Abstract
Background:
Hyponatremia has been shown to be a prognostic factor in heart failure (HF) with preserved ejection fraction (HFpEF). Serum sodium (sNa) cut-off, however, is not defined in HFpEF. Therefore, we investigated the relationship between sNa and HF-related events (cardiovascular death and hospitalization for HF decompensation) in HFpEF patients.
Methods and Results:
We assessed cardiac function using echocardiography and measured sNa in HFpEF patients with New York Heart Association class II (n=321) or III (n=84) in a compensated condition after implementing medical therapy for HF. During a mean follow-up of 27 months, 73 patients developed HF-related events. On multivariate Cox hazard analysis including established predictors in HF, sNa level as a continuous variable was identified as an independent predictor for HF-related events in HFpEF (per 1.0 mmol/L: HR, 0.93; 95% CI: 0.87–0.98; P<0.01). Kaplan-Meier analysis demonstrated significantly higher probability of HF-related events in the lower sNa group (sNa <140 mmol/L) than in the higher sNa group (sNa ≥140 mmol/L; P<0.001, log-rank test). Further, the low-normal sNa group (135 mmol/L<sNa<140 mmol/L) was significantly associated with HF-related events compared with the higher sNa group (P<0.001, log-rank test).
Conclusions:
sNa as a continuous variable was independently correlated with future HF-related events in HFpEF. Low-normal sNa could provide important prognostic information for practical risk stratification in HFpEF. (Circ J 2016; 80: 411–417)
Recent studies have shown that approximately half of patients with heart failure (HF) have a normal left ventricular ejection fraction (LVEF). It has been shown that approximately 15% of patients with HF with preserved LVEF (HFpEF) are re-admitted within 1 year because of HF exacerbation, and the mortality for HFpEF may be as high as 22–29% at 1 year, 23% at 3 years and 43–65% at 5 years.1–6
In the Irbesartan in Patients with Heart Failure and Preserved Ejection Fraction (I-PRESERVE) study, 60% of the deaths in HFpEF patients were due to cardiovascular causes, with sudden death and HF being the most common. That study identified 5 prognostic factors in outpatients with HFpEF (PF5): age; diabetes; New York Heart Association (NYHA) classification; history of hospitalization for HF; and LVEF.7,8
In other study, it was reported that predictors of 5-year mortality in HFpEF patients were older age, stroke, chronic obstructive pulmonary disease, diabetes, cancer, hyponatremia (<135 mmol/L) on admission, and low glomerular filtration rate on admission.9
Despite the substantial improvement in evidence-based treatment of HF, effective treatment for HFpEF is still uncertain. More information is needed on the pathophysiological conditions that correlate with clinical outcome in patients with HFpEF.
Hyponatremia, which is clinically defined as serum sodium (sNa) <135 mmol/L, is the most common electrolyte disorder in patients with HF. The most common reason for hyponatremia is dilution in patients with HF or liver cirrhosis.10
Several clinical studies have shown that hyponatremia is one of the powerful prognostic factors of mortality and re-hospitalization in HF.11−17
In patients with HFpEF, it has been estimated that approximately only 10–25% of cases of hyponatremia are reported.14,18
Several recent clinical studies demonstrated that hyponatremia is an independent predictor of mortality in HFpEF patients and that sNa at admission for acute HF predicted the likelihood of cardiovascular death, with a relative risk for cardiovascular death of 1.08 for each 1-mmol/L decrease.14,19–23
In HFpEF patients after treatment according to the guideline, we investigated the prognostic significance of sNa as a continuous variable and low-normal sNa (135 mmol/L<sNa<140 mmol/L) for HF-related clinical events.
Methods
Subjects and Protocol
We diagnosed HF using Framingham criteria24
and screened HF patients who were referred for treatment and diagnosis of HF at Kumamoto University Hospital between June 2006 and March 2012. All patients with HF were free of non-cardiac causes of HF-like symptoms, such as chronic obstructive pulmonary disease and end-stage renal disease including hemodialysis. Patients with reduced LVEF, acute coronary syndrome, severe valvular heart disease, hypertrophic obstructive cardiomyopathy, significant inflammatory disease, liver cirrhosis, or neoplasms were excluded. The diagnosis of HFpEF was based on guidelines from the European Society of Cardiology.25,26
Patients who were initially hospitalized for acute HF were registered after they were treated according to the guideline.27,28
Patients with HFpEF were followed prospectively at the outpatient clinic every month until July 2012 or until an endpoint occurred. Clinical events were also evaluated by annual telephone interview with each patient. The study complied with the Declaration of Helsinki regarding investigation in humans, was approved by the institutional review committee, and was conducted in accordance with the guidelines of the institutional ethics committee. Written informed consent was obtained from all patients. This study was registered at the University Hospital Medical Information Network Clinical Trials Registry (UMIN000009100).
Diagnosis of HFpEF
We applied the following diagnostic criteria of the European Working Group to HFpEF. A diagnosis of HFpEF requires three obligatory conditions: (1) signs or symptoms of congestive HF and Framingham criteria for HF; (2) normal or mildly abnormal systolic LV function (LVEF >50% and LV end-diastolic volume index <97 ml/m2); and (3) evidence of LV diastolic dysfunction (LVDD).25,26
In this study, signs or symptoms of congestive HF included rales, pulmonary edema, leg edema, hepatomegaly, dyspnea on exertion, fatigue, and impaired exercise capacity as assessed on exercise test.25,26
We defined LVDD as (1) ratio of early transmitral flow velocity to tissue Doppler early diastolic mitral annular (medial) velocity (E/e’) ≥15 or (2) 8<E/e’<15 and B-type natriuretic peptide (BNP) >200 pg/ml.25,26,29–31
Primary Endpoint
The primary endpoint was an HF-related event as a composite of cardiovascular death and hospitalization for HF decompensation. These events were followed at the monthly outpatient visit and were confirmed by review of medical records and direct contact with the physicians, patients or their families.
Endpoint Definitions
Cardiovascular death was defined as death due to myocardial infarction, stroke, or HF (within 28 days of onset), or documented sudden death in the absence of non-cardiovascular causes. A diagnosis of hospitalization for HF decompensation was made if the patient was admitted with symptoms typical of HF and had objective signs of worsening HF requiring i.v. drug treatment. For subjects experiencing two or more events, only the first event was considered in the analysis.
Biochemistry
Both compensated HF patients and decompensated acute HF patients were registered in the present study. At hospital discharge point when patients were in a compensated condition after implementation of medical therapy for HF, venous samples were obtained in a stable, fasting state and in the early morning to measure sNa, BNP and other biochemical markers. The main purpose of the present study was to investigate the relationship between the occurrence of future HF-related events and sNa in the HFpEF patients who were optimally treated according to the guideline and were already in a stable condition. Therefore, we selected sNa at discharge. BNP was analyzed using a commercially available assay (Abbott Japan, Matsudo, Japan) in the hospital clinical laboratory. Estimated glomerular filtration ratio (eGFR) was calculated using the Japanese Society of Nephrology formula.32
Echocardiography
In each patient, 2-D transthoracic echocardiography was performed by blinded sonographers according to the current guidelines,33
when patients were in a compensated condition after implementation of medical therapy for HF.
We used commercially available ultrasound systems (Vivid 7; GE-Vingmed Ultrasound, Horton, Norway; and Aplio XG; Toshiba, Tokyo, Japan). LVEF was measured using the biplane modified Simpson method. E/e’ ratio was calculated as described previously.34
Statistical Analysis
Skewed variables are expressed as median (IQR). Differences between two groups were tested using the chi-squared test for categorical variables. Categorical data are presented as frequencies and percentages. Differences in continuous variables were analyzed using the unpaired t-test or Mann-Whitney U-test, as appropriate.
Significant factors of HF-related events were identified using a univariate Cox proportional hazards model. We also constructed four forced inclusion multivariate models to determine the significance of sNa as a continuous valuable for the prediction of future HF-related events. We constructed the following combination of significant factors in the univariate Cox analysis to avoid the over-fit problem with limited outcomes (HF-related events: 73) in the present study: model 1, age, gender, diabetes mellitus, hypertension, atrial fibrillation, eGFR, LVEF, and sNa as clinically relevant variables;13
model 2, PF5 in I-PRESERVE study (age, NYHA classification, history of hospitalization for HF, diabetes mellitus, LVEF), BNP, and sNa as clinically important variables in patients with HFpEF;7,8,35
and model 3, sNa, serum potassium, eGFR, use of loop or thiazide diuretics, spironolactone, angiotensin-converting enzyme inhibitor (ACEI) or angiotensin II receptor blocker (ARB), or β-blocker as therapeutic variables for HFpEF patients
We defined lower sNa as <140 mmol/L based on the results of Meta-Analysis Global Group in Chronic heart failure (MAGGIC) study.13
In the present study, the association between sNa and the HF-related events rate was investigated using Kaplan-Meier estimates (sNa cut-off, 140 mmol/L). Then, we further divided the HFpEF patients as follows: hyponatremia group, sNa ≤135 mmol/L; low-normal sNa group, 135 mmol/L<sNa<140 mmol/L; lower sNa group, sNa <140 mmol/L; and higher sNa group, sNa ≥140 mmol/L, and analyzed them using Kaplan-Meier estimates.
To account for confounding variables, a propensity score adjustment was calculated for each patient using a logistic regression model in which the dependent variable was the lower sNa group (<140 mmol/L). Independent variables included in the propensity score model were age, sex, NYHA classification, hospitalization for HF, hypertension, diabetes mellitus, atrial fibrillation, ln BNP, eGFR, LVEF, and use of loop or thiazide diuretics, spironolactone, ACEI or ARB, or β-blocker. Multivariate Cox proportional hazard model with sNa as a continuous valuable and the propensity score were also analyzed.
The HFpEF patients were categorized as having persistent hyponatremia for sNa persistently <135 mmol/L at admission and throughout the hospital course.36
Using univariate Cox proportional hazards model, we evaluated the association between persistent hyponatremia and HF-related events. Further, we also constructed a forced inclusion multivariate model (model 1, age, gender, diabetes mellitus, hypertension, atrial fibrillation, eGFR, LVEF, and persistent hyponatremia) to determine the significance of persistent hyponatremia for the prediction of future HF-related events.
Statistical Analysis
P<0.05 denoted statistical significance. Statistical analysis was performed using SPSS version 20 (SPSS, Tokyo, Japan) and SAS version 9.1.3 (SAS Institute).
Results
Subjects
A total of 407 patients with HFpEF were enrolled in this study. The distribution of sNa in this study is shown in
Figure 1. Mean sNa was 139.8±3.0 mmol/L (median, 140 mmol/L; range, 121–148 mmol/L) (Table 1).
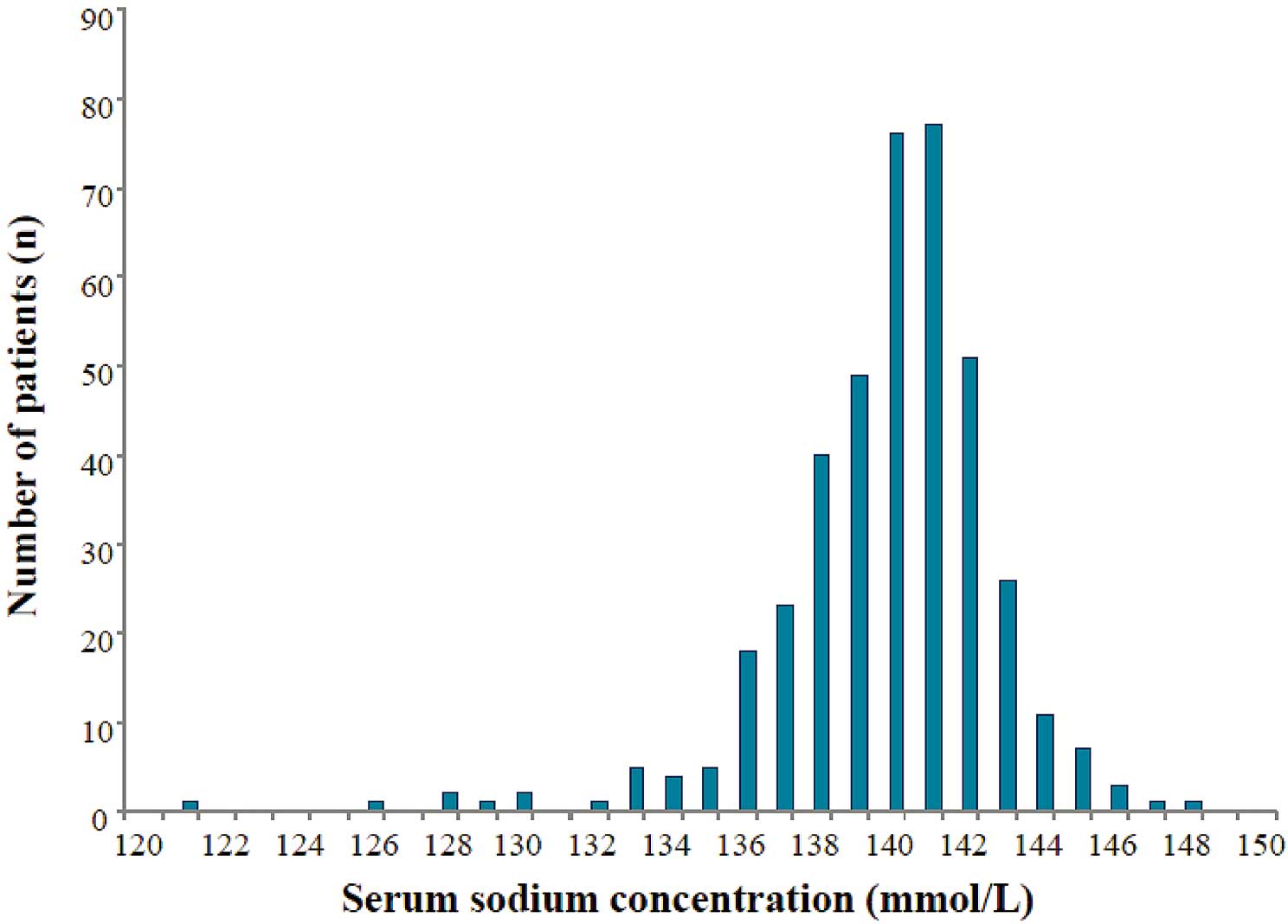
Table 1.
Baseline Patient Characteristics
| |
All patients (n=405) |
| Age (years) |
71.7±9.8 |
| Male |
205 (50.6) |
| BMI (kg/m2) |
24.0±3.8 |
| Diabetes mellitus |
128 (31.6) |
| Hypertension |
397 (98.0) |
| Dyslipidemia |
257 (63.5) |
| Ischemic etiology |
196 (48.4) |
| Atrial fibrillation |
127 (31.4) |
| History of hospitalization for HF |
100 (24.7) |
| NYHA classification (II, III) |
321, 84 |
| Systolic BP (mmHg) |
130.6±22.6 |
| Diastolic BP (mmHg) |
72.7±14.0 |
| Heart rate (beats/min) |
71.7±17.0 |
| Serum sodium (mmol/L) |
139.8±3.0 |
| Serum potassium (mmol/L) |
4.4±0.5 |
| eGFR (ml/min/1.73 m2) |
60.8±19.2 |
| BNP (pg/ml) |
111 [38–286] |
| LVDd (mm) |
44.4±5.6 |
| LVDs (mm) |
28.0±5.5 |
| LVEF (%) |
62.8±6.3 |
| Loop or thiazide diuretics |
120 (29.6) |
| Loop diuretics |
91 (22.5) |
| Thiazide diuretics |
40 (9.9) |
| Aldosterone antagonists or blockers |
55 (13.6) |
| β-blockers |
213 (52.6) |
| ACEI or ARB |
260 (64.2) |
Data given as mean±SD, median (IQR), or n (%). Loop or thiazide diuretics included dual treatment with loop and thiazide diuretics. ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BMI, body mass index; BNP, B-type natriuretic peptide; BP, blood pressure; eGFR, estimated glomerular filtration rate; HF, heart failure; LVDd, left ventricular end-diastolic dimension; LVDs, left ventricular end-systolic dimension; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; sNa, serum sodium.
The lower sNa group (<140 mmol/L, n=152) consisted mainly of men, had a significantly higher prevalence of atrial fibrillation, history of hospitalization for HF, higher NYHA classification, and was also more frequently treated with loop diuretics, aldosterone antagonists, and β-blockers compared with the higher sNa group. The lower sNa group had significantly higher serum potassium and BNP, and lower eGFR compared with the higher sNa group (Table S1).
Follow-up
Two patients were lost to follow-up. Data for 405 patients with HFpEF were available for analyzing HF-related events. The follow-up period was 1−70 months (mean, 26.5 months). At follow-up, 73 endpoints occurred among all patients with HFpEF. Details of the HF-related events are shown in
Table 2. The frequency of HF-related events was significantly higher in the lower sNa group compared with the higher sNa group (n=41, 27.0% vs. n=32, 12.6%; P<0.01).
Table 2.
Outcome in HFpEF
| |
All patients
(n=405) |
Lower sNa group
(sNa <140 mmol/L)
(n=152) |
Higher sNa group
(sNa ≥140 mmol/L) (
n=253) |
P-value |
| HF-related events |
73 (18.0) |
41 (27.0) |
32 (12.6) |
<0.01 |
| Cardiovascular death |
12 (3.0) |
9 (5.9) |
3 (1.2) |
0.01 |
| Hospitalization for HF decompensation |
61 (15.1) |
32 (21.1) |
29 (11.5) |
0.01 |
Data given as n (%). HFpEF, HF with preserved left ventricular ejection fraction. Other abbreviations as in Table 1.
The results of univariate and multivariate Cox proportional hazards analyses for the HF-related events are summarized in
Table 3. Among the significant variables on univariate analysis, sNa as a continuous variable was still an independent factor for HF-related events (per 1.0 mmol/L: HR, 0.87; 95% CI: 0.82−0.91; P=0.01). Using the three forced inclusion models with various clinical parameters in the multivariate Cox hazard analysis, sNa still significantly predicted HF-related events (Table 3).
Table 3.
Predictors of HF-Related Events
| Variable |
Univariate regression |
Multiple regression
using forced inclusion
model 1 |
Multiple regression
using forced inclusion
model 2 |
Multiple regression
using forced inclusion
model 3 |
| HR |
95% CI |
P-value |
HR |
95% CI |
P-value |
HR |
95% CI |
P-value |
HR |
95% CI |
P-value |
| Age (per year) |
1.01 |
0.99–1.04 |
0.33 |
|
|
|
1.01 |
0.98–1.03 |
0.57 |
|
|
|
| Sex (male) |
0.95 |
0.60–1.50 |
0.81 |
|
|
|
|
|
|
|
|
|
NYHA classification
III vs. II |
5.16 |
3.26–8.19 |
<0.01 |
1.93 |
1.10–3.38 |
0.02 |
1.75 |
1.01–3.04 |
0.04 |
|
|
|
History of hospitalization
for HF (yes) |
3.85 |
2.43–6.11 |
<0.01 |
1.75 |
1.04–2.95 |
0.04 |
2.09 |
1.28–3.41 |
<0.01 |
|
|
|
| Hypertension (yes) |
0.46 |
0.14–1.46 |
0.19 |
|
|
|
|
|
|
|
|
|
| Diabetes mellitus (yes) |
0.91 |
0.55–1.50 |
0.70 |
|
|
|
1.03 |
0.62–1.69 |
0.92 |
|
|
|
| Atrial fibrillation (yes) |
2.46 |
1.55–3.89 |
<0.01 |
1.47 |
0.91–2.37 |
0.11 |
|
|
|
|
|
|
| sNa (per mmol/L) |
0.87 |
0.82–0.91 |
<0.01 |
0.92 |
0.87–0.98 |
0.01 |
0.93 |
0.87–0.98 |
<0.01 |
0.89 |
0.84–0.96 |
<0.01 |
Serum potassium (per
mmol/L) |
1.95 |
1.23–3.10 |
0.01 |
1.28 |
0.78–2.08 |
0.33 |
|
|
|
1.10 |
0.69–1.75 |
0.70 |
| eGFR (per ml/min/1.73 m2) |
0.98 |
0.97–0.99 |
<0.01 |
0.99 |
0.98–1.01 |
0.28 |
|
|
|
0.99 |
0.97–1.00 |
0.03 |
| Ln (BNP) (per 0.05) |
1.04 |
1.03–1.05 |
<0.01 |
1.03 |
1.01–1.04 |
<0.01 |
1.03 |
1.02–1.04 |
<0.01 |
|
|
|
| LVEF (per %) |
0.94 |
0.91–0.98 |
0.03 |
0.99 |
0.95–1.03 |
0.64 |
0.98 |
0.95–1.03 |
0.44 |
|
|
|
Loop or thiazide diuretics
(yes) |
4.16 |
2.61–6.64 |
<0.01 |
|
|
|
|
|
|
2.71 |
1.56–4.71 |
<0.01 |
Aldosterone antagonists or
blockers (yes) |
3.87 |
2.34–6.40 |
<0.01 |
|
|
|
|
|
|
1.62 |
0.88–2.96 |
0.12 |
| ACEI or ARB (yes) |
1.76 |
1.04–2.97 |
0.03 |
|
|
|
|
|
|
0.96 |
0.55–1.68 |
0.89 |
| β-blocker (yes) |
1.83 |
1.13–2.94 |
0.01 |
|
|
|
|
|
|
1.64 |
1.01–2.67 |
0.04 |
Model 1 included age, gender, diabetes mellitus, hypertension, atrial fibrillation, eGFR, LVEF, and sNa as clinical relevantly variables. Model 2 included age, NYHA class, history of hospitalization for HF, diabetes mellitus, LVEF, BNP, and sNa as clinically important variables in patients with HF with normal ejection fraction. Model 3 included sNa, serum potassium, eGFR, and use of diuretic, spironolactone, ACEI or ARB, or β-blocker as therapeutic variables in patients with HF with normal ejection fraction. Abbreviations as in Table 1.
We carried out Cox proportional hazards analysis using the lower sNa group (sNa <140 mmol/L). The lower sNa group was significantly associated with HF-related events (HR, 1.77; 95% CI: 1.09–2.88; P=0.02) in the model with propensity score adjustment.
Next, we carried out Cox proportional hazards analysis using the hyponatremia group (sNa ≤135 mmol/L). The hyponatremia group was also significantly associated with HF-related events (HR, 2.41; 95% CI: 1.05–5.51; P=0.04) in the model with propensity score adjustment.
There were 20 patients with hyponatremia (sNa ≤135 mmol/L) at admission, and 5 with persistent hyponatremia. On univariate Cox proportional hazards analysis, persistent hyponatremia was a significant variable for HF-related events (HR, 6.38; 95% CI: 1.99−20.44; P=0.002).
On multivariate Cox hazard analysis (model 1), persistent hyponatremia was still significantly correlated with HF-related events (HR, 6.64; 95% CI: 2.01−21.94; P<0.01).
Kaplan-Meier Analysis
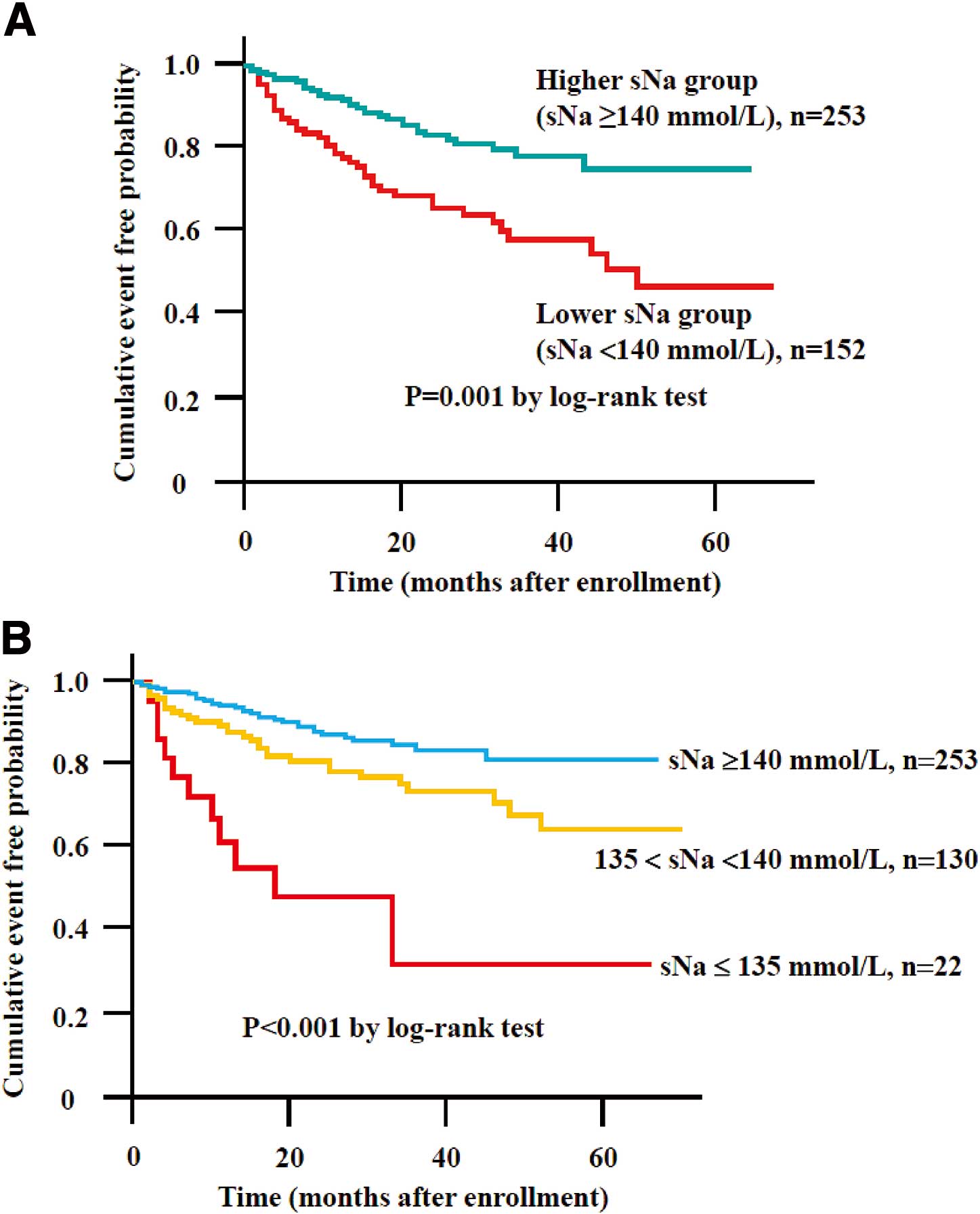
On Kaplan-Meier analysis a significantly higher probability of HF-related events was noted in the lower sNa group (sNa <140 mmol/L) compared with the higher sNa group (sNa ≥140 mmol/L; log-rank test, P<0.001;
Figure 2A). On Kaplan-Meier analysis there was a gradual increase in HF-related events with decreasing sNa (Figure 2B), and the low-normal sNa group (135 mmol/L<sNa<140 mmol/L) had a higher probability of HF-related events than the higher sNa group (sNa ≥140 mmol/L).
Discussion
Among the prospective cohort of patients with HFpEF, we focused on the association between sNa as a continuous variable and HF-related events. On univariate and multivariate Cox hazard analysis, sNa as a continuous variable was a significant and independent predictor of future HF-related events in HFpEF patients.
We further focused on the association between lower sNa (sNa <140 mmol/L), which consisted of the hyponatremia group and the low-normal sNa group, and HF-related events. On Kaplan-Meier analysis the lower sNa group was significantly associated with a higher risk of HF-related events. On Cox proportional hazards analysis in the model with propensity score adjustment, the lower sNa group was significantly associated with HF-related events. Lower sNa (sNa <140 mmol/L) was an important predictor of future HF related-events. Moreover, low-normal sNa (135 mmol/L<sNa<140 mmol/L) was associated with HF-related events on Kaplan-Meier analysis. The present study therefore first confirms that low-normal sNa is an important pathophysiological condition predicting future HF-related events in patients with HFpEF. sNa is a cheap and readily available daily blood test that could be used to evaluate future risk stratification in HFpEF.
The I-PRESERVE study reported that the predictors of outcome in outpatients with HFpEF were PF5 (age, diabetes, NYHA class, history of hospitalization for HF, LVEF) and NT-proBNP.7
In the present study, on multivariate Cox hazard analysis using forced inclusion models with these established predictors, sNa still significantly predicted HF-related events, indicating that sNa is an independent and important predictor of HF-related events in HFpEF patients.
Hyponatremia is clinically defined as sNa <135 mmol/L.13,37
In the present study, we focused on lower sNa (sNa <140 mmol/L) based on the results of previous studies.13,20
Kaplan-Meier analysis showed that the lower sNa group (sNa <140 mmol/L) had a significantly higher probability of HF-related events.
On multivariate Cox hazard analysis the lower sNa group (sNa <140 mmol/L) was significantly associated with HF-related events in the model with propensity score adjustment, indicating that lower sNa even within the normal clinical range is significantly correlated with future HF-related events in HFpEF patients. Therefore, the prognostic risk of HFpEF patients with lower sNa <140 mmol/L should be borne in mind in clinical practice.
Among the prospective HFpEF patient cohort, we evaluated the association between persistent hyponatremia and HF-related events. In the univariate and multivariate Cox hazards analysis, persistent hyponatremia was a significant predictor of future HF-related events in HFpEF patients. The present HFpEF patient consisted of patients hospitalized for examination and diagnosis of HF or treatment of acute HF, and the number of acute HF patients was not large (n=53, 13.1%) compared with other studies. This might be the reason why the number of patients with persistent hyponatremia in the present study was lower than in other studies.
Mean sNa in the present study (139.8±3.0 mmol/L) was higher than that in a recent meta-analysis that noted an association between hyponatremia and prognosis of patients hospitalized for HF (mean sNa, 137.5±4.4 mmol/L).22
This could be because that study included more decompensated acute HF patients than the present study, and they measured sNa of HFpEF patients at admission. The present mean sNa (139.8±3.0 mmol/L), however, was similar to that in a recent study that investigated the association between hyponatremia and the prognosis of outpatients for HFpEF (mean sNa, 139±3.5 mmol/L).23,38
The distribution of sNa in the present study is concordant with that in ambulatory HF outpatients, which included already treated HF patients without decompensated acute HF.23,38
Despite the substantial improvement in evidence-based treatment for HF, effective treatment for HFpEF is still uncertain. To investigate an effective treatment for HFpEF, we may need to stratify HFpEF patients according to pathophysiologic profile. The pathophysiologic profile of HF patients with hyponatremia is different to that of HF patients without hyponatremia, because it involves multifunctional conditions: activation of the renin-angiotensin-aldosterone system, upregulation of the sympathetic nervous system and excessive vasopressin release.37
The pathophysiologic mechanisms of low sNa, and sNa cut-off in patients with HFpEF should be carefully investigated in future studies to stratify HFpEF patients according to pathophysiologic profile.
Despite several large-scale clinical trials indicating the prognostic importance of hyponatremia, we do not know whether treatment targeting hyponatremia improves the clinical outcome in HFpEF patients. In the Study of Ascending Levels of Tolvaptan in Hyponatremia trial, tolvaptan, a V2
receptor antagonist, was effective in HF patients with hypervolemic and euvolemic hyponatremia.39–42
In The Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study With Tolvaptan trial (EVEREST), tolvaptan reduced body weight, electrolyte abnormality, and congestive symptoms but not all-cause mortality, cardiovascular mortality, or HF hospitalizations in patients with HF with reduced EF.17,43–46
The EVEREST study did not target hyponatremia for treatment, therefore only 8% of the patients in that study had hyponatremia. We need to investigate the effect of intervention for hyponatremia and low-normal sNa on the HF-related events in HFpEF patients.
The present study has some limitations. First, it was a single-center design with a small patient population. Therefore, a large multiracial and multicenter study is required. Second, there were fewer patients with sNa <135 mmol/L than other studies. This was thought to be because the patient samples were collected after implementation of medical therapy. Third, only approximately one-third of patients with HFpEF were given loop or thiazide diuretics, which is much lower than one would expect. We could not extensively evaluate the association between loop or thiazide diuretic dose and low-normal sNa.
Conclusions
sNa level as a continuous variable was independently correlated with the occurrence of future HF-related events in patients with HFpEF. Low-normal sNa could successfully predict future HF-related events. sNa could provide independent and important prognostic information in HFpEF patients.
Source of Funding
This study was supported in part by a Grant-in-aid for Scientific Research (No. C25461086 for S.S., and No.24790770 for E.Y.) from the Ministry of Education, Culture, Sports, Science and Technology in Japan and the Salt Science Research Foundation grant in Japan (No.1237 for E.Y.).
Disclosures
The authors declare no conflicts of interest.
Supplementary Files
Supplementary File 1
Table S1.
Baseline patient characteristics vs. sNa (n=405)
Please find supplementary file(s);
http://dx.doi.org/10.1253/circj.CJ-15-0878
References
- 1.
Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 2006; 355: 251–259.
- 2.
Bhatia RS, Tu JV, Lee DS, Austin PC, Fang J, Haouzi A, et al. Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med 2006; 355: 260–269.
- 3.
Steinberg BA, Zhao X, Heidenreich PA, Peterson ED, Bhatt DL, Cannon CP, et al. Trends in patients hospitalized with heart failure and preserved left ventricular ejection fraction: Prevalence, therapies, and outcomes. Circulation 2012; 126: 65–75.
- 4.
Zhou J, Shi H, Zhang J, Lu Y, Fu M, Ge J, et al. Rationale and design of the beta-blocker in heart failure with normal left ventricular ejection fraction (beta-PRESERVE) study. Eur J Heart Fail 2010; 12: 181–185.
- 5.
Tarantini L, Oliva F, Cantoni S, Cioffi G, Agnoletto V, Alunni G, et al. Prevalence and prognostic role of anaemia in patients with acute heart failure and preserved or depressed ventricular function. Intern Emerg Med 2013; 8: 147–155.
- 6.
Ushigome R, Sakata Y, Nochioka K, Miyata S, Miura M, Tadaki S, et al. Temporal trends in clinical characteristics, management and prognosis of patients with symptomatic heart failure in Japan: Report From the CHART Studies. Circ J 2015; 79: 2396–2407.
- 7.
Zile MR, Gaasch WH, Anand IS, Haass M, Little WC, Miller AB, et al. Mode of death in patients with heart failure and a preserved ejection fraction: Results from the Irbesartan in Heart Failure With Preserved Ejection Fraction Study (I-PRESERVE) Trial. Circulation 2010; 121: 1393–1405.
- 8.
Udelson JE. Heart failure with preserved ejection fraction. Circulation 2011; 124: e540–e543, doi:10.1161/CIRCULATIONAHA.111.071696.
- 9.
Tribouilloy C, Rusinaru D, Mahjoub H, Soulière V, Lévy F, Peltier M, et al. Prognosis of heart failure with preserved ejection fraction: A 5 year prospective population-based study. Eur Heart J 2008; 29: 339–347.
- 10.
Sturdik I, Adamcova M, Kollerova J, Koller T, Zelinkova Z, Payer J. Hyponatremia is an independent predictor of in-hospital mortality. Eur J Intern Med 2014; 25: 379–382.
- 11.
Barsheshet A, Shotan A, Cohen E, Garty M, Goldenberg I, Sandach A, et al. Predictors of long-term (4-year) mortality in elderly and young patients with acute heart failure. Eur Heart J 2010; 12: 833–840.
- 12.
Choi DJ, Han S, Jeon ES, Cho MC, Kim JJ, Yoo BS, et al. Characteristics, outcomes and predictors of long-term mortality for patients hospitalized for acute heart failure: A report from the Korean heart failure registry. Korean Circ J 2011; 41: 363–371.
- 13.
Rusinaru D, Tribouilloy C, Berry C, Richards AM, Whalley GA, Earle N, et al. Relationship of serum sodium concentration to mortality in a wide spectrum of heart failure patients with preserved ejection fraction: An individual patient data meta-analysis: Meta-Analysis Global Group in Chronic heart failure (MAGGIC). Eur J Heart Fail 2012; 14: 1139–1146.
- 14.
Bettari L, Fiuzat M, Shaw LK, Wojdyla DM, Metra M, Felker GM, et al. Hyponatremia and long-term outcomes in chronic heart failure: An observational study from the Duke Databank for Cardiovascular Diseases. J Card Fail 2012; 18: 74–81.
- 15.
Klein L, O’Connor CM, Leimberger JD, Gattis-Stough W, Piña IL, Felker GM, et al. Lower serum sodium is associated with increased short-term mortality in hospitalized patients with worsening heart failure: Results from the Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of Chronic Heart Failure (OPTIME-CHF) study. Circulation 2005; 111: 2454–2460.
- 16.
Lee DS, Austin PC, Rouleau JL, Liu PP, Naimark D, Tu JV. Predicting mortality among patients hospitalized for heart failure: Derivation and validation of a clinical model. JAMA 2003; 290: 2581–2587.
- 17.
Hamaguchi S, Kinugawa S, Tsuchihashi-Makaya M, Matsushima S, Sakakibara M, Ishimori N, et al. Hyponatremia is an independent predictor of adverse clinical outcomes in hospitalized patients due to worsening heart failure. J Cardiol 2014; 63: 182–188.
- 18.
Liu M, Fang F, Yu CM. Noncardiac comorbidities in heart failure with preserved ejection fraction: Commonly ignored fact. Circ J 2015; 79: 954–959.
- 19.
Mentz RJ, Broderick S, Shaw LK, Fiuzat M, O’Connor CM. Heart failure with preserved ejection fraction: Comparison of patients with and without angina pectoris (from the Duke Databank for Cardiovascular Disease). J Am Coll Cardiol 2014; 63: 251–258.
- 20.
Deubner N, Berliner D, Frey A, Güder G, Brenner S, Fenske W, et al. Dysnatraemia in heart failure. Eur J Heart Fail 2012; 14: 1147–1154.
- 21.
Kinugasa Y, Kato M, Sugihara S, Hirai M, Yamada K, Yanagihara K, et al. Geriatric nutritional risk index predicts functional dependency and mortality in patients with heart failure with preserved ejection fraction. Circ J 2013; 77: 705–711.
- 22.
Rusinaru D, Buiciuc O, Leborgne L, Slama M, Massy Z, Tribouilloy C. Relation of serum sodium level to long-term outcome after a first hospitalization for heart failure with preserved ejection fraction. Am J Cardiol 2009; 103: 405–410.
- 23.
Bavishi C, Ather S, Bambhroliya A, Jneid H, Virani SS, Bozkurt B, et al. Prognostic significance of hyponatremia among ambulatory patients with heart failure and preserved and reduced ejection fractions. Am J Cardiol 2014; 113: 1834–1838.
- 24.
McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: The Framingham Study. N Engl J Med 1971; 285: 1441–1446.
- 25.
Paulus WJ, Tschöpe C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, et al. How to diagnose diastolic heart failure: A consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J 2007; 28: 2539–2550.
- 26.
How to diagnose diastolic heart failure: European Study Group on Diastolic Heart Failure. Eur Heart J 1998; 19: 990–1003.
- 27.
JCS Joint Working Group. Guidelines for treatment of acute heart failure (JCS 2011): Digest version. Circ J 2013; 77: 2157–2201.
- 28.
Sato N, Kajimoto K, Keida T, Mizuno M, Minami Y, Yumino D, et al. Clinical features and outcome in hospitalized heart failure in Japan (from the ATTEND Registry). Circ J 2013; 77: 944–951.
- 29.
Ommen SR, Nishimura RA, Appleton CP, Miller FA, Oh JK, Redfield MM, et al. Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: A comparative simultaneous Doppler-catheterization study. Circulation 2000; 102: 1788–1794.
- 30.
Akiyama E, Sugiyama S, Matsuzawa Y, Konishi M, Suzuki H, Nozaki T, et al. Incremental prognostic significance of peripheral endothelial dysfunction in patients with heart failure with normal left ventricular ejection fraction. J Am Coll Cardiol 2012; 60: 1778–1786.
- 31.
Lubien E, DeMaria A, Krishnaswamy P, Clopton P, Koon J, Kazanegra R, et al. Utility of B-natriuretic peptide in detecting diastolic dysfunction: Comparison with Doppler velocity recordings. Circulation 2002; 105: 595–601.
- 32.
Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 2009; 53: 982–992.
- 33.
Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification: A report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 2005; 18: 1440–1463.
- 34.
Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr 2009; 10: 165–193.
- 35.
Daniels LB, Maisel AS. Natriuretic peptides. J Am Coll Cardiol 2007; 50: 2357–2368.
- 36.
Gheorghiade M, Rossi JS, Cotts W, Shin DD, Hellkamp AS, Piña IL, et al. Characterization and prognostic value of persistent hyponatremia in patients with severe heart failure in the ESCAPE Trial. Arch Intern Med 2007; 167: 1998–2005.
- 37.
Farmakis D, Filippatos G, Parissis J, Kremastinos DT, Gheorghiade M. Hyponatremia in heart failure. Heart Fail Rev 2009; 14: 59–63.
- 38.
Balling L, Schou M, Videbæk L, Hildebrandt P, Wiggers H, Gustafsson F; Danish Heart Failure Clinics Network. Prevalence and prognostic significance of hyponatraemia in outpatients with chronic heart failure. Eur J Heart Fail 2011; 13: 968–973.
- 39.
Ishikawa S, Schrier RW. Pathophysiological roles of arginine vasopressin and aquaporin-2 in impaired water excretion. Clin Endocrinol 2003; 58: 1–17.
- 40.
Schrier RW, Gross P, Gheorghiade M, Berl T, Verbalis JG, Czerwiec FS, et al. Tolvaptan, a selective oral vasopressin V2-receptor antagonist, for hyponatremia. N Engl J Med 2006; 355: 2099–2112.
- 41.
Imamura T, Kinugawa K, Minatsuki S, Muraoka H, Kato N, Inaba T, et al. Urine osmolality estimated using urine urea nitrogen, sodium and creatinine can effectively predict response to tolvaptan in decompensated heart failure patients. Circ J 2013; 77: 1208–1213.
- 42.
Imamura T, Kinugawa K, Shiga T, Kato N, Muraoka H, Minatsuki S, et al. Novel criteria of urine osmolality effectively predict response to tolvaptan in decompensated heart failure patients: Association between non-responders and chronic kidney disease. Circ J 2013; 77: 397–404.
- 43.
Konstam MA, Gheorghiade M, Burnett JC Jr, Grinfeld L, Maggioni AP, Swedberg K, et al. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: The Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study With Tolvaptan Clinical Status Trials. JAMA 2007; 297: 1319–1331.
- 44.
Gheorghiade M, Konstam MA, Burnett JC Jr, Grinfeld L, Maggioni AP, Swedberg K, et al. Short-term clinical effects of tolvaptan, an oral vasopressin antagonist, in patients hospitalized for heart failure: The Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study With Tolvaptan Clinical Status Trials. JAMA 2007; 297: 1332–1343.
- 45.
Gheorghiade M, Gattis WA, O’Connor CM, Adams KF Jr, Elkayam U, Barbagelata A, et al. Effects of tolvaptan, a vasopressin antagonist, in patients hospitalized with worsening heart failure: A randomized controlled trial. JAMA 2004; 291: 1963–1971.
- 46.
Gheorghiade M, Gottlieb SS, Udelson JE, Konstam MA, Czerwiec F, Ouyang J, et al. Vasopressin v(2) receptor blockade with tolvaptan versus fluid restriction in the treatment of hyponatremia. Am J Cardiol 2006; 97: 1064–1067.