2016 Volume 80 Issue 11 Pages 2327-2335
2016 Volume 80 Issue 11 Pages 2327-2335
Background: Since cardiovascular disease accounts for one-quarter of deaths in the Japanese population, we developed a nationwide database using the administrative case-mix Diagnostic Procedure Combination (DPC) system (ie, the Japanese Registry Of All cardiac and vascular Diseases (JROAD)-DPC) to reveal the current status of cardiovascular medicine in Japan.
Methods and Results: The JROAD-DPC database included 704,593 health records’ data of 2012 from 610 certificated hospitals of the Japanese Circulation Society. The 35,824 patients with acute myocardial infarction (AMI) and 108,665 patients with heart failure (HF) were admitted to hospitals. Increased hospital case volume was associated with reduced in-hospital mortality rates for both AMI and HF (P for trend <0.001). Although there was little variation among AMI patients in terms of aspirin use at discharge (median prescription rate, 83.0%; interquartile range [IQR], 76.9–88.0%), there were wide variations in the proportions of patients prescribed β-blockers (BB) and angiotensin-converting enzyme inhibitors (ACEI)/angiotensin-receptor blockers (ARB) at discharge (BB, 41.4%, IQR 27.6–55.7%; ACEI/ARB, 52.0%, IQR 40.3–62.3%). In patients with HF, there were between-hospital variations in medications at discharge (BB, 38.1%, IQR, 27.8–47.6%; ACEI/ARB, 41.0%, IQR 31.7–49.1%).
Conclusions: A nationwide administrative database of patients with cardiovascular diseases (JROAD-DPC) provided useful information that will contribute to improved quality of medical care, especially in the aging society of Japan, where HF has become an important health problem. (Circ J 2016; 80: 2327–2335)
Japanese people have the longest life expectancy in the world. Over the past 50 years, the percentage of the population that is elderly has increased 4-fold, from 5.7% in 1960 to 23.1% in 2010.1 This rate of change is the fastest worldwide. Cardiovascular disease (CVD) accounts for 25.5% of deaths in the Japanese population, as determined by the survey by the Ministry of Health, Labour and Welfare Health statistics in 2010.2 A nationwide database is required to better prevent the onset and progression of CVD. Comprehensive information from Japan, with its rapidly aging population, will be crucial for developing future perspectives in other countries.
Editorial p 2289
The Japanese Registry Of All cardiac and vascular Diseases (JROAD) was launched in 2004 to assess the clinical activity of each Japanese institution with cardiovascular beds, and to provide adequate feedback to teaching hospitals to improve patient care. However, JROAD does not have individual patient data, which would allow for more powerful and reliable investigations of nationwide data. To complement JROAD, we developed a nationwide claim database, JROAD-DPC, using data from the Japanese Diagnosis Procedure Combination/Per Diem Payment System (DPC/PDPS).
Data from DPC/PDPS lists the lump-sum medical expenses evaluated based on diagnostic and procedural costs beginning in 2002.3 The DPC database includes the following individual data: unique hospital identifier, patient age and sex, main diagnoses and comorbidities, drugs and devices, diagnostic and therapeutic procedures, length of stay, and discharge status. Following the same methodology used in the J-ASPECT nationwide stroke registry,4,5 the combination of JROAD and DPC-based claim data may be helpful to clarify the real-world practice of Japanese CVD other than stroke.
The objectives of this study were therefore to perform a nationwide claim survey using the DPC discharge database (ie, JROAD-DPC), and to reveal the current status of cardiovascular medicine in Japan.
Nearly all teaching hospitals with cardiovascular beds except for stroke participated in JROAD to meet the Japanese Circulation Society (JCS) requirement that JCS-certified teaching hospitals provide cardiology training for physicians who wish to be JCS board-certified cardiologists and undertake the JCS board test.6 Class A JCS-certified teaching hospitals need more than 2 JCS board-certified cardiologists and 30 cardiovascular beds, and class B need more than 1 JCS board-certified cardiologists and 15 cardiovascular beds.
This cross-sectional survey used the DPC discharge database from institutions participating in the JROAD study. Of the 1,612 hospitals that responded to JROAD, 610 agreed to participate in the DPC discharge database study. Computer software was developed to identify patients in the annual de-identified discharge database who were hospitalized because of CVD except for stroke. This identification was based on the International Classification of Diseases (ICD)-10 diagnosis codes related to acute myocardial infarction (AMI: I21.0, I21.1, I21.2, I21.3, I21.4, and I21.9) and heart failure (HF: I50.0, I50.1, and I50.9).4 Data for CVD other than stroke were also collected. The ICD codes for data collection are summarized in Table S1 and Table S2. The information of primary summary indexes related to AMI and HF from JROAD-DPC is also summarized in Table S3.
Hospitalization records between April 1, 2012, and March 31, 2013, were collected; however, patients with scheduled admissions were excluded from the analysis. The following data were extracted from the database: unique hospital identifier, patient age and sex, diagnoses, comorbidities at admission, in-hospital use of medications (antihypertensive agents, hypoglycemic agents, cholesterol-lowering agents, and antiplatelet agents), smoking, Killip classification, New York Heart Association (NYHA) classification on admission, and discharge status. The Killip and NYHA classifications on admission were determined by the physician, and data on all medication use were collected electronically from the claim data. Comorbidities were determined primarily from the ICD-10 codes, but were also checked against the medications and procedures each patient was receiving/undergoing to determine if these were compatible with the code data. Smoking was evaluated based on the physician’s record and rated patients as active or inactive smokers.
It is well known that admission to higher-volume hospitals, which diagnose and treat more cases per year, reduces mortality rates for numerous surgical conditions and medical diseases, including AMI and HF.7,8 The annual case volumes of AMI and HF patients were calculated from the total annual number of condition-specific cases in each hospital in the DPC data. For the purposes of characterizing the sample, hospitals were categorized into quartiles by annual case volumes of AMI and HF.
Ethics StatementThis research plan was designed by the authors and approved by the Institutional Review Board of the National Cerebral and Cardiovascular Center, which waived the requirement for individual informed consent according to the “opt-out” principle. Each hospital anonymized patients’ ID by the code change equations made by each hospital in the original DPC data, which was sent to the Ministry of Health, Labour and Welfare. It notified patients through homepages or posters in each hospital that their information was being collected by this study. Patients could opt-out their information from the database if they wished to exclude it.
Statistical AnalysisAll continuous variables are presented as mean±SD or median (interquartile range; IQR) if the distribution of variables was skewed. The unpaired t-test or Wilcoxon rank-sum test was used to compare groups. Analysis of variance was used to compare means across multiple groups. Non-continuous and categorical variables are presented as frequencies or percentages and were compared using the χ2 test. The Tukey-Kramer test was used to compare continuous variables, and the χ2 test with Bonferroni correction was used for categorical variables. To determine the association between case volume and in-hospital mortality rates in patients hospitalized for AMI and HF, we used hierarchical logistic regression models3,9 to estimate odds ratios (ORs) for in-hospital deaths. To rule out mild cases, we excluded hospitals that had less than 10 cases and patients who were discharged alive within 3 days of admission. This was consistent with a previous study by the Center for Medicare and Medicaid Service, which kept anonymous data excluding small cases.8 We also referred to the National Database of Health Insurance Claims and Specific Health Checkups of Japan publication rule, which excludes small case volume data to avoid specification of individuals.10 We categorized hospitals into quartiles based on case volume: very low (VL), low (L), high (H), and very high (VH). Quartiles were analyzed for trend with the Cochran-Armitage trend test. The main outcome measure was in-hospital or 30-day death. Each model included 2 hierarchy levels (hospital and patient) for considering the random effects of hospital variation; fixed effects of case volume; and patient effects of age, sex, Charlson comorbidity index, Killip class for AMI, and NYHA class for HF. The difference between participating and non-participating hospitals in the DPC discharge study was determined by the unpaired t-test or Wilcoxon rank-sum test. The correlation of case volume between JROAD and JROAD-DPC was measured using Spearman correlation coefficient. Variations in drug prescription patterns related to AMI and HF quality indicators were assessed by coefficient of variation (CV; %). The Bonferroni method was used to adjust the P values in multiple testing, where appropriate, particularly for the demographic variables difference between participating and non-participating hospitals. The analyses were performed using SAS 9.2 (SAS Institute, Cary, NC, USA) and STATA 12 (Stata Corp, College Station, TX, USA).
The DPC system was introduced in 1,505 of the 7,493 hospitals in Japan (ie, DPC hospitals).11 A total of 1,612 hospitals participated in JROAD; 610 of the 1,116 that used the DPC system agreed to participate in the JROAD-DPC study. DPC hospitals accounted for 479,539 of the 898,166 hospital beds in Japan. Altogether, this study included 261,812 beds (29.1% of the total in Japan) in the 610 participating hospitals (Figure 1). JROAD-DPC participating hospitals covered 59.4% (28,851/48,550) of beds in CVD wards for specialized care. The JROAD-DPC database included a total of 704,593 health record data elements. Data of 35,824 patients with AMI and 108,665 with HF were extracted based on ICD-10 codes. Figure S1 shows the correlation of annual AMI patient numbers between JROAD and JROAD-DPC in 610 hospitals. JROAD reported the number of AMI patients hospitalized between January 1, 2012, and December 31, 2012, from individual institutes, whereas JROAD-DPC extracted the AMI patients hospitalized between April 1, 2012, and March 31, 2013. The correlation between institutional reports and claim data was 0.946 (P<0.001), with a median difference of 0.499 and mean relative difference of –5.29%.

Study flow chart of JROAD-DPC study. JROAD, Japanese Registry Of All cardiac and vascular Diseases; DPC, Diagnostic Procedure Combination; JCS, Japanese Circulation Society.
The 97 questionnaires of practice volume and structural indictors in the JROAD survey were similar between participating hospitals (n=482) and non-participating hospitals (n=375) among JCS-certified teaching hospitals (class A) with DPC information (Table S4). Among the class A and B JCS-certified teaching hospitals, there were only differences in in-hospital deaths following AMI between JROAD-DPC participating and non-participating hospitals (Table S4). In the overall analysis of JCS-certified teaching hospitals (classes A and B) and other general hospitals, 5 of 97 questionnaires of practice volume and structural indictors differed between JROAD-DPC participating and non-participating institutes (Table S4).
Furthermore, when comparing the case numbers of AMI and HF between JROAD-DPC and JROAD, JROAD-DPC covered 51.7% (35,824 /69,235) of annual AMI cases and 51.1% (108,665/212,765) of annual HF cases in JCS-certified institutes.
Distribution of Patient Age and Severity of AMI and HF Assessed by the DPC Discharge DatabaseThe DPC discharge database for AMI consisted of 25,788 male (72.0%) and 10,036 female (28.0%) hospitalized patients. Figure 2A shows the distribution of ages. The peak age groups were 60–69 years in men and 80–89 years in women. The average ages of the male and female AMI patients were 67±13 and 77±13 years, respectively. Figure 2B shows the distribution of AMI patients by Killip class. The Killip 1 classification was the most common. The overall in-hospital mortality rate in patients with AMI was 14.5%. By Killip class, it was 1.7% for Killip 1, 4.0% for Killip 2, 13.5% for Killip 3, and 47.5% for Killip 4. It was significantly higher in Killip 4 than in Killip 1 (P<0.001).

Age distribution of males and females (A) and Killip classification (B) among 35,824 patients with acute myocardial infarction. Age distribution of males and females (C) and NYHA classification (D) among 108,665 patients with heart failure. NYHA, New York Heart Association.
The DPC discharge database for HF consisted of 57,368 male (52.8%) and 51,297 female (47.2%) hospitalized patients. As shown in Figure 2C, the peak age was 80–89 years in men and women. The average ages of the male and female HF patients were 75±13 and 81±12 years, respectively. Figure 2D shows the distribution of HF patients by NYHA class. The NYHA I classification was the least common in all groups. The overall in-hospital mortality rate for HF was 11.3%. By NYHA class, it was 2.3% for NYHA I, 3.3% for NYHA II, 6.8% for NYHA III, and 17.9% for NYHA IV. It was significantly higher in NYHA class IV than in class I (P<0.001).
Case Volume-Outcome RelationshipWe categorized hospitals into quartiles according to the annual case volume of AMI patients (Table S5), as follows: 15 (IQR 10–17) in the VL group, 32 (IQR 18–40) in the L group, 57 (IQR 41–70) in the H group, and 109 (IQR 71–259) in the VH group. An inverse trend for the risk of death was observed across quartiles (Figure 3A) (all P for trend of 7-day mortality and 30-day mortality and in-hospital mortality, <0.001). After adjusting for age, sex, Charlson score, and Killip class, and comparing with the VL group as a reference, higher hospital volume was associated with lower mortality rates (all P for trend <0.001; Table).
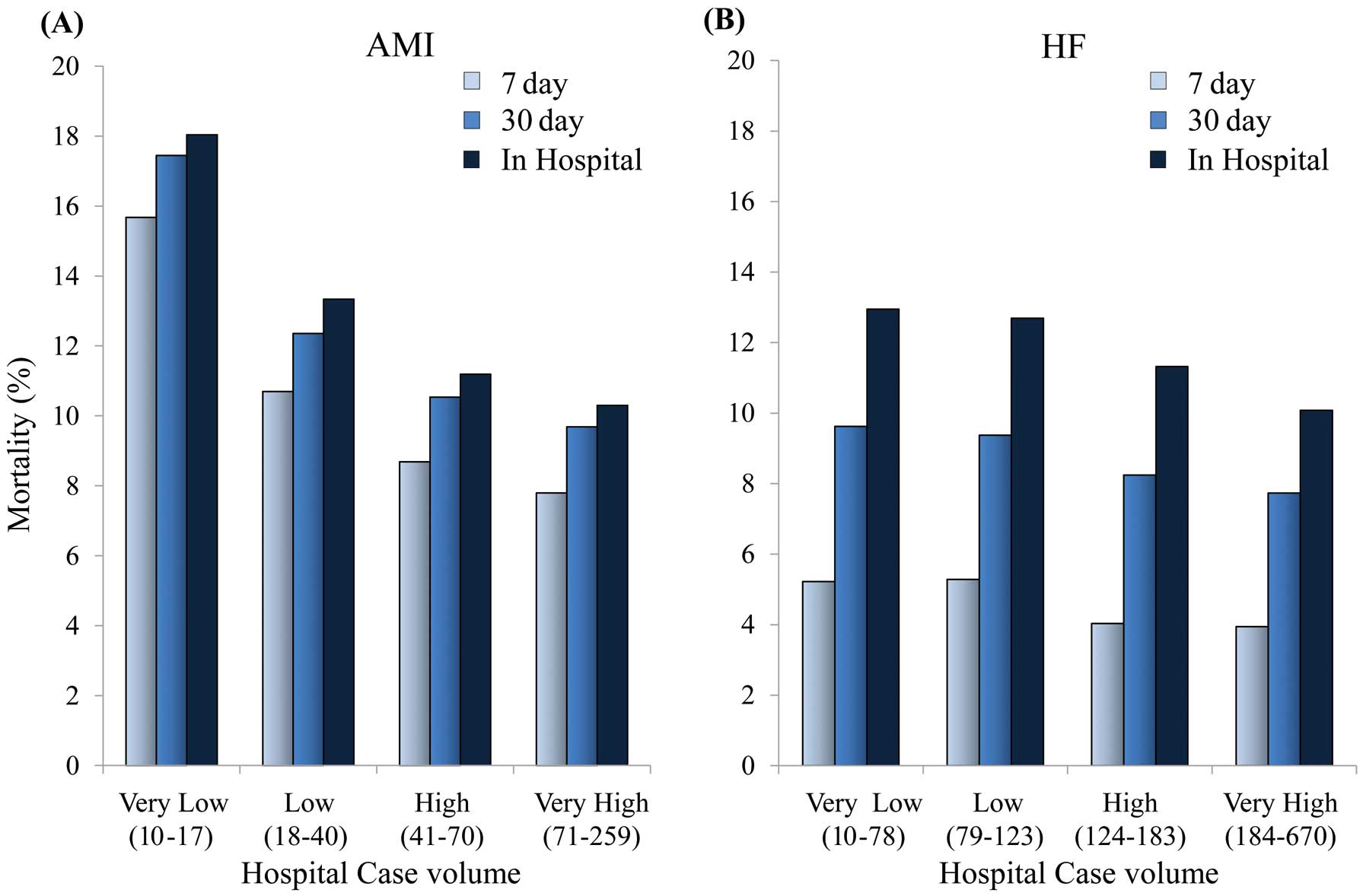
Relationship between mortality rates and hospital case volume (quartiles) in patients with acute myocardial infarction (A) and heart failure (B).
| Quartiles* | N (total cases) | 7-day mortality | 30-day mortality | In-hospital mortality | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | Univariate | Multivariate | ||||||||
| OR (95% CI) |
P for trend | OR (95% CI) |
P for trend | OR (95% CI) |
P for trend | OR (95% CI) |
P for trend | OR (95% CI) |
P for trend | OR (95% CI) |
P for trend | ||
| AMI | |||||||||||||
| Very low (10–17) | 1,272 | Ref. | <0.001 | Ref. | 0.002 | Ref. | <0.001 | Ref. | 0.006 | Ref. | <0.001 | Ref. | 0.004 |
| Low (18–40) | 4,407 | 0.60 (0.49–0.74) | 0.77** (0.53–1.13) | 0.67 (0.55–0.81) | 0.87** (0.61–1.24) | 0.70 (0.57–0.85) | 0.93** (0.66–1.31) | ||||||
| High (41–70) | 7,882 | 0.51 (0.42–0.63) | 0.63** (0.44–0.92) | 0.56 (0.46–0.68) | 0.71** (0.50–1.00) | 0.57 (0.47–0.69) | 0.74** (0.53–1.04) | ||||||
| Very high (71–259) | 15,888 | 0.45 (0.37–0.55) | 0.60** (0.41–0.86) | 0.51 (0.42–0.61) | 0.68** (0.48–0.96) | 0.52 (0.43–0.63) | 0.71** (0.51–0.98) | ||||||
| HF | |||||||||||||
| Very low (10–21) | 7,903 | Ref. | <0.001 | Ref. | <0.001 | Ref. | <0.001 | Ref. | 0.003 | Ref. | <0.001 | Ref. | <0.001 |
| Low (22–46) | 15,631 | 1.01 (0.89–1.15) | 1.02† (0.83–1.27) | 0.97 (0.88–1.07) | 0.99† (0.84–1.16) | 0.97 (0.90–1.06) | 0.98† (0.85–1.13) | ||||||
| High (47–80) | 22,463 | 0.76 (0.67–0.87) | 0.79† (0.64–0.98) | 0.84 (0.77–0.93) | 0.86† (0.73–1.01) | 0.86 (0.79–0.93) | 0.87† (0.75–1.00) | ||||||
| Very high (81–259) | 38,415 | 0.75 (0.66–0.84) | 0.75† (0.61–0.92) | 0.79 (0.72–0.86) | 0.83† (0.71–0.97) | 0.75 (0.70–0.81) | 0.79† (0.69–0.90) | ||||||
*No. of cases in each hospitals. **Adjusted for age, sex, Charlson’s comorbidity index and Killip class. †Adjusted for age, sex, Charlson’s comorbidity index and NYHA class. AMI, acute myocardial infarction; CI, confidence intervals; HF, heart failure; OR, odds ratios.
We also categorized hospitals into quartiles according to the annual case volume of HF patients (Table S6), as follows: 63 (IQR 10–78) in the VL group, 102 (IQR 79–123) in the L group, 153 (IQR 124–183) in the H group, and 253 (IQR 184–670) in the VH group. An inverse trend for the risk of death was observed across quartiles (Figure 3B) (all P for trend of 7-day mortality, 30-day mortality and in-hospital mortality, <0.001). Multivariate analysis also showed that higher hospital volume was associated with lower mortality rates (all P for trend <0.001; Table).
Medications at Discharge of Japanese Patients With AMI and HFIn patients with AMI, the prescription rates of aspirin and statins at discharge were 83.0% (IQR 76.9–88.0%, CV 18.7%) and 66.9% (IQR 54.5–75.6%, CV 24.7%), respectively, with minimal hospital-level variation. However, there were wide variations in the prescription rates of β-blockers (BB) and angiotensin-converting enzyme inhibitors (ACEI)/angiotensin-receptor blockers (ARB) at discharge (BB, 41.4% [IQR 27.6–55.7%, CV 28.1%]; ACEI/ARB, 52.0% [IQR 40.3–62.3%, CV 98.9%]) (Figures 4A–D). We found similar results for HF patients, with wide variations in the prescription rates of BB and ACEI/ARB at discharge (BB, 38.1% [IQR 27.8–47.6%, CV 62.4%]; ACEI/ARB, 41.0% [31.7–49.2%, CV 29.8%]) (Figures 4E,F).

Distribution among hospitals (n=610) of prescription rates for aspirin (A), β-blockers (B), ACEI/ARB (C), and statins (D) for patients with acute myocardial infarction. Distribution among hospitals (n=610) of prescription rates for ACEI/ARB (E) and β-blockers (F) for patients with heart failure. ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin-receptor blocker.
The major findings of JROAD-DPC were as follows: (1) HF was nearly 3-fold as common as AMI and has become an important health problem with the aging of the population, and (2) differences in in-hospital mortality rates of AMI and HF were associated in part with variations in hospital case volumes at discharge. To the best of our knowledge, the present JROAD-DPC study is unique in its analysis of a large number of health record elements from a nationwide claim-based database that can be combined with comprehensive care capability resources (ie, hospitals, beds, and cardiologists) from JROAD for all JCS-certified teaching hospitals. It covers all CVD except for stroke. This type of nationwide registry with a large number of cases offers a unique opportunity to assess the current status of CVD care in Japan and benchmarking for quality of care. This type of nationwide database is emerging in other countries. For example, the NCDR (National Cardiovascular Data Registry) comprises 7 distinct quality measurement and improvement programs developed by the American College of Cardiology Foundation, resisted over several millions of CVD cases12 and published important studies regarding patient care and outcomes.13–15
JROAD-DPC: Development of Nationwide Claim-Based Database for CVDThe strength of this study stems from the large data set obtained through the Japanese administrative case-mix DPC system, whereby individual patients’ records are collected systematically nationwide using a uniform reporting format. The large sample size should increase the reliability of our results. Although the DPC system is similar to the Nationwide Inpatient Sample in the USA, it has a few distinct advantages. First, physicians are required to provide complete diagnostic code data until the time of patient discharge. The diagnostic accuracy of cardiologists within the DPC system seems to be confirmed.16,17 Second, the DPC system can distinguish post-hospitalization sequelae from comorbidities that were already present at admission.
In the analysis for data of 97 practical volume and structural indicators in the JROAD survey, there was no difference between participating and non-participating hospitals among class A JCS-certified hospitals. However, there was 1 (in-hospital death following AMI) difference among classes A and B JCS-certified hospitals; 5 (number of patients with chronic HF, number of in-hospital deaths from CVD except for stroke, in-hospital deaths following AMI, number of beds in cardiology department, pacemaker implantation) out of 97 questionnaires differed among hospitals containing other general hospitals. In this analysis, 1,021 (91%) of 1,116 hospitals were JCS-certified (classes A and B hospitals). Combined, our data represent “real-world” clinical practice of JCS-certified hospitals specialized for CVD other than stroke.
Comparison of the JROAD-DPC With Regional Registry Studies of AMI and HFIn this study, data for 35,824 AMI patients demonstrated a male predominance (~70%) and an age difference between sexes of approximately 10 years. These nationwide database findings confirm the results of previous regional registry studies. For instance, the MIYAGI-AMI registry, which included 43 hospitals, reported that 72% of AMI patients were male and the average ages of male and female patients were 65±13 and 75±11 years, respectively.18 The overall mortality rate of 14.5% in our study is higher than in the regional registries: 7.8% in 2008 in the MIYAGI-AMI registry18 and 9.6% in 2011 in the KACE registry.19 Our finding of severity-specific mortality based on Killip class was consistent with a previous report on AMI.20
We analyzed data of 108,665 patients with HF, 3-fold more than with AMI. The overall in-hospital mortality rate for HF was 11.3%, and 17.9% in patients in NYHA class IV. Japanese HF patients were relatively old, particularly the women, who had an average age of over 80 years. The CHART-2 registry from 24 hospitals reported that the average ages of males and females with stage C/D HF were 68±12 and 72±12 years, respectively.21 The patients in the CHART-2 registry were enrolled in 2006, and the difference in study period compared with our investigation may partly explain the disparity in patient ages.
Significant Inverse Association Between Hospital Case Volume and In-Hospital OutcomesThe DPC data can provide a relatively complete perspective on in-hospital mortality that additionally takes into account severity and complications during hospitalization. The 7-day, 30-day, and in-hospital mortality of AMI patients decreased with increasing hospital case volumes, even after adjusting for baseline characteristics and settings. These results confirm and expand the findings of previously published studies from the USA, which has a different healthcare system.8,22 With these studies as a reference point, our data demonstrated a lower 30-day mortality in Japan than in the USA. As shown in Figure 3A, most fatal events occurred early during hospitalization, suggesting that prompt medical care contributed to better cardiovascular outcomes. A previous comparative study of AMI that analyzed 3 Japanese, 4 European, 4 American, and 2 worldwide databases demonstrated that the rate of primary percutaneous coronary intervention (PCI) was higher (75–94%) in Japan than in the other countries (5.5–49.6%), particularly in low-volume hospitals.23 The difference in mortality rates between Japan and the USA may be related to the primary access to PCI hospitals, but further international comparisons under quality-controlled conditions are needed.
We also found a significant inverse association between case volume and mortality in HF patients. The rate of decline in mortality as related to case volume was slower in patients with HF than in those with AMI (Figure 3). Unlike death from AMI, that related to HF increased with duration of hospitalization. Although many studies have reported an inverse association between hospital case volume and death in diseases treated using surgical or interventional techniques (eg, primary PCI),24–31 few studies have assessed the association between case volume and death in HF. The present findings suggest the importance of multidisciplinary treatment for HF patients who are relatively old and have various risk factors and underlying heart diseases (eg, HF with preserved or reduced ejection fraction).
Assessment of Quality Indicators for AMI and HF in Japanese PatientsThe present study showed that in AMI patients, BB and ACEI/ARB were prescribed less frequently and with wider variations between hospitals than aspirin and statins (Figure 3); a similar finding was also observed in a prior study in the USA.32 Also, as shown in Figure 4E and Figure 4F, there were wide variations in BB and ACEI/ARB prescriptions at hospital discharge for patients with HF (Figures 4E,F).
The American Heart Association launched the “Get With The Guidelines” program in the USA in 2000, with a subsequent substantial improvement in the quality of care over a broad range of cardiovascular conditions in multiple settings.32–34 One study of the program’s effects found that median hospital adherence to AMI and HF composite measures was 93% (IQR 87–97%) and 92% (IQR 85–96%), respectively.35 As shown in the present study, however, in Japan there seems to be greater variation among hospitals in the quality of care for AMI or HF.
Our findings have important implications. First, AMI patients should be sent to core regional hospitals that achieve high volumes, even among the JCS-certified hospitals. Second, we can obtain the greatest benefits by targeting quality improvement efforts in hospitals with smaller volumes.
Study LimitationsOur data has several important limitations. First, the DPC system focused on JCS-certified hospitals; although these institutions accounted for 29% of all hospital beds in Japan, the applicability of our findings to non-certified hospitals is unclear. Second, although there were significant correlations in annual AMI patient numbers as determined by institutional reports and claim data (Figure S1), the data collection period was not always consistent. Further studies using a well-defined time period are needed. Data validation with chart review and re-evaluation of discrepant cases could improve the precision of JROAD-DPC. Third, neither JROAD nor JROAD-DPC covers stroke. As stroke is major contributor to the morbidity and mortality in CVD in Japan, future collaboration with other registries for stroke (eg, J-ASPECT study)4 seems preferable. Fourth, the target of JROAD-DPC is JCS-certified teaching hospitals with the DPC system. Therefore, generalization of the results from JROAD-DPC is limited for small clinics or non-specialist hospitals.
A nationwide administrative JROAD-DPC database of patients with CVD provided useful information that will contribute to improved quality of medical care, especially in the aging society of Japan, where HF has become an important health problem.
The present work was supported in part by a grant from the Ministry of Health, Labour and Welfare of Japan [H26-Ippan-001] (S.Y.), and was supported by the Japanese Circulation Society. The funders had no role in the design and conduct of the study or in the collection, management, analysis, and interpretation of the data, the preparation, review, or approval of the manuscript and the decision to submit the manuscript for publication.
All authors have completed the ICMJE Form for Disclosure of Potential Conflicts of Interest.
H.O. reports personal fees from Actelion Pharmaceuticals Japan Ltd, grants from Astellas Pharma Inc, personal fees from Astra Zeneca K.K., grants and personal fees from Bayer Yakuhin, Ltd, personal fees from Boehringer Ingelheim Japan, grants from Bristol-Myers Squibb Company, grants from Chugai Pharmaceutical Co, Ltd, grants and personal fees from Daiichi Sankyo Co, Ltd, grants from Dainippon Sumitimo Pharma Co, Ltd, personal fees from Eisai Co, Ltd, personal fees from Kowa Company, Ltd, personal fees from Kyowa Hakko Kirin Co, Ltd, personal fees from Mitsubishi Tanabe Pharma, grants from Mochida Pharmaceutical Co, Ltd, grants and personal fees from MSD K.K., grants from Novartis Pharma K.K., grants from Ono Pharmaceutical Co, Ltd, grants and personal fees from Otsuka Pharmaceutical Co, Ltd, grants and personal fees from Pfizer Japan Inc, grants and personal fees from Sanofi K.K., grants from Shionogi & Co, Ltd, grants and personal fees from Takeda Pharmaceutical Co, Ltd, personal fees from Teijin Pharma Co, Ltd, outside the submitted work. S.Y. reports grants and personal fees from Takeda, personal fees from Daiichi-Sankyo, personal fees from Astra-Zeneca, grants and personal fees from Bristol-Myers grants from Otsuka, grants from Boehringer Ingel, outside the submitted work. H.T. reports grants from MSD, grants and personal fees from Takeda, grants and personal fees from Daiichi-Sankyo, grants from Teijin, grants and personal fees from Nippon Boehringer, grants and personal fees from Bayer, grants from BMS, grants and personal fees from Tanabe-Mitsubishi, outside the submitted work. I.K. reports grants and personal fees from Takeda Pharmaceutical Co, Ltd, grants and personal fees from Nippon Boehringer Ingelheim Co, Ltd, personal fees from MSD K.K, grants and personal fees from Astellas Pharma Inc, grants from Genzyme Japan K.K., grants and personal fees from Mitsubishi Tanabe Pharma Corporation, personal fees from Pfizer Japan Inc, grants and personal fees from Daiichi-Sankyo, outside the submitted work. Y. Saito reports grants and personal fees from MSD Co, Ltd, grants and personal fees from Mitsubishi Tanabe Pharma Corporation, grants and personal fees from Daiichi Sankyo Company Ltd, grants and personal fees from Takeda Pharmaceutical Co, Ltd, grants and personal fees from Novartis Pharma K.K., grants and personal fees from Shionogi & Co, Ltd, grants and personal fees from Astellas Pharma Inc, grants and personal fees from AstraZeneca K.K., grants and personal fees from Otsuka Pharmaceutical Co, Ltd, grants from St. Jude Medical Japan Co, Ltd, grants and personal fees from Kyowa Hakko Kirin Co, Ltd, grants and personal fees from Pfizer Japan Inc, grants and personal fees from Eisai Co, Ltd, grants and personal fees from Kowa Pharmaceutical Co Ltd, grants from Sanofi-Aventis K.K., grants and personal fees from Dainippon Sumitomo Pharma Co, Ltd, grants and personal fees from Toa Eiyo Ltd, grants and personal fees from Bayer Yakuhin, Ltd, grants from Baxter Limited, grants and personal fees from Mochida Pharmaceutical Co, Ltd, outside the submitted work.
Supplementary File 1
Table S1. Diagnosis codes for data extraction in JROAD-DPC
Table S2. Procedure codes for data extraction in JROAD-DPC
Table S3. Summary of JROAD-DPC data associated with AMI and HF
Table S4. Characteristics of JROAD-DPC participating and non-participating hospitals
Table S5. Characteristics of patients with AMI according to hospital case volume in JROAD-DPC
Table S6. Characteristics of patients with HF according to hospital case volume in JROAD-DPC
Figure S1. Correlation of annual AMI patient numbers between JROAD and JROAD-DPC in 610 hospitals.
Please find supplementary file(s);
http://dx.doi.org/10.1253/circj.CJ-16-0196