2016 Volume 80 Issue 5 Pages 1067-1072
2016 Volume 80 Issue 5 Pages 1067-1072
With ongoing progress in the prevention and treatment of coronary artery disease (CAD), a continued decrease in prevalence and lethality is expected in high-income countries. Prevention will include lipid-lowering, antithrombotic and anti-inflammatory therapies. With respect to the former, potent, safe and prolonged drugs (such as generic forms of PCSK9 inhibitors relying on monoclonal antibodies or miRNA) should result in a decreased incidence of acute coronary syndromes. Another key aspect will be the ability to identify genetic predictors of CAD and therefore implement targeted personalized prevention early in life. Curative treatment will involve a short course of potent and reversible antithrombotics, but long-term therapy will rely on the ability to stabilize or even regress plaque (eg, using PCSK9 inhibition or modified high-density lipoprotein infusions or anti-inflammatory therapies). Antithrombotic therapy will rely on highly reversible agents (or agents with specific titratable antagonists), and on personalized therapies in which the doses, combinations and duration of therapy will be determined differentially for each patient on the basis of clinical characteristics, genetic profiling and biomarkers. Finally, the need for revascularization in stable CAD will be rare, given the expected progress in prevention. The main challenge, 20 years from now, is likely to be the provision of such effective care at acceptable costs in low- and middle-income countries. (Circ J 2016; 80: 1067–1072)
There is currently a decrease in both the incidence and lethality of coronary artery disease (CAD) in industrialized countries, which is well documented by large-scale studies such as the WHO MONICA study.1 Both the epidemiological evidence and the modelling have suggested that progress is largely related to prevention (explaining the reduced incidence) and, to a lesser extent, to improved curative treatment (explaining the reduced lethality) of acute myocardial infarction (MI).2
The current paradigm for the management of atheromatous CAD is to implement widespread prevention by addressing the main modifiable risk factors for atherosclerosis (smoking, high blood pressure, diabetes mellitus, hypercholesterolemia) and improving subjects lifestyle by encouraging a healthy diet, maintenance of normal body weight and regular exercise.3 There is solid evidence that preventing or stopping smoking, normalizing blood pressure, lowering plasma cholesterol and, in certain circumstances and with certain treatments, treating diabetes, is associated with a reduction in the incidence of coronary events. Because CAD is silent for decades, this “prevention phase” can last many years. At this stage, patients can develop exertional angina because of severe narrowing of the coronary arteries reducing the coronary flow reserve, but the current thinking is that, despite the existence of plaques in the coronary arteries, most patients will not die from CAD until, because of rupture or superficial erosion of the plaque fibrous cap, thrombosis superimposes atherosclerosis causing an acute MI or sudden death.
For those patients with evidence of disease (either following an acute coronary syndrome, or coronary revascularization or arterial imaging), more intensive pharmacotherapy is generally recommended, relying on lifetime treatment with antiplatelet agents, with low-dose aspirin being the first-line agent, statins, and in most cases an angiotensin-converting enzyme inhibitor and a β-blocker.4 Recently, the role of systematic chronic β-blockade in patients with stable CAD has been questioned. Although β-blockers remain the cornerstone of treatment for patients with exertional angina, or for patients with heart failure, their role in asymptomatic patients is uncertain: most of the evidence for their benefit stems from trials performed in patients with acute MI antedating the widespread reperfusion era and because observational analyses suggest that their benefit on outcomes in chronic stable patients is probably confined to patients with prior MI.5–8 Likewise, angiotensin-converting enzyme inhibitors have established benefits in patients with diabetes, chronic kidney disease, left ventricular dysfunction or heart failure, but their role in stable patients with CAD and none of these conditions is uncertain.9
Finally, most patients with evidence of CAD will be, at one point or another, candidates for myocardial revascularization using either percutaneous coronary intervention or coronary artery bypass grafting, with the former currently representing the vast majority of revascularization procedures. The need for revascularization can arise because of either acute coronary syndrome or evidence of severe myocardial ischemia, generally accompanied by angina symptoms, in the context of medical therapy.
The most likely immediate advance in preventive treatment in patients with CAD is likely to stem from the ability to dramatically lower plasma cholesterol well below the levels achieved currently by maximal statin therapy, using the novel PCSK9 inhibitors, alirocumab, evolocumab and bococizumab. It is well established that plasma low-density lipoprotein (LDL) cholesterol levels predict cardiovascular event rates in patients with documented CAD, and that LDL lowering, whether by statins or by ezetimibe10 reduces the risk.11 There is already robust evidence that bimonthly to monthly subcutaneous injections of the monoclonal antibodies targeting PCSK9 achieve major reductions in plasma LDL cholesterol levels, which are sustained for several weeks. The safety profile of these agents appears, so far, to be good, with minimal local injection site reactions and no identified general toxicity. There remain unknowns regarding the long-term safety of lowering LDL cholesterol well below the 70 mg/dl level. Statin trials have generally shown that “lower is better” and that the patients achieving the lowest LDL values not only have the lowest cardiovascular event rates but also no more safety issues. However, PCSK9 inhibitors achieve even lower LDL values, which can routinely stay below 30 mg/dl. Questions are pending regarding the safety of such low levels achieved permanently, in particular with respect to cognitive and neurologic side effects.12 Analyses of the small number of subjects with mutated loss of function PCSK9, who live with permanently low levels of LDL cholesterol, have not shown cause for concern, but these studies are far from definitive. Establishing safety will require not only long-term follow-up from large outcome trials but also careful post-marketing surveillance. However, currently, the most pressing question is whether there is a gain in efficacy (and therefore a substantial reduction in cardiovascular event rates) when these agents are added to maximally tolerated statin treatment. Post hoc analyses of safety trials have suggested potential benefits (Figure 1),13 but need to be confirmed by the ongoing large outcome trials, some of which will most likely report in the coming months (Figures 2,3).14,15 In parallel, trials are exploring the effects of PCSK9 induced LDL lowering on plaque structure and volume. Previous studies with potent statin therapy have suggested that dramatic LDL lowering can be associated with reductions in plaque volume.16 Overall, if PCSK9 inhibitors “work” (ie, not only achieve marked reductions in LDL plasma levels, but also dramatic reduction in cardiovascular event rates without major safety concerns), they have the potential to change the natural history and affect the epidemiology of CAD.

Post hoc analysis describing the Kaplan-Meier estimates for time to first adjudicated major cardiovascular events in the long-term safety trial with alirocumab or placebo (derived from reference 12). The data are derived from the safety analysis. The outcome of interest is the primary outcome for the ongoing ODYSSEY OUTCOMES trial (ie, the composite of coronary death, nonfatal myocardial infarction, fatal and nonfatal ischemic stroke, and unstable angina requiring hospitalization). HR, hazard ratio; LLT, lipid-lowering therapy.

Schematic of the ongoing ODYSSEY OUTCOMES randomized trial (derived from reference 14). ACS, acute coronary syndrome; CAD, coronary artery disease; LDL-C, low-density lipoprotein cholesterol; MI, myocardial infarction.

Schematic of the ongoing FOURIER trial (derived from reference 13). CVD, cardiovascular disease; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; Pts, patients; Rx, treatment; Sq, subcutaneous.
Apart from monoclonal antibodies, there are other methods of lowering PCSK9 levels, such as RNA interference. It has already been shown that RNA interference can achieve marked PCSK9 knock-down and that this is associated with prolonged reduction in LDL cholesterol levels over several months,17 potentially opening the door to treatment every 6 months or every year with a single injection. However, the safety and efficacy of these approaches is not yet established and there are, specifically, concerns regarding potential liver toxicity.
Finally, in addition to LDL lowering with PCSK9 inhibition, there are other promising methods of interfering with lipid metabolism in order to prevent atherothrombosis; for example, it is possible to improve cholesterol efflux capacity (a phenomenon that has been shown to have a potent correlation with the risk of cardiovascular events18) with agents such as modified high-density lipoprotein (HDL) particles.19 Whether such approaches are really likely to sustainably reduce cardiovascular risk is still uncertain.
Anti-Inflammatory TherapiesIn the current paradigm for CAD, chronic stable coronary atheromatous plaques suddenly become unstable following rupture or erosion of the fibrous cap. However, the mechanisms underlying such an abrupt phenomenon are unclear. It is generally thought that local or systemic inflammatory processes play a role: first there is good evidence that chronic low-level systemic inflammation (as measured, for instance, using high-sensitivity C-reactive protein levels) is associated with greater cardiovascular risk.20 There is also evidence that patients with chronic inflammatory diseases have an increased cardiovascular risk. In addition, there is also evidence of temporal associations between acute inflammation/infection and cardiovascular events.21 However, to disentangle the many potential confounders of the association between inflammation and cardiovascular events, intervention trials are needed. Some small trials have suggested potential benefit from anti-inflammatory agents such as colchicine.22 And, indeed, several large-scale randomized trials are ongoing, testing various anti-inflammatory strategies to reduce cardiovascular risk in patients with CAD, such as canakinumab (an interleukin 1β inhibitor), low-dose methotrexate, etc.23,24 If this approach works, there would potentially be the ability to positively affect not only the substrate for acute events (by preventing plaque buildup with LDL lowering) but also the phenomena leading to acute superimposed thrombosis. However, it will take several years to determine definitively if this approach works or not.
Targeted and Personalized Antithrombotic TherapiesThere are many options for preventive and curative antithrombotic therapy in patients with atherothrombosis, but they prevent ischemic events at the price of an increased risk of bleeding. The main conundrum is the need to balance the ischemic and bleeding risks on an individual level, in order to select the optimal combination, intensity and duration of therapy for each patient. Until now, the mainstay of long-term prevention has been single antiplatelet therapy with low-dose aspirin,25 but with the advent of other potent antiplatelet and anticoagulant agents, more options have opened, both for secondary prevention26 and for primary prevention. It is highly likely that, in the future, rather than relying on a single agent at a single dose given continuously for all patients, treatment will be adjusted in terms of number of agents, dose and duration to the expected benefit/risk ratio as measured by calculators. A good example is the dual antiplatelet therapy (DAPT) risk score to guide the duration of DAPT with aspirin and an oral P2Y12 receptor blocker after stenting,27 which can assist clinical decision making (Figure 4), but remains a rather crude tool compared with what will be achievable once biomarkers and genetic information can be built into the risk assessment tools. Another path forward will be the ability to reverse antithrombotics, using either very short-lived agents (eg, cangrelor, an intravenous antagonist of the platelet P2Y12 receptor, which has shown promise to reduce the periprocedural thrombotic complications of percutaneous coronary intervention)28 or with specific antagonists.29
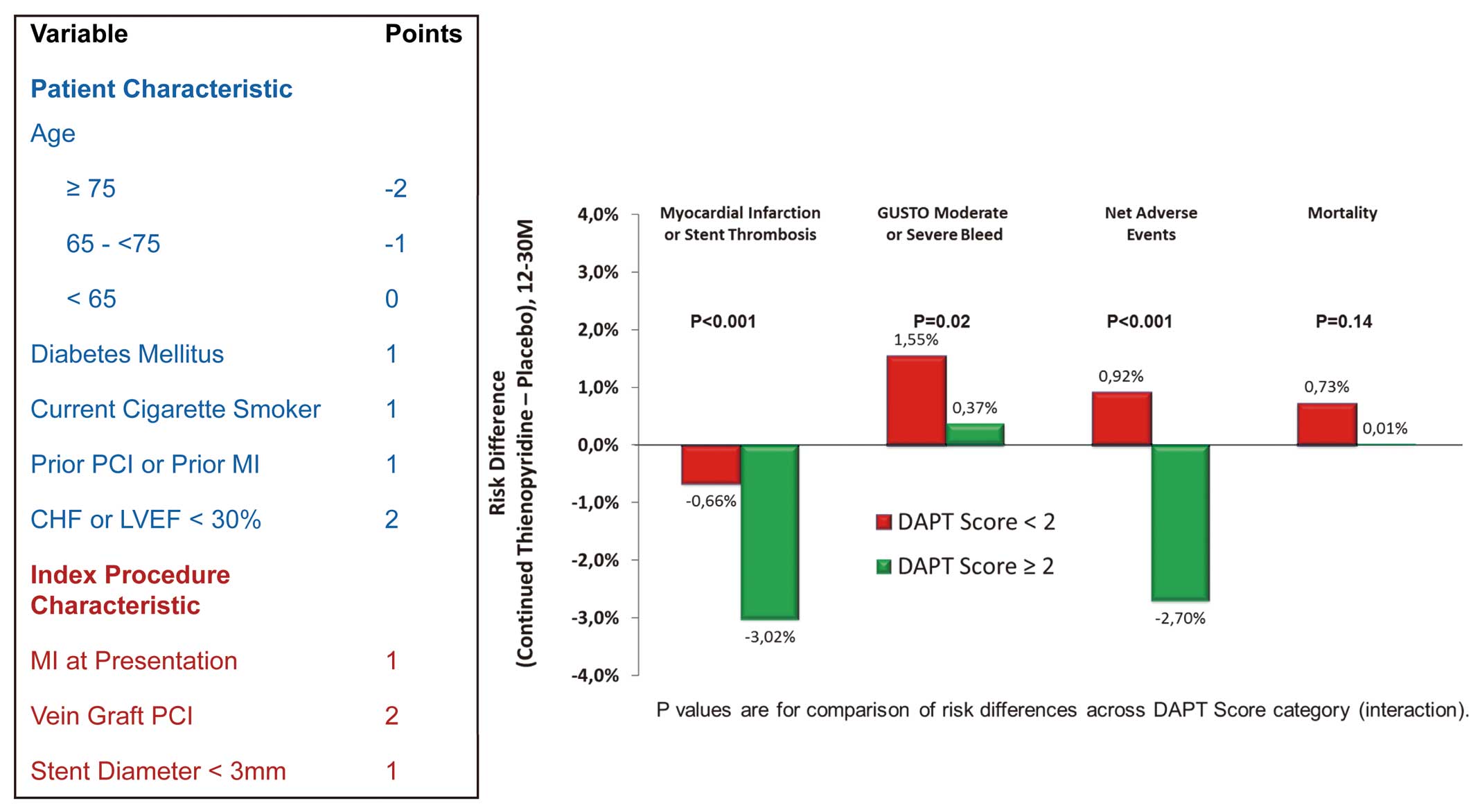
Computation of the DAPT risk score and its effect on the efficacy and safety of dual antiplatelet therapy according to whether the score is above or below the median value of 2 (derived from reference 26). CHF, chronic heart failure; LVEF, left ventricular ejection fraction; MI, myocardial infarction; PCI, percutaneous coronary intervention.
Given the anticipated progress in prevention, with effective prevention of the generation of atheromatous plaques, and effective prevention of plaque rupture or erosion, it is possible that a major decline in the incidence of coronary events might be witnessed in the next decades, at least in areas and countries where effective (but costly) therapies will be available on a large scale. This is likely to reduce dramatically the need for myocardial revascularization, which would then appear to be confined to the acute treatment of occlusion or impending coronary occlusion in the context of acute coronary syndromes. For those patients who will still need percutaneous coronary intervention, permanent stenting is likely to be replaced by biodegradable devices, once the efficacy and safety of the latter is improved.30,31
Genome-Guided PreventionThe current model of prevention for cardiovascular diseases is rather crude: patients at risk of developing coronary events in the next few years are identified by measurement of conventional risk factors and calculators and modifiable risk factors are corrected as much as possible using “standardized” therapies. However, we already have the ability to extract critical genetic information to predict not only the cardiovascular risk of each individual, but also their response to such commonly used therapies as statins.32,33 In the future, it is likely that such approaches will rapidly expand to allow both personalized prevention but also to start prevention much earlier in life by addressing the lifetime cumulative risk rather than the current approach, which addresses relatively short-term risk and therefore is heavily affected by age and results in abstention from intensive preventive treatment for several decades.
The future of prevention and treatment of CAD appears bright: most of the recent gains in lifetime expectancy in industrialized countries such as the USA are derived from gains in prevention and treatment of cardiovascular disease, and this trend will continue. However, implementation of the sophisticated risk assessment and treatment strategies outlined here will require substantial resources. This has to be contrasted with the observation that although the incidence of cardiovascular disease is declining in high-income countries, the bulk of the health burden from CAD now rests on low- and middle-income countries, which account for the vast majority of cardiovascular deaths worldwide and which explains why CAD is still projected to remain the number 1 cause of death in the world in 2030. Findings solutions to make the effective preventive and curative strategies described here available to the entire world is clearly the challenge of the next decades.
G.D. has no disclosures. P.G.S. has the following disclosures:
ConsultancyAnnual income from a single company or organization, as an officer or consultant, which exceeds an annual total of 1,000,000 yen
- AstraZeneca, Bayer, Boehringer-Ingelheim, Bristol-Myers-Squibb, CSL-Behring, Daiichi-Sankyo, GlaxoSmithKline, Janssen, Lilly, Novartis, Pfizer, Regeneron, Roche, Sanofi, Servier, The Medicines Company
Remuneration (eg, Lecture Fees)Remuneration for attending meetings (presentations), paid for time and effort of the activity, which exceeds an annual total of 500,000 yen, per company or organization
- AstraZeneca, Bayer, Bristol-Myers-Squibb, Lilly, Sanofi, Servier, The Medicines Company
Research FundingResearch funds (trust research funds, joint research funds etc) provided by a single company or organization which exceeds an annual total of 1,000,000 yen
- Merck, Sanofi, Servier
Travel Expenses, Gifts, and Others, Which Are Unrelated to ResearchTravel expenses, gifts and other contributions which exceed an annual total of 50,000 yen from one single company or organization
- AstraZeneca, Servier