2016 Volume 80 Issue 2 Pages 450-460
2016 Volume 80 Issue 2 Pages 450-460
Background: The effects of eicosapentaenoic acid (EPA) on coronary artery disease have been previously reported; however, those of the addition of EPA to strong statins on coronary plaque components and local inflammatory cytokines are not known.
Methods and Results: A total of 95 patients who had been treated with strong statin for at least 6 months were randomized into 2 groups: an EPA group (additional treatment with EPA at 1,800 mg/day, n=48) or a control group (no additional treatment, n=47), for 6 months. The tissue characteristics of target coronary plaque in each patient were analyzed using IB-IVUS before and after treatment. We also measured plasma levels of inflammatory cytokines sampled in the coronary sinus (CS) and peripheral vein. A significant reduction in lipid volume (18.5±1.3 to 15.0±1.5 mm3, P=0.007) and a significant increase in fibrous volume (22.9±0.8 to 25.6±1.1 mm3, P=0.01) were observed in IB-IVUS image analyses in the EPA group, but no significant changes in the plaque components in the control group. CS levels of pentraxin 3 and monocyte chemoattractant protein-1 were lower after than before treatment with EPA (3.3±2.1 to 2.6±1.2 ng/ml, 120.4±26.2 to 110.2±26.8 pg/ml, P=0.015 and P=0.008, respectively); however, there were no significant changes in those inflammatory cytokines between pre- and post-treatment in the control group.
Conclusions: The addition of EPA was associated with reduced lipid volume in coronary plaques and decreased inflammatory cytokines. (Circ J 2016; 80: 450–460)
Many epidemiologic studies have shown a significant inverse association between increased intake of long-chain n-3 polyunsaturated fatty acids, especially eicosapentaenoic acid (EPA) and docosahexaenoic acid, and cardiovascular or cerebrovascular disease.1–7 A large-scale intervention clinical trial (Japan EPA Lipid Intervention Study: JELIS) that included hypercholesterolemic patients showed that long-term use of EPA (1,800 mg/day), in addition to statin therapy, significantly reduced the incidence of major coronary events.8 Subanalysis of JELIS also showed that EPA reduced the incidence of recurrent stroke or coronary artery disease in the high-risk group.9,10 Proposed mechanisms to explain these findings include reduced triglyceride concentrations,11 antiarrhythmic effects,12 decreased platelet aggregation,13 improved vascular reactivity,14 decreased inflammation15 and lowered blood pressure.16 However, the effects of EPA on the coronary plaque components and inflammatory cytokines are not known. Our hypothesis was that the addition of EPA to strong statin treatment could further stabilize plaque components and the local inflammatory reaction of coronary arteries in patients with stable angina.
Editorial p 321
Integrated backscatter intravascular ultrasound (IB-IVUS) has recently become available, by which plaque properties can be diagnosed by combining spectral parameters of posterior-scattering signals of IVUS.17–23 We aimed to assess the additional effects of EPA on coronary plaque by IB-IVUS and to measure local plasma levels of inflammatory cytokines in patients who had been treated with strong statins.
This study was designed as a blocked-randomization, open-label, single-center study that included dyslipidemic patients with stable angina pectoris planned to be treated with a bare-metal stent because previous coronary angiography (CAG) had shown significant coronary stenosis, defined as ≥75% angiographic stenosis and mild stenosis defined as <50% in the same coronary branch, and who had been treated with the typical Japanese dose of a strong statin (atorvastatin at 10 mg/day; n=37, pitavastatin at 2 mg/day; n=36, or rosuvastatin at 2.5 mg/day; n=22) for more than 6 months (Figure 1A). Patients with any of the following were excluded from the study: (1) hypersensitivity to high-purity EPA, (2) acute coronary syndrome (ACS), (3) a history of percutaneous coronary intervention (PCI) or coronary artery bypass surgery, (4) renal insufficiency with baseline serum creatinine >2.0 mg/dl, (5) hemodialysis and (6) highly calcified lesions defined as the calcium arc ≥180°. We also excluded patients with severe culprit lesions (long lesion or small reference diameter) that should be treated with a drug-eluting stent (DES). The DES has been reported to induce persistent inflammatory changes of the vascular wall for a long period through combined drug and polymer actions.24,25 It markedly influences inflammatory cytokines, so we excluded patients treated with DES. All patients underwent PCI, and also had IB-IVUS analyses performed in the treated coronary vessel immediately and 6 months after the PCI. Additional EPA treatment was initiated within 24 h after baseline IVUS observation. Peripheral blood samples were collected at the time of admission for PCI and follow-up CAG respectively. The ejection fraction was measured echocardiographically, using the biplane modified Simpson’s rule at the time of admission for PCI. The primary endpoint evaluated in this study was the change in lipid and fibrous plaque components assessed by IB-IVUS after the addition of EPA to existing strong statin therapy. The secondary endpoint was the change in local inflammatory cytokines (described later) levels sampled in the coronary sinus (CS). Patients were observed monthly during regular outpatient visits; there were no cases of death, acute myocardial infarction (AMI), or target vessel revascularization.

Study protocol and procedure of IVUS analysis and blood sampling. (A) Study flowchart. (B) IVUS measurement. Representative case: analytical region proximal to the stent placed in the RCA. (C) IVUS analysis was performed using the proximal end of the stent as an index. Target plaques had to be >5 mm from the intervention site. (D) Blood sampling to measure inflammatory cytokine levels in the CS. AP, angina pectoris; BMS, bare-metal stent; CAG, coronary angiography; CS, coronary sinus; EPA, eicosapentaenoic acid; IVUS, intravascular ultrasound; PCI, percutaneous coronary intervention; pt(s), patient(s); RCA, right coronary artery.
Written informed consent was given by all the patients, and the study protocol was approved by the Institutional Review Board of Tokushima University Hospital.
IVUS Data AcquisitionFor IVUS observation, ViewIT (40 MHz) (Terumo Co, Tokyo, Japan) was used. Data were collected at 0.5 mm/s auto pullback and analyzed using the IVUS console. For the prevention of coronary spasm, we administered an intracoronary optimal dose of isosorbide dinitrate before measurements. Targeted mild stenosis (<50% diameter stenosis on quantitative CAG) was selected by the presence of an easily definable branch or by the distance from the stent edge, to ensure reliable comparisons between baseline and 6-month follow-up. When slight stenosis was present at 2 or more sites, we selected the lesions with more severe stenosis while excluding those with inadequate IVUS data. Plaques analyzed in this study had to be more than 5 mm from the intervention site. For each plaque we selected an area defined by the narrowest site in the plaque as determined by IVUS, as well as 5 mm both proximally and distally (total length, 10 mm), and extracted 10 frames in 1-mm intervals using a motorized pullback system (Figures 1B,C). Cross-sectional lumen area, cross-sectional vessel area within the external elastic membrane, and plaque area (external elastic membrane area minus lumen area) were determined using software attached to the IVUS system. Plaque volume was calculated using integration.
The definitions of IB values for each histologic category were determined by comparing histologic images, as reported in a previous study.17 Plaque components were classified into 4 types by combining the spectral parameters of posterior-scattering signals of IVUS: lipid pool, fibrosis, dense fibrosis or calcification. The percent area of each component was automatically measured in each plaque. The percent volume was calculated using integration. The IVUS measurements were conducted independently by 2 observers who were blinded to each patient’s clinical characteristics. Measurements of lipid volume were performed in a group of 30 randomly selected records by 1 observer and then repeated on 2 separate days by 2 observers who were unaware of the patient’s clinical characteristics and the measurements of the others and of the study time point.
Blood Sampling and Measurement of Inflammatory CytokinesBlood sampling was performed by the femoral approach before performing PCI or follow-up CAG. Samples to measure plasma levels of inflammatory cytokines [pentraxin (PTX)3, monocyte chemoattractant protein (MCP)-1, high-sensitivity C-reactive protein (hs-CRP), tumor necrosis factor (TNF)α, interleukin-6, matrix metalloproteinase (MMP)-3, MMP-9] were taken from the CS (Figure 1D) and femoral vein using a coronary venographic catheter (UNITE 5Fr CS 100 cm, Asahi Intecc, Aichi, Japan).
Statistical AnalysisIn setting patient numbers, we could not find prior IB-IVUS results to use as a background; therefore, we could not make assumptions about the changes in lipid volume in the EPA-administered and control groups. Instead, patient numbers were determined in an exploratory fashion.
All data are expressed as mean±SD. Testing for significant differences of each parameter between baseline and 6 months was performed using the paired Student’s t-test and testing between groups was performed using the unpaired Student’s t-test. Significance was indicated by P<0.05. Statistical analyses were performed using StatView 5.0 (SAS Institute Inc, Cary, NC, USA).
Between May 2009 and December 2014, 95 patients who were eligible based on the inclusion criteria were enrolled in this study. They were allocated to 2 groups using computer-assisted permuted-block randomization26 with random block size of 4–6: an EPA group (additional treatment with EPA at 1,800 mg/day, n=48) and a control group (no additional treatment, n=47). In total, 36 patients were excluded from this study during the follow-up period because of (1) inadequate IVUS findings (32 patients), (2) no angiographic mild stenosis (1 patient), (3) EPA hypersensitivity (1 patient), (4) refusing follow-up blood sampling from CS (1 patient), and (5) refusing follow-up IVUS study (1 patient). We analyzed the final population of 59 patients (EPA group, n=29; control group, n=30) (Figure 1A).
Baseline CharacteristicsThere were no significant differences in the baseline characteristics of the 2 groups (Table 1).
| EPA (n=29) | Control (n=30) | P value | |
|---|---|---|---|
| Age (years) | 68.1±10.1 | 69.4±10.7 | 0.49 |
| Male sex | 21 (72) | 19 (63) | 0.45 |
| Hypertension | 29 (100) | 30 (100) | – |
| DM type 2 | 15 (53) | 15 (50) | 0.89 |
| Current smoker | 0 (0) | 0 (0) | – |
| Systolic BP (mmHg) | 121.2±9.1 | 119.5±11.9 | 0.14 |
| Diastolic BP (mmHg) | 66.8±7.3 | 66.6±10.6 | 0.98 |
| EPA (μg/ml) | 59.9±23.5 | 71.6±38.7 | 0.13 |
| AA (μg/ml) | 163.7±43.9 | 169.7±38.0 | 0.51 |
| EPA/AA | 0.3±0.2 | 0.4±0.2 | 0.14 |
| HDL-C (mg/dl) | 50.5±13.9 | 54.3±16.2 | 0.40 |
| LDL-C (mg/dl) | 97.7±20.6 | 100.0±31.1 | 0.85 |
| Triglycerides (mg/dl) | 151.3±73.2 | 128.6±58.9 | 0.16 |
| FBS (mg/dl) | 122.7±37.7 | 121.0±30.7 | 0.96 |
| HbA1c (%) | 6.1±1.3 | 6.0±0.7 | 0.16 |
| LVEF (%) | 61.1±10.3 | 62.4±8.3 | 0.32 |
| Culprit coronary artery | |||
| LAD (%) | 12 (41) | 11 (37) | 0.71 |
| LCx (%) | 7 (24) | 9 (30) | 0.61 |
| RCA (%) | 10 (34) | 10 (33) | 0.93 |
| LMT (%) | 0 (0) | 0 (0) | – |
| Medications | |||
| Aspirin | 29 (100) | 30 (100) | – |
| Thienopyridine | 29 (100) | 30 (100) | – |
| ACEI | 9 (31) | 7 (23) | 0.51 |
| ARB | 7 (24) | 11 (37) | 0.30 |
| CCB | 13 (45) | 13 (43) | 0.91 |
| β-blocker | 8 (28) | 9 (30) | 0.84 |
Data given as mean±SD or n (%). EPA group, strong statin plus EPA; control group, strong statin only. AA, arachidonic acid; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BP, blood pressure; CCB, calcium-channel blocker; DM, diabetes mellitus; EPA, eicosapentaenoic acid; FBS, fasting blood sugar; HbA1c, hemoglobin A1c; HDL-C, high-density-lipoprotein cholesterol; LAD, left anterior descending; LCx, left circumflex; LDL-C, low-density-lipoprotein cholesterol; LMT, left main trunk; LVEF, left ventricular ejection fraction; RCA, right coronary artery.
Serum EPA level and EPA/AA ratio were significantly increased in the EPA group; however, there were no significant changes in this level or this ratio in the control group. There were no significant changes in serum AA, lipid level, glucose and blood pressure between baseline and follow-up in either group (Table 2).
| Baseline | Follow-up | P value | |
|---|---|---|---|
| EPA (μg/ml) | |||
| EPA | 59.9±23.5 | 174.0±47.6* | <0.0001 |
| Control | 71.6±38.7 | 67.6±39.3 | 0.65 |
| AA (μg/ml) | |||
| EPA | 151.2±43.9 | 143.5±38.5 | 0.09 |
| Control | 169.7±38.0 | 178.9±39.6 | 0.27 |
| EPA/AA | |||
| EPA | 0.3±0.2 | 1.3±0.5** | <0.0001 |
| Control | 0.4±0.2 | 0.4±0.3 | 0.51 |
| HDL-C (mg/dl) | |||
| EPA | 50.5±13.9 | 49.4±14.7 | 0.56 |
| Control | 54.3±16.2 | 55.5±18.1 | 0.50 |
| LDL-C (mg/dl) | |||
| EPA | 97.7±20.6 | 91.4±20.6 | 0.22 |
| Control | 100.0±31.1 | 88.3±19.2 | 0.10 |
| Triglycerides (mg/dl) | |||
| EPA | 157.3±73.2 | 138.1±94.2 | 0.10 |
| Control | 128.6±58.9 | 119.5±62.3 | 0.53 |
| FBS (mg/dl) | |||
| EPA | 122.7±37.7 | 126.3±50.1 | 0.21 |
| Control | 121.0±30.7 | 118.1±34.5 | 0.71 |
| HbA1c (%) | |||
| EPA | 6.1±1.3 | 6.0±1.1 | 0.60 |
| Control | 6.0±0.7 | 6.0±0.9 | 0.86 |
| Systolic BP (mmHg) | |||
| EPA | 121.2±9.1 | 122.5±9.4 | 0.12 |
| Control | 117.5±11.9 | 118.1±9.4 | 0.71 |
| Diastolic BP (mmHg) | |||
| EPA | 66.8±7.3 | 66.9±8.2 | 0.80 |
| Control | 66.6±10.6 | 67.0±8.5 | 0.87 |
Data given as mean±SD. *P<0.001, vs. control group; **P=0.002, vs. control group. There were no significant differences in the other parameters between groups at baseline or follow-up. Abbreviations as in Table 1.
There were no significant differences between the 2 groups in vessel volume, lumen volume and plaque volume at baseline. These parameters did not change significantly between baseline and follow-up (Table 3A). Changes on the IVUS images of coronary plaque in representative cases in the EPA group and control group are shown in Figures 2 and 3, respectively. There were also no significant differences between the 2 groups in the volume of the 4 IB components at baseline. However, significant increases in fibrous volume and reductions in lipid volume were observed after treatment with EPA, despite no significant changes in the parameters in the control group. There were no significant changes in dense fibrous volume or calcified volume in both groups. After 6 months, there were significant differences in fibrous volume and lipid volume, but no significant differences in dense fibrous volume and calcified volume between the 2 groups (Table 3A). There were significant differences in both the nominal and percent change in fibrous and lipid volumes between the 2 groups (Table 3B). The intraobserver correlation coefficient of lipid volume was r=0.94 (P<0.01). The interobserver correlations of lipid volume by the 2 physicians who conducted IVUS measurement independently was r=0.90 (P<0.01).
| A | Baseline | Follow-up | P value |
|---|---|---|---|
| Vessel volume (mm3) | |||
| EPA | 138.8±21.5 | 128.9±19.2 | 0.28 |
| Control | 133.2±15.3 | 137.9±11.2 | 0.34 |
| Lumen volume (mm3) | |||
| EPA | 95.1±12.5 | 87.1±7.2 | 0.45 |
| Control | 88.4±6.3 | 93.6±2.1 | 0.27 |
| Plaque volume (mm3) | |||
| EPA | 43.5±7.8 | 42.9±9.9 | 0.62 |
| Control | 44.8±9.2 | 44.3±12.4 | 0.66 |
| Fibrous volume (mm3) | |||
| EPA | 22.9±0.8 | 25.6±1.1¶ | 0.01 |
| Control | 24.0±1.0 | 21.8±1.9 | 0.15 |
| Lipid volume (mm3) | |||
| EPA | 18.5±1.3 | 15.0±1.5§ | 0.007 |
| Control | 17.8±1.3 | 19.3±2.1 | 0.29 |
| Dense fibrosis volume (mm3) | |||
| EPA | 1.0±0.3 | 1.2±0.5 | 0.52 |
| Control | 1.9±0.3 | 2.4±0.6 | 0.83 |
| Calcified volume (mm3) | |||
| EPA | 1.1±0.1 | 1.1±0.2 | 0.45 |
| Control | 1.1±0.1 | 0.8±0.3 | 0.51 |
| B | EPA | Control | P value |
| Nominal change | |||
| Vessel volume (mm3) | −9.9±3.2 | 4.7±3.3 | 0.10 |
| Lumen volume (mm3) | −8.1±4.1 | 5.2±4.5 | 0.12 |
| Plaque volume (mm3) | −0.6±2.8 | −0.5±3.7 | 0.80 |
| Fibrous volume (mm3) | 2.7±0.1 | −2.2±0.8 | 0.01 |
| Lipid volume (mm3) | −3.5±0.2 | 1.5±1.0 | 0.005 |
| Dense fibrosis volume (mm3) | 0.2±0.3 | 0.5±0.3 | 0.21 |
| Calcified volume (mm3) | −0.1±0.1 | −0.3±0.2 | 0.33 |
| Percent change | |||
| Fibrous volume (%) | 11.7±8.3 | −9.2±5.3 | 0.01 |
| Lipid volume (%) | −18.9±9.2 | 8.4±4.5 | 0.002 |
| Dense fibrosis volume (%) | 20.0±10.2 | 26.3±13.2 | 0.62 |
| Calcified volume (%) | −8.9±3.2 | −27.3±11.7 | 0.08 |
(A) EPA group, strong statin plus eicosapentaenoic acid; control group, strong statin only. (B) Data given as mean±SD. ¶P=0.014 vs. control, §P=0.013 vs. control. There were no significant differences in the other parameters between the 2 groups at baseline or follow-up. EPA group, strong statin plus EPA; control group, strong statin only. EPA, eicosapentaenoic acid; IVUS, intravascular ultrasound.

Representative case in the EPA group. Color-coded maps of coronary arterial plaque constructed using IB-IVUS in the EPA group. Percent volume of the fatty (lipid pool) and fibrous (fibrosis) components changed from 48.6% to 36.4% and from 49.1% to 61.3%, respectively, showing stabilization of the plaque on IB imaging. IB-IVUS, integrated backscatter intravascular ultrasound; EPA, eicosapentaenoic acid.

Representative case in the control group. Color-coded maps of coronary arterial plaque constructed using IB-IVUS in the control group. Percent volume of the fatty (lipid pool) and fibrous (fibrosis) components changed from 48.2% to 46.5% and from 45.8% to 48.5%, respectively, not showing apparent stabilization of the plaque on IB imaging. IB-IVUS, integrated backscatter intravascular ultrasound.
The CS levels of PTX3 and MCP-1 were lower after than before treatment in the EPA group, but there were no significant changes in cytokine levels between baseline and follow-up in the control group. Peripheral vein cytokine levels showed no significant changes between baseline and follow-up in both groups (Table 4).
| Baseline | Follow-up | P value | |
|---|---|---|---|
| CS | |||
| PTX3 (ng/ml) | |||
| EPA | 3.3±2.1 | 2.6±1.2 | 0.015 |
| Control | 2.8±2.6 | 2.5±1.4 | 0.62 |
| MCP-1 (pg/ml) | |||
| EPA | 120.4±26.2 | 110.2±26.8 | 0.008 |
| Control | 111.5±32.8 | 103.8±30.8 | 0.22 |
| hs-CRP (mg/L) | |||
| EPA | 0.2±0.4 | 0.1±0.2 | 0.38 |
| Control | 0.1±0.2 | 0.1±0.1 | 0.47 |
| TNF-α (pg/ml) | |||
| EPA | 2.5±1.9 | 3.1±2.1 | 0.65 |
| Control | 3.6±4.1 | 2.4±3.0 | 0.33 |
| IL-6 (pg/ml) | |||
| EPA | 1.6±2.3 | 1.7±2.1 | 0.80 |
| Control | 2.9±5.3 | 4.4±9.4 | 0.58 |
| MMP-3 (ng/ml) | |||
| EPA | 13.1±10.9 | 12.8±9.2 | 0.32 |
| Control | 10.8±6.2 | 11.4±8.1 | 0.45 |
| MMP-9 (ng/ml) | |||
| EPA | 23.6±12.8 | 28.1±19.8 | 0.51 |
| Control | 42.3±33.3 | 36.5±30.4 | 0.54 |
| PV | |||
| PTX3 (ng/ml) | |||
| EPA | 2.8±2.1 | 2.2±1.4 | 0.15 |
| Control | 2.8±2.4 | 2.5±1.4 | 0.59 |
| MCP-1 (pg/ml) | |||
| EPA | 116.5±20.9 | 114.1±20.1 | 0.21 |
| Control | 109.1±33.3 | 102.7±27.9 | 0.43 |
| hs-CRP (mg/L) | |||
| EPA | 0.1±0.3 | 0.1±0.1 | 0.40 |
| Control | 0.1±0.2 | 0.1±0.1 | 0.18 |
| TNF-α (pg/ml) | |||
| EPA | 3.6±1.5 | 2.9±1.9 | 0.35 |
| Control | 2.2±1.7 | 1.7±1.5 | 0.28 |
| IL-6 (pg/ml) | |||
| EPA | 1.4±1.9 | 1.5±1.8 | 0.85 |
| Control | 9.3±31.7 | 2.4±3.7 | 0.31 |
| MMP-3 (ng/ml) | |||
| EPA | 13.4±12.1 | 13.0±8.2 | 0.14 |
| Control | 11.2±7.0 | 10.9±7.3 | 0.72 |
| MMP-9 (ng/ml) | |||
| EPA | 24.3±13.3 | 26.5±18.2 | 0.29 |
| Control | 38.5±28.5 | 36.2±27.3 | 0.73 |
Data given as mean±SD. There were no significant differences in the inflammatory cytokine levels between the 2 groups at baseline or follow-up. EPA group, strong statin plus EPA; control group, strong statin only. CS, coronary sinus; EPA, eicosapentaenoic acid; hs-CRP, high-sensitivity C-reactive protein; IL-6, interleukin-6; MCP-1, monocyte chemoattractant protein-1; MMP-3, matrix metalloproteinase-3; PTX3, pentraxin 3; TNF-α, tumor necrosis factor α; MMP-9, matrix metalloproteinase-9; PV, peripheral vein.
Figure 4 shows the correlations between the change in lipid volume and the change in cytokines. The nominal change in lipid volume was significantly correlated with PTX3 (r=0.366, P=0.04) and MCP-1 (r=0.459, P=0.01) in the EPA group, but not in the control group.
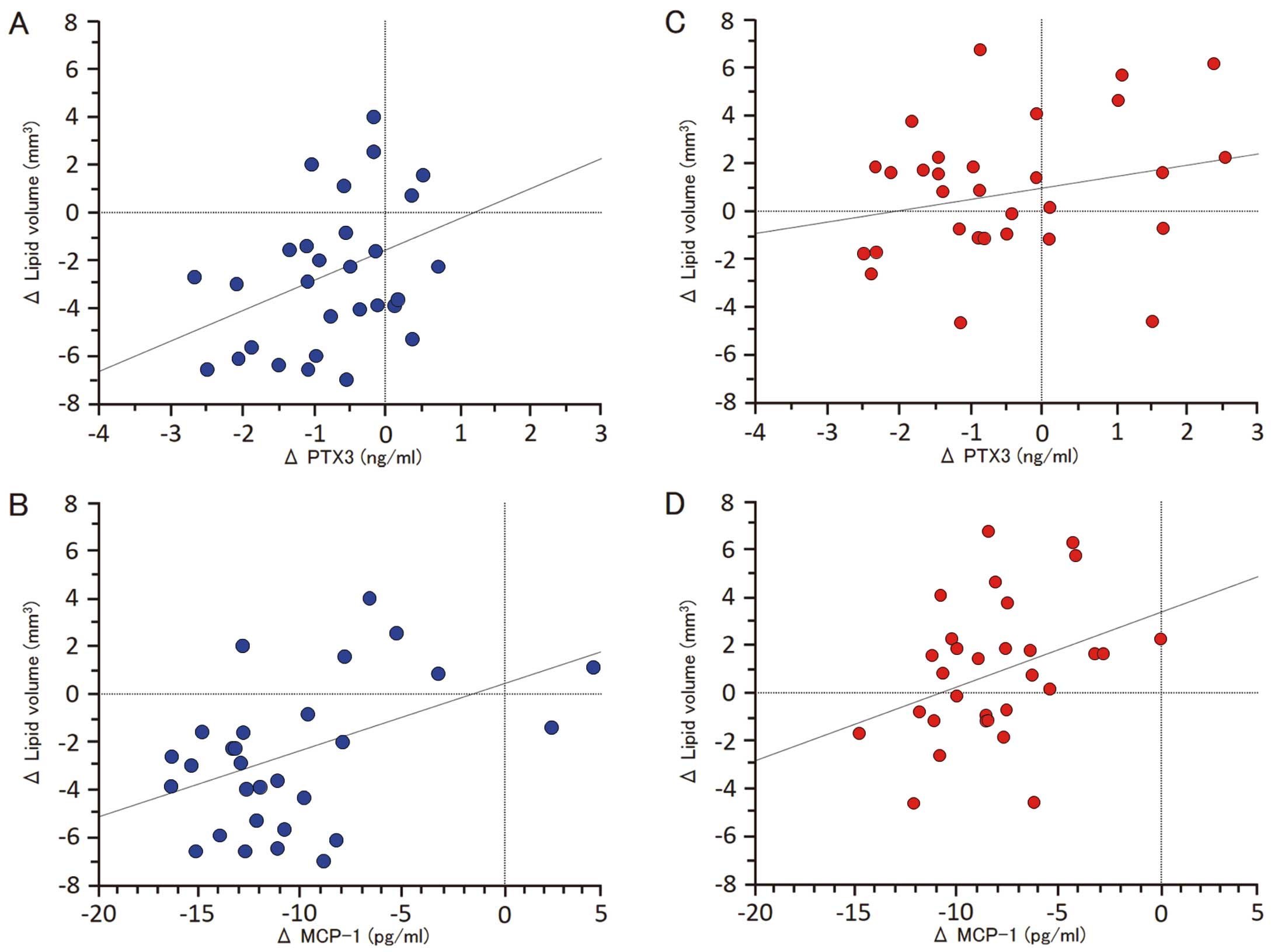
Correlations between the change in lipid volume and the change in cytokines. (A,B) Significantly positive correlation between the nominal change in lipid volume and PTX3 (r=0.366, P=0.04) or MCP-1 (r=0.459, P=0.01) in the EPA group. (C,D) No significant correlations between the nominal change in lipid volume and PTX3 (r=0.239, P=0.222) or MCP-1 (r=0.359, P=0.05) in the control group. MCP-1, monocyte chemoattractant protein-1; PTX3, pentraxin 3.
Several cohort studies have shown that low consumption of fish is associated with coronary artery disease mortality.1–7 On the other hand, the clinical benefit of lipid-lowering therapy with statins has been conclusively demonstrated by numerous large-scale randomized clinical trials for primary and secondary prevention.27–31 In the JELIS trial,8 18,645 patients (14,981 in primary prevention and 3,664 in secondary prevention) with hypercholesterolemia were randomized to receive statin alone or statin plus highly purified EPA. At the end of 5 years, those randomized to EPA plus statin showed a 19% lower occurrence of major cardiovascular events. However, no patients treated with strong statins were included in the JELIS trial. To our knowledge, this is the first study to evaluate the change in coronary plaque components and local inflammatory cytokines after adding purified EPA ester to the treatment of dyslipidemic patients who were already being treated with strong statins.
Plaque Vulnerability and EPAA previous pathologic study of vulnerable plaque after ACS demonstrated that a large lipid core could be used as a histologic marker of plaque vulnerability.32 Lee et al reported that a low plasma level of EPA was an independent predictor of all-cause mortality following AMI.33 Kashiyama et al reported a relationship between coronary plaque vulnerability and the serum EPA/AA ratio using coronary angioscopy, and concluded that a low serum EPA level and a low EPA/AA ratio were associated with high plaque vulnerability.34 Ueeda et al also demonstrated a significant correlation between serum EPA level and the extent of soft plaque evaluated by multidetector-row computed tomography in AMI patients.35 A recent study reported that the concomitant use of EPA and rosuvastatin stabilized coronary thin-cap fibroatheroma better than the statin alone on serial optical coherence tomography (OCT).36 In the present study, we demonstrated that combination treatment of EPA and a strong statin therapy stabilized plaque components assessed by IB-IVUS to a greater degree than did strong statin therapy alone. Our results may coincide with the OCT analyses.
Effect of Addition of EPA to Strong Statin on Plaque ComponentsPrevious studies have revealed the clinical importance of in vivo evaluation of vulnerable plaques by intravascular imaging, such as virtual histology IVUS, IB-IVUS and OCT.37–40 It has also been demonstrated by IB-IVUS that lipid-lowering therapy with statins stabilizes plaques by reducing the fatty component and increasing the fibrous component.40,41 Measurement of the lipidic components by IB-IVUS has proven useful in both diagnosis17,42–44 and assessment of the prognosis of coronary plaques as a risk for coronary events.45,46 In the present study, we demonstrated that the EPA+strong statin group had a larger decrease in the lipidic components and a greater increase in fibrous components assessed by IB-IVUS without lowering serum lipid levels. Thus, we propose that a greater decrease in lipidic components and a larger increase in fibrous components assessed by IB-IVUS indicate that combined EPA and strong statin therapy stabilizes vulnerable coronary plaques better than strong statin therapy alone.
In the control group, the lipid volume serially increased, although there was not a significant difference. In this study, to eliminate the influence of serial changes related to statin, the effects of EPA addition were investigated in patients who responded to statin therapy, showing stable effects. Briefly, the subjects were patients who had taken a strong statin for ≥6 months. In these subjects, the effects of the strong statin had reached a steady state, and there was no significant decrease in the lipid volume during the observation period. Other factors, such as lifestyle, may have contributed to numerical changes.
Cytokines and Vulnerable PlaquesIt has already been reported that arterial inflammation plays an important role in promoting plaque vulnerability, and that hs-CRP and PTX3 are useful markers of arterial inflammation.47,48 In the present study, EPA add-on therapy significantly lowered PTX3 and MCP-1 levels in the CS more than strong statin therapy alone did over the short term. PTX3 is produced directly by macrophages and vascular smooth muscle cells in the vicinity of atherosclerotic lesions, whereas hs-CRP is produced in the liver in response to inflammatory cytokines. Therefore, PTX3 levels in the CS reflect the local inflammatory response of the coronary artery more directly than other systemic markers of inflammation, such as hs-CRP. MCP-1 is also produced predominantly by macrophages and endothelial cells and is a potent chemotactic factor for monocytes.49 Our results suggest that EPA added to a strong statin stabilizes plaque components better than strong statin alone therapy through suppression of coronary inflammation. The presence of EPA promotes the secretion of anti-inflammatory prostaglandins and less potent leukotrienes, resulting in a shift to a milieu of less inflammatory mediators. EPA also decreases the agonist-induced activation of nuclear factor (NF)-κB and peroxisome proliferator activated receptor (PPAR)-γ. Activation of the transcription factor NF-κB is associated with atherosclerotic progression by upregulation of cytokine gene expression.50 Activated NF-κB and its target genes are found in the atherosclerotic vessel wall. Another transcription factor, PPAR-γ, also plays a role in acute inflammation control.51 Therefore, it is possible that this is one pathway by which EPA exerts its antiinflammatory effects.
It was also reported that high EPA content in plaques is associated with reduced numbers of foam cells and T cells, less inflammation and increased stability.52 These findings show that EPA could have beneficial effects on the stability of coronary plaques, suggesting an adjuvant effect of strong statins in terms of their protective effects against coronary inflammation.
Changes in Local Inflammatory Cytokine LevelsIn the present study, inflammatory cytokine levels in the CS were lower after than before additional EPA treatment; however, no significant changes were observed in the femoral vein. Blood sampling from the femoral vein is likely to be affected when the patient has a collagen, malignant or other infectious disease leading to a systemic inflammatory reaction. Therefore, we consider that blood sampling from the CS reflected micro-changes in the cardiac inflammatory reaction. Local anti-inflammatory effects of additional EPA assessed by blood sampling from the CS were associated with the anti-arteriosclerotic effects assessed by IB imaging.
Clinical ImplicationsThe present study findings suggest that the addition of EPA further stabilizes coronary plaques in patients with stable angina who are treated with strong statins. In addition, EPA has been reported to transform dysfunctional high-density lipoprotein to the functional form.53 Additional EPA therapy may reduce the residual risk of strong statin therapy in patients with stable angina. The results suggest the usefulness of EPA for secondary prevention in these patients.
Study LimitationsFirst, it was performed at a single center and the study population was relatively small. Second, acoustic shadowing by a guide wire or calcification may affect tissue characterization. Therefore, lesions that were masked by acoustic shadowing were not included in this study. Third, thrombus has several IB values according to its formation. A fresh thrombus and the lipid pool have similar IB values and organized thrombus and fibrosis also have similar IB values. It is possible that fresh thrombus at baseline was misclassified as lipid pool (blue) and organized thrombus at follow-up as fibrosis (green). This is a limitation of analyses using IB-IVUS. Fourth, all patients underwent IB-IVUS analyses performed in the vessel undergoing the intervention. Ideally, the analytical lesion should be selected in non-culprit vessels. However, analytical plaques in this study had to be more than 5 mm away from the intervention site; therefore, we think that these plaques were less affected by the PCI procedures. Fifth, the plaque capacity was small at most coronary sites investigated in the present study; it remains to be clarified whether or not EPA addition is effective for advanced atheroma. However, the results suggest that EPA addition inhibits the deterioration of initial atheroma. Furthermore, a study using serial OCT observation reported the efficacy of EPA addition for the stabilization of atheroma.36 This is consistent with our findings. In the future, the effects on advanced atheroma and on plaque stabilization should be further investigated.
In patients with dyslipidemia treated with strong statins, the addition of EPA was associated with a reduction in the lipid content of plaques in the coronary arteries as observed by IB-IVUS, as well as with a decrease in inflammatory cytokines.
We thank the nursing and technical staff of the Cardiac Catheterization Laboratory at Tokushima University Hospital for their expert help in conducting this study.
M.S. received lecture fees from Mochida Pharmaceutical Co, Ltd, AstraZeneca K.K., and Shionogi & Co, Ltd. Otherwise, no disclosures in regard to this manuscript.