2016 Volume 80 Issue 4 Pages 1041-1043
2016 Volume 80 Issue 4 Pages 1041-1043
Background: Pulmonary valve replacement (PVR) is a common reoperation, typically required approximately 10 years following right ventricular outflow tract reconstruction and especially true in cases of tetralogy of Fallot. However, an improved prosthetic valve is required.
Methods and Results: A fresh decellularized pulmonary allograft was used for PVR to correct pulmonary valve regurgitation in a 35-year-old man 33 years following tetralogy of Fallot repair. The postoperative course and short-term valve function were excellent. This is the first case of a decellularized pulmonary allograft in Japan.
Conclusions: Fresh decellularized pulmonary allografts have the potential to become a new source of material for PVR in patients who have undergone right ventricular outflow tract reconstruction. (Circ J 2016; 80: 1041–1043)
Pulmonary valve regurgitation (PR) is the most common clinically important complication, typically occurring about 10 years after tetralogy of Fallot (TOF) repair. PR causes right ventricular (RV) enlargement as well as other complications, such as left ventricular dysfunction, arrhythmias, heart failure, and death.1–3 Pulmonary valve replacement (PVR) is recommended before the occurrence of RV dysfunction, but the timing is still controversial because biological valves and cryopreserved homografts have relatively short durability with some late disadvantages, especially in adolescents.4,5 Herein, we report the first clinical application of a decellularized fresh pulmonary allograft for PVR in Japan.
The patient was a 35-year-old man diagnosed with TOF at birth. He underwent TOF repair using a mono-cusped transannular patch with mini-right ventriculotomy (Method III)6 at 2 years of age in Osaka University Hospital. The postoperative course was uneventful for a few decades, but PR gradually progressed, and RV enlargement was recognizable. The ECG showed normal sinus rhythm, with QRS duration of 160 ms. Echocardiography showed RV enlargement, moderate to severe PR, and trivial tricuspid valve regurgitation. Cardiac magnetic resonance imaging showed a pulmonary valve regurgitant fraction of 50.8%, a significantly enlarged RV (RV end-diastolic volume index of 160 ml/m2) with depressed contractility, and RV ejection fraction of 30%. Accordingly, we decided to perform PVR with a recommendation of a fresh decellularized pulmonary allograft for the following reasons: (1) the relatively young age of the patient meant he might otherwise require multiple PVR in his lifetime if he received a commercially available biological valve; (2) long term anticoagulation therapy after PVR was not required; and (3) absence of pulmonary hypertension. He agreed to our recommendation, and we received approval from the Ethical Committee at Osaka University to use this approach.
The fresh pulmonary allograft was provided by the German Society for Tissue Transplantation (Hannover, Germany) and decellularized by CorLife GbH (Hannover, Germany) (Figure 1). This decellularized human pulmonary valve (ESPOIR pulmonary valve) has been approved in Germany (approval no.: PEI.G.11634.01.1) for heart valve replacement since 2013, and ESPOIR pulmonary valves are currently being used in an investigative trial (NCT 02035540, ClinicalTrials.gov) in Europe. The operation was performed using re-median sternotomy, aortic and bicaval cannulation, and normothermic cardiopulmonary bypass. The pulmonary trunk was opened longitudinally. The original bicuspid pulmonary valve was dysplastic and shrunken, but no calcification was observed. A fresh decellularized pulmonary allograft with a 29-mm diameter was used for PVR. We resected the original valve leaflets and anastomosed the distal end of the allograft with a continuous suture. The proximal end of the allograft was anastomosed at the pulmonary valve annular position with a continuous suture in the same fashion as a pulmonary homograft implantation. Weaning from cardiopulmonary bypass was accomplished without any problems and the patient did not require blood transfusion. He was extubated 3 h after the operation and discharged from the hospital on postoperative day 10. Anticoagulation therapy was as for traditional bioprosthetic valves, and warfarin was discontinued 2 months after PVR. At 6 months after the operation, echocardiography and cardiac MRI showed no PR, no pulmonary valve stenosis, and good cardiac function (Figure 2).

Fresh decellularized pulmonary allograft (provided by the German Society for Tissue Transplantation [Hannover, Germany] and decellularized by CorLife [Hannover, Germany]).
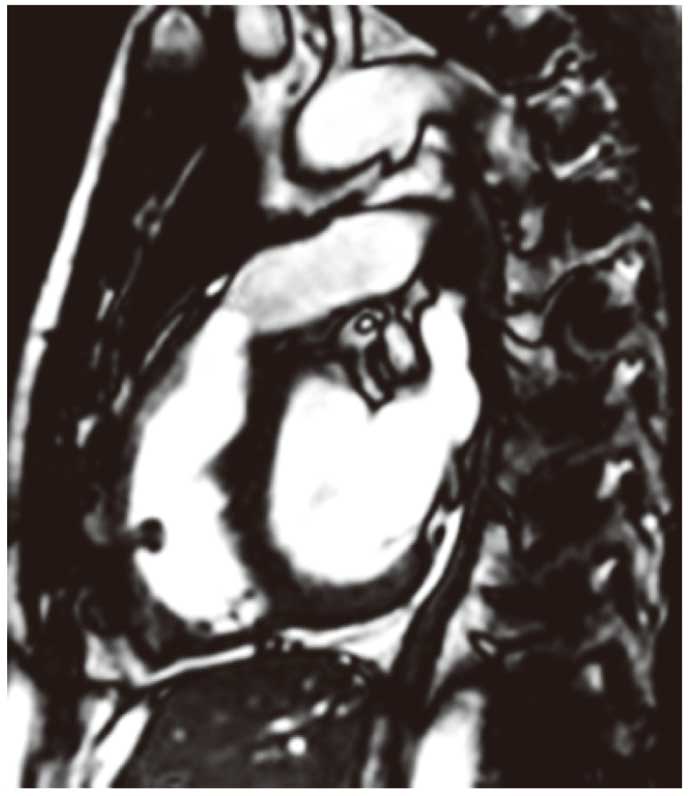
Cardiac magnetic resonance image taken 6 months after implantation of the decellularized allograft (end-diastolic phase). PA flow max: 1.73 m/s, PA forward flow: 78 ml/beat, PA regurgitant flow: 0 ml/beat. PA, pulmonary artery.
Over the 10 years following TOF repair, PVR is the most common reoperation. In our hospital, approximately 34% of patients who survive TOF repair undergo PVR within the following 40 years.
Bioprosthetic valves are commonly used for PVR because aggressive anticoagulation therapy is not needed, but the valves may deteriorate after a decade or more. Freedom from RV outflow tract reintervention after PVR is approximately 60% at 10 years,5 and structural failure of bioprosthetic valves is more common in younger patients. Mechanisms underlying the deterioration of bioprosthetic valves at a young age are poorly understood, but may be related to a stronger immunologic response during childhood.7 Moreover, intrinsic deterioration and valve calcification is more frequent in adolescents and young adults than in older patients. Because the number of reinterventions is a risk factor for death at long-term follow-up,8 the decision for optimal timing of PVR is crucial. Patients typically expected to undergo PVR are relatively young; therefore, there is a strong need for new-concept valves with markedly improved durability. Recently, Miyazaki et al9 reported the usefulness of fan-shaped expanded polytetrafluoroethylene (ePTFE) valved conduits with bulging sinuses at a decade follow-up. The ePTFE valves were covered by thin fibrous tissue and revealed focal regions of endothelialization, suggesting good biocompatibility; furthermore, they impeded cellular penetration and calcification. However, durability over a longer term (decades long) follow-up is unknown, and the ePTFE valve does not undergo adaptive growth in the follow-up period.
Cebotari et al10 reported a retrospective clinical follow-up of the surgical implantation of a new-concept biological valve (fresh decellularized allograft) in children and young adults; these new valves were compared mostly with xenograft and allograft valves in pediatric cardiac surgery. The fresh decellularized allografts maintained the extracellular matrix formation, provided superior performance, and showed very promising early results concerning durability and adaptive growth. Moreover, the authors observed no evidence of significant early activation of the cellular immune response with the use of fresh decellularized pulmonary allografts, which corresponded with the excellent mid-term results of these valves in clinical use.11 Furthermore, there appeared to be autologous regeneration of these valves.
In conclusion, we present the first successful Japanese case of a decellularized pulmonary allograft (provided from Germany) being used to treat PVR. Longer-term follow-up and further studies are needed, but this new-concept biological valve may provide promising long-term results for young patients with pulmonary valve insufficiency.
The authors earnestly thank Axcel Haverich, MD (Professor of Division of Cardiothoracic, Transplantation, and Vascular Surgery, Hannover Medical School, Hannover, Germany) and his colleagues for their cooperation in providing this decellularized fresh pulmonary allograft.