Association of the baseline clinical and laboratory characteristics with all-cause mortality during follow up (event rate: 6.3%)
Table S5.
Association of the baseline clinical and laboratory characteristics with the risk of death or recurrence of MI during follow up (event rate: 10.9%)
Table S6.
Association of the baseline clinical and laboratory characteristics with the risk of any adverse cardiovascular event
Table S7.
Prognostic impact of the miR-122-5p/133b ratio and LVEF with adjustment for the pattern of pharmacological treatment by multivariate cox regression analyses
Table S8.
Univariate cox regression analyses evaluating the prognostic value of the out-of-proportion increase of circulating miR-122, as defined by the ratio between miR-122-5p and the various analysed cardiac-specific and muscle-enriched miRNAs
Figure S1.
Pearson’s correlation of circulating miR-122-5p levels (A) and miR-122/133b ratio (B) with interleukin-6 levels.
Figure S2.
Comparison of the white blood cell count during the initial 72 h after coronary angioplasty with respect to the tertile distribution of circulating miR-122-5p at baseline.
Figure S3.
Comparison of the neutrophil count during the initial 72 h after coronary angioplasty with respect to the tertile distribution of circulating miR-122-5p at baseline.
Figure S4.
Comparison of the C-reactive protein concentration during the initial 72 h after coronary angioplasty with respect to the tertile distribution of circulating miR-122-5p at baseline.
Figure S5.
Prognostic accuracy of the left ventricular ejection fraction (LVEF), miR-122-5p and miR-122/133b ratio with respect to the prediction of long-term outcome, including (A) all-cause mortality, (B) death or recurrence of myocardial infarction and (C) any adverse cardiovascular event.
Please find supplementary file(s);
http://dx.doi.org/10.1253/circj.CJ-16-0568
References
- 1.
Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 1993; 75: 843–854.
- 2.
Quiat D, Olson EN. MicroRNAs in cardiovascular disease: From pathogenesis to prevention and treatment. J Clin Invest 2013; 123: 11–18.
- 3.
De Rosa S, Curcio A, Indolfi C. Emerging role of microRNAs in cardiovascular diseases. Circ J 2014; 78: 567–575.
- 4.
Fukushima Y, Nakanishi M, Nonogi H, Goto Y, Iwai N. Assessment of plasma miRNAs in congestive heart failure. Circ J 2011; 75: 336–340.
- 5.
Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A 2008; 105: 10513–10518.
- 6.
Hata S. Need and importance of biomarkers to predict acute myocardial infarction and its prognosis. Circ J 2012; 76: 2090–2091.
- 7.
Adachi T, Nakanishi M, Otsuka Y, Nishimura K, Hirokawa G, Goto Y, et al. Plasma microRNA 499 as a biomarker of acute myocardial infarction. Clin Chem 2010; 56: 1183–1185.
- 8.
Ai J, Zhang R, Li Y, Pu J, Lu Y, Jiao J, et al. Circulating microRNA-1 as a potential novel biomarker for acute myocardial infarction. Biochem Biophys Res Commun 2010; 391: 73–77.
- 9.
Cheng Y, Tan N, Yang J, Liu X, Cao X, He P, et al. A translational study of circulating cell-free microRNA-1 in acute myocardial infarction. Clin Sci (Lond) 2010; 119: 87–95.
- 10.
Corsten MF, Dennert R, Jochems S, Kuznetsova T, Devaux Y, Hofstra L, et al. Circulating MicroRNA-208b and MicroRNA-499 reflect myocardial damage in cardiovascular disease. Circ Cardiovasc Genet 2010; 3: 499–506.
- 11.
D’Alessandra Y, Devanna P, Limana F, Straino S, Di Carlo A, Brambilla PG, et al. Circulating microRNAs are new and sensitive biomarkers of myocardial infarction. Eur Heart J 2010; 31: 2765–2773.
- 12.
Kuwabara Y, Ono K, Horie T, Nishi H, Nagao K, Kinoshita M, et al. Increased microRNA-1 and microRNA-133a levels in serum of patients with cardiovascular disease indicate myocardial damage. Circ Cardiovasc Genet 2011; 4: 446–454.
- 13.
Wang GK, Zhu JQ, Zhang JT, Li Q, Li Y, He J, et al. Circulating microRNA: A novel potential biomarker for early diagnosis of acute myocardial infarction in humans. Eur Heart J 2010; 31: 659–666.
- 14.
Devaux Y, Vausort M, Goretti E, Nazarov PV, Azuaje F, Gilson G, et al. Use of circulating microRNAs to diagnose acute myocardial infarction. Clin Chem 2012; 58: 559–567.
- 15.
Eitel I, Adams V, Dieterich P, Fuernau G, de Waha S, Desch S, et al. Relation of circulating microRNA-133a concentrations with myocardial damage and clinical prognosis in ST-elevation myocardial infarction. Am Heart J 2012; 164: 706–714.
- 16.
Gidlof O, Smith JG, Miyazu K, Gilje P, Spencer A, Blomquist S, et al. Circulating cardio-enriched microRNAs are associated with long-term prognosis following myocardial infarction. BMC Cardiovasc Disord 2013; 13: 12.
- 17.
Zhang R, Niu H, Ban T, Xu L, Li Y, Wang N, et al. Elevated plasma microRNA-1 predicts heart failure after acute myocardial infarction. Int J Cardiol 2013; 166: 259–260.
- 18.
Devaux Y, Vausort M, McCann GP, Kelly D, Collignon O, Ng LL, et al. A panel of 4 microRNAs facilitates the prediction of left ventricular contractility after acute myocardial infarction. PLoS One 2013; 8: e70644, doi:10.1371/journal.pone.0070644.
- 19.
Matsumoto S, Sakata Y, Nakatani D, Suna S, Mizuno H, Shimizu M, et al. A subset of circulating microRNAs are predictive for cardiac death after discharge for acute myocardial infarction. Biochem Biophys Res Commun 2012; 427: 280–284.
- 20.
Zampetaki A, Willeit P, Tilling L, Drozdov I, Prokopi M, Renard JM, et al. Prospective study on circulating microRNAs and risk of myocardial infarction. J Am Coll Cardiol 2012; 60: 290–299.
- 21.
Esau C, Davis S, Murray SF, Yu XX, Pandey SK, Pear M, et al. MiR-122 regulation of lipid metabolism revealed by in vivo antisense targeting. Cell Metab 2006; 3: 87–98.
- 22.
Elmen J, Lindow M, Schutz S, Lawrence M, Petri A, Obad S, et al. LNA-mediated microRNA silencing in non-human primates. Nature 2008; 452: 896–899.
- 23.
Gao W, He HW, Wang ZM, Zhao H, Lian XQ, Wang YS, et al. Plasma levels of lipometabolism-related miR-122 and miR-370 are increased in patients with hyperlipidemia and associated with coronary artery disease. Lipids Health Dis 2012; 11: 55.
- 24.
Andersson P, Gidlof O, Braun OO, Gotberg M, van der Pals J, Olde B, et al. Plasma levels of liver-specific miR-122 is massively increased in a porcine cardiogenic shock model and attenuated by hypothermia. Shock 2012; 37: 234–238.
- 25.
Dong S, Cheng Y, Yang J, Li J, Liu X, Wang X, et al. MicroRNA expression signature and the role of microRNA-21 in the early phase of acute myocardial infarction. J Biol Chem 2009; 284: 29514–29525.
- 26.
Huang X, Huang F, Yang D, Dong F, Shi X, Wang H, et al. Expression of microRNA-122 contributes to apoptosis in h9c2 myocytes. J Cell Mol Med 2012; 16: 2637–2646.
- 27.
Bostjancic E, Zidar N, Glavac D. MicroRNAs and cardiac sarcoplasmic reticulum calcium ATPase-2 in human myocardial infarction: Expression and bioinformatic analysis. BMC Genomics 2012; 13: 552.
- 28.
Boeri M, Verri C, Conte D, Roz L, Modena P, Facchinetti F, et al. MicroRNA signatures in tissues and plasma predict development and prognosis of computed tomography detected lung cancer. Proc Natl Acad Sci U S A 2011; 108: 3713–3718.
- 29.
Meder B, Keller A, Vogel B, Haas J, Sedaghat-Hamedani F, Kayvanpour E, et al. MicroRNA signatures in total peripheral blood as novel biomarkers for acute myocardial infarction. Basic Res Cardiol 2011; 106: 13–23.
- 30.
Goretti E, Vausort M, Wagner DR, Devaux Y. Association between circulating microRNAs, cardiovascular risk factors and outcome in patients with acute myocardial infarction. Int J Cardiol 2013; 168: 4548–4550.
- 31.
Liebetrau C, Mollmann H, Dorr O, Szardien S, Troidl C, Willmer M, et al. Release kinetics of circulating muscle-enriched microRNAs in patients undergoing transcoronary ablation of septal hypertrophy. J Am Coll Cardiol 2013; 62: 992–998.
- 32.
Widera C, Gupta SK, Lorenzen JM, Bang C, Bauersachs J, Bethmann K, et al. Diagnostic and prognostic impact of six circulating microRNAs in acute coronary syndrome. J Mol Cell Cardiol 2011; 51: 872–875.
- 33.
Kaudewitz D, Lee R, Willeit P, McGregor R, Markus HS, Kiechl S, et al. Impact of intravenous heparin on quantification of circulating microRNAs in patients with coronary artery disease. Thromb Haemost 2013; 110: 609–615.
- 34.
Bauters C, Kumarswamy R, Holzmann A, Bretthauer J, Anker SD, Pinet F, et al. Circulating miR-133a and miR-423-5p fail as biomarkers for left ventricular remodeling after myocardial infarction. Int J Cardiol 2013; 168: 1837–1840.
- 35.
Wei T, Folkersen L, Ehrenborg E, Gabrielsen A. MicroRNA 486-3p as a stability marker in acute coronary syndrome. Biosci Rep 2016; 36: e00351, doi:10.1042/BSR20160023.
- 36.
Matsumoto S, Sakata Y, Suna S, Nakatani D, Usami M, Hara M, et al. Circulating p53-responsive microRNAs are predictive indicators of heart failure after acute myocardial infarction. Circ Res 2013; 113: 322–326.
- 37.
Chiam K, Wang T, Watson DI, Mayne GC, Irvine TS, Bright T, et al. Circulating serum exosomal miRNAs as potential biomarkers for esophageal adenocarcinoma. J Gastrointest Surg 2015; 19: 1208–1215.
- 38.
Arroyo JD, Chevillet JR, Kroh EM, Ruf IK, Pritchard CC, Gibson DF, et al. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci U S A 2011; 108: 5003–5008.





