2016 Volume 80 Issue 6 Pages 1470-1477
2016 Volume 80 Issue 6 Pages 1470-1477
Background: It remains to be determined whether balloon pulmonary angioplasty (BPA) improves biventricular cardiac functions and pulmonary flow in patients with chronic thromboembolic pulmonary hypertension (CTEPH).
Methods and Results: We enrolled 30 consecutive patients with inoperable CTEPH who underwent BPA, and carried out serial cardiac magnetic resonance imaging (CMR; M/F, 9/21; median age, 65.2 years). No patient died during the treatment or follow-up period. BPA significantly improved WHO functional class (III/IV, 83.0 to 4.0%), 6-min walking distance (330.2±168.7 to 467.3±114.4 m), mean pulmonary artery pressure (40.8±10.7 to 23.2±4.94 mmHg), pulmonary vascular resistance (9.26±4.19 to 3.35±1.40 WU) and cardiac index (2.19±0.64 to 2.50±0.57 L·min·m2; all P<0.01). CMR also showed improvement of right ventricular (RV) ejection fraction (EF; 41.3±12.4 to 50.7±8.64%), left ventricular (LV) end-diastolic volume index (72.1±14.0 to 81.6±18.6 ml/m2) and LV stroke volume index (41.0±9.25 to 47.8±12.3 ml/m2; all P<0.01). There was a significant correlation between change in RVEF and LVEF (Pearson’s r=0.45, P=0.01). Average velocity in the main pulmonary artery was also significantly improved (7.50±2.43 to 9.79±2.92 cm/s, P<0.01).
Conclusions: BPA improves biventricular functions and pulmonary flow in patients with inoperable CTEPH. (Circ J 2016; 80: 1470–1477)
Chronic thromboembolic pulmonary hypertension (CTEPH) is characterized by the presence of organized thrombi in the pulmonary circulation and poor prognosis due to progressive right ventricular (RV) failure.1,2 Pulmonary endarterectomy (PEA), which surgically removes organized thrombi, can cure CTEPH,1,3–8 but distal-type CTEPH is not suitable for PEA,8 and these inoperable CTEPH patients have poor prognosis due to progressive RV failure. As an alternative therapy, Feinstein et al originally developed balloon pulmonary angioplasty (BPA) in 2001.9 and we and others have recently showed that BPA improves pulmonary hemodynamics, exercise capacity and prognosis in inoperable CTEPH.10–12
Editorial p 1326
In CTEPH, RV pressure overload causes RV dysfunction and dilation with resulting poor prognosis.13–15 Precise evaluation of RV function, however, still remains difficult because of anatomical complexity of the RV chamber. Recently, cardiac magnetic resonance imaging (CMR) has been widely used to evaluate RV configuration and function because it non-invasively provides 3-D RV imaging, high-resolution evaluation of RV structure and accurate functional assessment without geometric assumptions.16 Kreitner et al used CMR to evaluate change in cardiac function and pulmonary flow after PEA in CTEPH patients.17 Van Wolferen et al reported that in patients with idiopathic pulmonary arterial hypertension (IPAH), both RV and left ventricular (LV) function as measured on CMR were improved after medical treatment, and that improvement of LV end-diastolic volume (LVEDV) was one of the independent predictors of mortality.18 Little is known, however, about the change in biventricular functions in inoperable CTEPH before and after BPA.
Thus, in the present study, the aim was evaluate the effects of BPA on biventricular functions and pulmonary arterial blood flow using CMR in patients with inoperable CTEPH.
The present study was approved by the ethics committee of Tohoku University (2014-1-875).
PatientsWe retrospectively enrolled 30 consecutive patients with inoperable CTEPH (M/F, 9/21; median age, 65.2 years) classified as World Health Organization (WHO) functional class (WHO-FC) II–IV despite optimal medical therapy. Based on the NICE criteria,19 diagnosis of CTEPH was done on medical history, physical examination, electrocardiogram (ECG), chest X-ray, echocardiography, lung ventilation/perfusion scintigraphy, right heart catheterization (RHC), computed tomography angiography or pulmonary artery (PA) angiography. All patients were diagnosed as inoperable by experienced surgeons because of the location of thrombi, surgical accessibility, age and comorbidity. The patients were treated with optimal medical therapy and BPA between July 2009 and July 2015. They also underwent RHC and CMR before and after BPA. Clinical assessment, including functional status, plasma brain natriuretic peptide (BNP) and 6-min walk distance (6MWD), was performed at the first admission and before each BPA procedure.
BPAFigure 1 shows the BPA procedure.10–12 We performed BPA via the internal jugular vein or femoral vein, depending on the catheter accessibility to the target vessels. After RHC, a 6-Fr guiding catheter with a 0.035-inch J-curved wire was advanced into a target artery. After crossing a 0.014-inch guidewire through the target lesion, we evaluated the morphology of lesions using optimal coherence tomography (OCT), and selected the balloon size based on the angiographic and OCT-derived diameter of the target vessel.10 We used 2–7-mm balloons to dilate target lesions to 50–75% of the vessel diameter.8 In one procedure, target lesion was limited to 1 or 2 segments in one lobe to minimize complications of BPA. We repeated BPA sessions until mean PA pressure (PAP) became <30 mmHg at a 4–8-week interval.10

Representative angiography in balloon pulmonary angioplasty (BPA) in a patient with chronic thromboembolic pulmonary hypertension. (A) Before BPA. Arrows, target legion. (B) Dilation to the target legion by balloon catheter. (C) Immediately after BPA. The target legion was dilated, and pulmonary arterial flow improved. (D) Follow-up for the target lesion. Pulmonary venous flow is also improved (arrows).
At the first admission and before each BPA procedure, we performed RHC to measure PAP, PA wedge pressure, cardiac output (CO), cardiac index (CI), stroke volume (SV), stroke volume index (SVI) and pulmonary vascular resistance (PVR). CO (CI) and SV (SVI) were determined using the Fick method.
CMRAll patients underwent repeated CMR at the first admission and after BPA. CMR was done with a whole body MR scanner at 1.5T (Intera Achiva 1.5T Nova Dual, Philips Medical Systems, Best, the Nederlands) with a 5-channel cardiac coil.16 Cine imaging was acquired using a steady-state free precession sequence (echo time, 3.1 ms; repetition time, 1.5 ms; flip angle, 60°; slice thickness, 10 mm; no. slices, 20; no. phases per 1 cardiac cycle, 20; field of view, 360 mm; matrix size, 192×192). Two-dimensional phase contrast imaging was acquired perpendicular to the main PA approximately 1–2 cm above the pulmonary valve in a double oblique plane to ensure orthogonal flow measurements. Typical sequence parameters of the retrograde ECG-gated phase contrast gradient echo sequence were as follows: echo time, 5.3 ms; repetition time, 8.24 ms; flip angle, 15°; no. phases per 1 cardiac cycle, 16; field of view, 360 mm; matrix size, 160×75; and velocity encoding, 50–150 cm/s. We traced the LV- and RV- endocardial and epicardial contours in the end-diastolic and end-systolic frames of short axial slices using a standalone workstation (Zio Station 2; Ziosoft, Tokyo, Japan). We measured LVEDV, LV end-systolic volume (LVESV), LV mass, LV ejection fraction (LVEF), RV end-diastolic volume (RVEDV), RV end-systolic volume (RVESV), RV ejection fraction (RVEF) and RV mass. The interventricular septum was assessed on the mid-chamber short axis images at phase of maximum septal displacement. Interventricular septal angle (ISA) was measured by determining the angle between the mid-point of the interventricular septum and the 2 hinge points; the mid-point of the LV free wall was also measured (Figure 2).20

(A) Calculation of interventricular septum angle (ISA). ISA was measured in the early diastolic phase of maximum septal displacement. (B) Flow time curve in the main pulmonary artery. Acceleration time (AT), cycle duration until peak flow; ejection time (ET), cycle duration until flow becomes 0 ml/s. ROI, region of interest.
We analyzed 2-D phase contrast imaging using the same workstation. Regions of interest (ROI) were traced manually in the PA on magnitude images and transferred to the phase images. Peak velocity, average velocity, maximum area and minimum area of the PA were computed automatically from the ROI. Acceleration time (AT) and ejection time (ET) were recorded manually from flow-time curves (Figure 2).21,22
Statistical AnalysisStatistical analysis was performed with JMP 11.0 (SAS Institute, Cary, NC, USA). Quantitative variables are expressed as mean±SD or median (IQR), and categorical variables as the number and percentages. Changes in clinical parameters, RHC parameters and CMR were evaluated using paired t-test, Wilcoxon signed ranks test or McNemar’s test, as appropriate. Pearson’s correlation coefficient (r) was used to evaluate correlations of biventricular functions on CMR. P<0.05 was considered to be statistically significant.
Twenty-five of 30 patients (83.3%) had WHO-FC III/IV (Table 1). Median duration from onset of symptoms to the first BPA was 20.5 months. As a vasodilator therapy prior to BPA, prostacyclin analogue, phosphodiesterase-5 inhibitor, endothelin receptor antagonist and soluble guanylate cyclase stimulator were used in 24 (80%), 29 (97%), 7 (23%) and 1 (3%) patients, respectively (Table 2). Of these patients, 18 (60%) received combination therapy (Table 2). A total of 152 BPA were performed (mean procedures per patient, 5.1±2.0). The complications, including bloody sputum and mild-moderate hemoptysis, occurred in 20 out of 152 BPA (13%), which were successfully treated non-invasively. Importantly, no patients died during the procedure or follow-up period. The mean period from the final BPA session to CMR was 9.0±8.7 months.
| Subjects | 30 |
| Age (years) | 65.2 (range, 34–83) |
| F/M | 21/9 |
| WHO-FC II/III/IV | 5/19/6 |
| Time from onset of symptoms to BPA (months) | 20.5 (IQR, 9.8–39.3) |
Data given as n, mean (range) or median (IQR). BPA, balloon pulmonary angioplasty; PH, pulmonary hypertension; WHO-FC, World Health Organization functional classification.
| Before BPA | After BPA | P-value | |
|---|---|---|---|
| WHO-FC III/IV | 25 (83.0) | 1 (4.0) | <0.01 |
| 6MWD (m)† | 330.2±168.7 | 467.3±114.4 | <0.01 |
| BNP (pg/ml) | 147.6 (37.8–305.5) | 40.8 (16.4–54.0) | <0.01 |
| Therapy for PH (including combination therapy) | 30 (100) | 25 (83) | |
| Bosentan | 4 (13) | 0 (0) | |
| Ambrisentan | 3 (10) | 6 (20) | |
| Riociguat | 1 (3) | 2 (7) | |
| Sildenafil | 22 (73) | 13 (43) | |
| Tadalafil | 7 (23) | 6 (20) | |
| Beraprost | 17 (57) | 12 (40) | |
| Epoprostenol | 7 (23) | 0 (0) | |
| RHC data | |||
| Heart rate (beats/min) | 66.6±11.0 | 60.0±10.9 | 0.01 |
| PCWP (mmHg) | 8.83±3.23 | 10.2±3.50 | 0.06 |
| Systolic PAP (mmHg) | 71.4±19.9 | 39.3±9.20 | <0.01 |
| Diastolic PAP (mmHg) | 24.6±7.19 | 14.7±4.00 | <0.01 |
| PAP (mmHg) | 40.8±23.2 | 23.2±4.94 | <0.01 |
| Cardiac index (L/min/m2) | 2.19±0.64 | 2.50±0.57 | <0.01 |
| Stroke volume index (ml/m2) | 32.8±9.32 | 42.1±9.76 | <0.01 |
| PVR (WU) | 9.26±4.19 | 3.35±1.40 | <0.01 |
| Pulse pressure | 46.8±15.2 | 24.5±6.67 | <0.01 |
Data given as n (%), mean±SD or median (IQR). †One patient could not perform 6-min walk test due to dyspnea (n=29). Pulse pressure is calculated as systolic PAP-diastolic PAP. 6MWD, 6-min walk distance; BNP, brain natriuretic peptide; CTEPH, chronic thromboembolic pulmonary hypertension; PAP, pulmonary artery pressure; PCWP, pulmonary capillary wedge pressure; PVR, pulmonary vascular resistance; RHC, right heart catheterization; WU, wood units. Other abbreviations as in Table 1.
After BPA, the number of patients with WHO-FC III/IV was significantly decreased (from 25/30 to 1/30, P<0.01; Table 2). Also, serum BNP was significantly decreased and 6MWD was significantly increased (Table 2). The percentage of patients treated with pulmonary vasodilators was reduced from 100% to 83% after BPA (Table 2). Additionally, the number of patients with combination therapy was decreased from 18 to 11. Importantly, all 7 patients treated with i.v. epoprostenol were successfully tapered off without any hemodynamic worsening.
RHC ParametersResults of RHC before and after BPA are listed in Table 2. PAP was markedly decreased after BPA (mean PAP, 40.8±10.7 to 23.2±4.9 mmHg, P<0.01). Pulse pressure and PVR were also decreased, and CI and SVI were increased after BPA (Table 2).
CMR ParametersCine images and PA flow curves before and after BPA are shown in Figure 3. All RV parameters except for RVSV index (RVSVI) were significantly improved after BPA (Table 3). Among the LV parameters, LVEDV index (LVEDVI), LVSV index (LVSVI) and LV mass index (LVMI) were significantly increased (Table 3). ISA was also significantly improved (Table 3). Figure 4 shows scatter plots between changes in RV and LV volumetric parameters before and after BPA. There were significant correlations between the changes in the following parameters: RVEF and LVEF; RVSVI and LVSVI; RVSVI and LVEDVI; ISA and LVEDVI; ISA and LVSVI (Figure 4). Parameters of PA flow before and after BPA were obtained in 22 patients (female, 67.0%; mean age, 67.8 years). Average velocity was significantly increased (Figure 3). Peak velocity (P=0.07) and ET (P=0.09) tended to be increased after BPA, whereas AT or AT/ET was unaltered (Table 4). Maximum, minimum and average PA area were significantly decreased (Table 4).

Cine cardiac images (end-diastolic and end-systolic frames of short axial slices) and flow curves in the main pulmonary artery of a patient with chronic thromboembolic pulmonary hypertension (A–C) before and (D–F) after balloon pulmonary angioplasty (BPA). (A,B) Before BPA, the right ventricle (RV) was dilated and compressed the left ventricle (LV), but (D,E) after BPA, the RV became smaller along with decompressed LV. (C,F) Peak and average velocity increased (peak velocity, 33.2 to 52.7 cm/s; average velocity, 2.10 to 2.60 cm/s) and ejection time became longer from 390 to 420 ms after BPA. ROI, region of interest.
| Before BPA | After BPA | P-value | |
|---|---|---|---|
| Right ventricle | |||
| RVEDVI (ml/m2) | 104.5±35.3 | 85.4±20.3 | <0.01 |
| RVESVI (ml/m2) | 64.8±33.9 | 42.5±14.0 | <0.01 |
| RVSVI (ml/m2) | 39.76±7.75 | 42.8±11.2 | 0.24 |
| RVEF (%) | 41.3±12.4 | 50.7±8.64 | <0.01 |
| RV mass index (g/m2) | 33.5±11.4 | 26.4±4.32 | <0.01 |
| Left ventricle | |||
| LVEDVI (ml/m2) | 72.1±14.0 | 81.6±18.6 | <0.01 |
| LVESVI (ml/m2) | 31.0±12.1 | 33.8±11.4 | 0.10 |
| LVSVI (ml/m2) | 41.0±9.25 | 47.8±12.3 | <0.01 |
| LVEF (%) | 57.7±10.7 | 59.5±8.52 | 0.21 |
| LV mass index (g/m2) | 53.7±8.90 | 56.8±9.95 | 0.02 |
| ISA (º)† | 157.5±13.5 | 150.2±12.3 | 0.02 |
Data given as mean±SD. †Short axis data not obtained in one patient for technical reasons (n=29). CMR, cardiac magnetic resonance imaging; ISA, interventricular septal angle; LVEDVI, left ventricular end-diastolic volume index; LVEF, left ventricular ejection fraction; LVESVI, left ventricular end-systolic volume index; LVSVI, left ventricular stroke volume index; RVEDVI, right ventricular end-diastolic volume index; RVEF, right ventricular ejection fraction; RVESVI, right ventricular end-systolic volume index; RVSVI, right ventricular stroke volume index. Other abbreviations as in Tables 1,2.
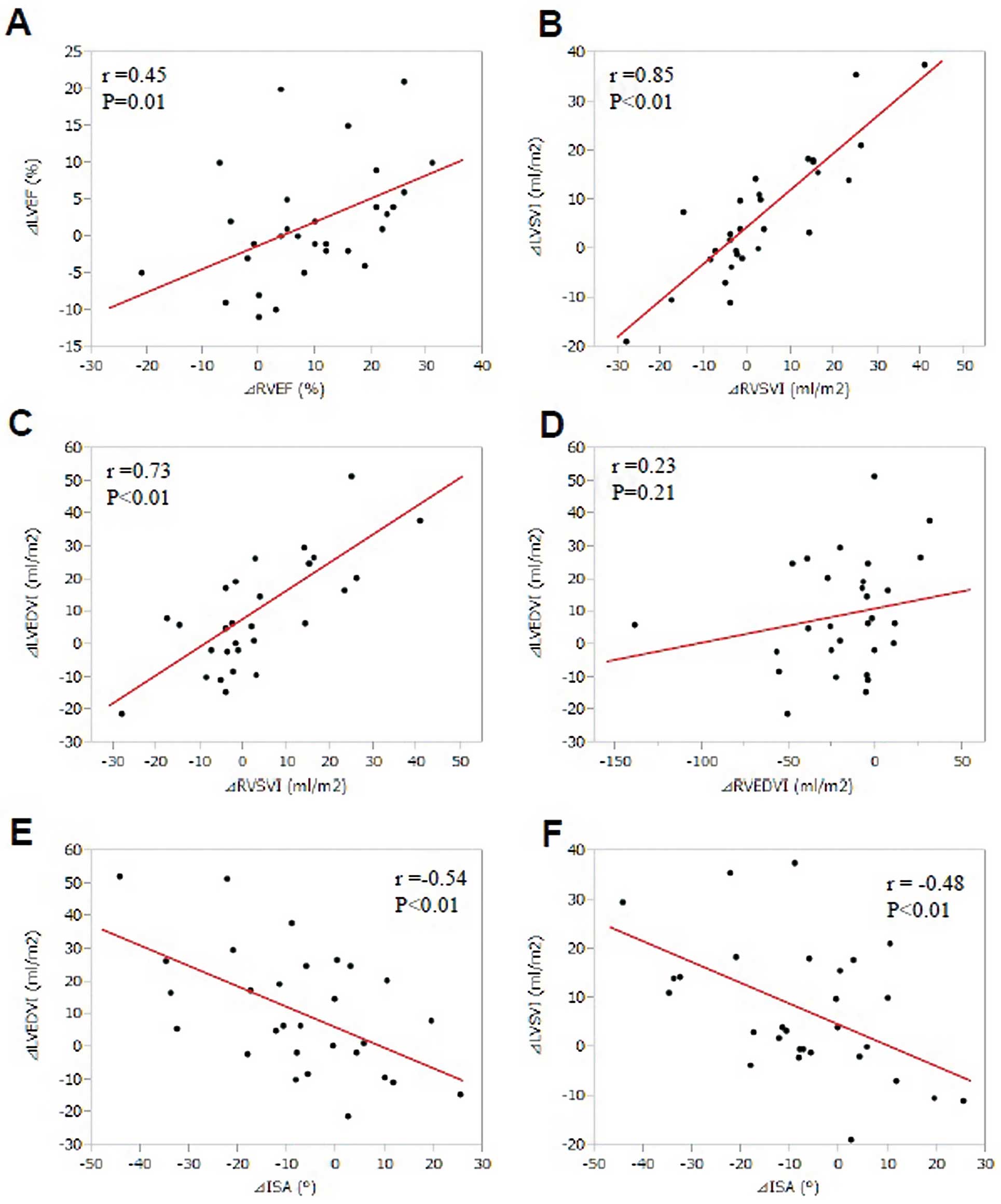
A significant correlation was noted between the changes in (A) right ventricular ejection fraction (∆RVEF) and those in left ventricular ejection fraction (∆LVEF) and between changes in (B) right ventricular stroke volume index (∆RVSVI) and those in left ventricular stroke volume index (∆LVSVI). A significant correlation was also noted between (C) ∆RVSVI and the changes in LV end-diastolic volume index (∆LVEDVI) but not between the change in (D) right ventricular end-diastolic volume index (∆RVEDVI) and ∆LVEDVI. A significant correlation was also noted between changes in (E) interventricular septal angle (∆ISA) and ∆LVEDVI or (F) ∆LVSVI.
| Before BPA | After BPA | P-value | |
|---|---|---|---|
| Peak velocity (cm/s) | 56.7±15.5 | 62.4±16.7 | 0.07 |
| Average velocity (cm/s) | 7.50±2.43 | 9.79±2.92 | <0.01 |
| AT (ms) | 111.8±41.5 | 111.8±27.1 | 0.94 |
| ET (ms) | 354.5±58.8 | 382.2±85.0 | 0.09 |
| AT/ET (%) | 28.4±12.9 | 37.9±11.7 | 0.84 |
| Maximum PA area (cm2) | 10.5±2.60 | 9.87±2.82 | <0.01 |
| Minimum PA area (cm2) | 8.51±2.14 | 7.37±2.17 | <0.01 |
| Average PA area (cm2) | 9.58±2.31 | 8.54±2.42 | <0.01 |
Data given as mean±SD. AT, acceleration time; ET, ejection time; PA, pulmonary artery. Other abbreviations as in Tables 1–3.
The major findings of the present CMR study CMR are (1) BPA significantly improved both RV and LV functions in patients with inoperable CTEPH; and (2) BPA also improved PA flow in those patients. To the best of our knowledge, this is the first CMR study to demonstrate the therapeutic effects of BPA on biventricular functions and PA flow pattern in patients with inoperable CTEPH.
Re-Evaluation of BPAIn 2001, Feinstein et al demonstrated for the first time that BPA reduced mean PAP and improved NYHA status and 6MWD in 18 patients.9 But, given that pulmonary edema occurred in 11 patients after BPA (61%) and one patient died from RV failure in their study, BPA did not become widespread. More than 10 years later, we and others have recently demonstrated that modified staged BPA is a safe and effective treatment for patients with inoperable CTEPH.10–12 Indeed, the present study also showed that BPA is a safe and effective treatment, as evidenced by the improvements in WHO status, serum BNP, 6MWD, PAP and PVR without major complications.23
BPA Improves Biventricular Functions in CTEPHAdvanced pulmonary hypertension causes chronic pressure and volume overload on the RV, resulting in RV hypertrophy and dilation.18,24 This RV remodeling is also noted in CTEPH patients and could lead to RV failure.6 RV dysfunction is one of the important prognostic determinants of PH.19 It was previously reported that PEA promptly improved pulmonary hemodynamics and RV function and induced RV reverse remodeling.6,25,26 It is controversial, however, as to whether PEA could exert acute effects on LV remodeling and function. Iino et al found no significant change in LV function at 6 months after PEA,25 whereas Hardziyenka et al noted reverse LV remodeling (increased LVEDVI and LVSVI) at 8 months after PEA.26 Reesink et al noted that LV function normalized owing to the restoration of pulmonary hemodynamics and RV function at least at 4 months after PEA.6 Based on the results of these PEA studies, 4–6 months may be needed to induce LV remodeling and improve LV function with PEA or BPA. Recently, Fukui et al reported the short-term beneficial effects of BPA on pulmonary hemodynamics and RV remodeling, but not LV remodeling, in CTEPH patients by using CMR.27 In the present study, we were able to demonstrate that BPA improves not only RV function but also LV function in CTEPH patients. The discrepancy in the effects of BPA on LV remodeling between the two studies could be explained by the facts that in the Fukui et al study, the hemodynamic improvement by BPA was not complete (mean PA pressure, from 39.4±7.6 to 27.3±8.5 mmHg, P<0.001) and the time from the final BPA session to CMR was short (4.0±0.8 months),27 whereas in the present study, hemodynamic improvement was complete (Table 2) and the duration between the final BPA session and CMR was relatively long (9.0±8.7 months).
Pressure overload and remodeling in the RV could cause LV dysfunction through ventricular interaction, ventricular interdependence, leftward septal shift and RV hypertrophy.18,28 Thus, biventricular dysfunctions usually occur in CTEPH patients.29,30 In the previous studies, however, the effects of PEA on LV function were not consistent. Iino et al found no significant change in LV function after PEA,25 whereas Hardziyenka et al noted reverse LV remodeling (increased LVEDVI and LVSVI) after the procedure.26 Galiè et al noted that two mechanisms are involved in the increase in LV size after effective medical treatment in pulmonary arterial hypertension (PAH): (1) increase in RVSV; and (2) decompression of the LV due to decreased RVEDV.28 In the present study, the extent of the improvement of RVSVI and RVEF were correlated to that of LVEDVI and the extent of the improvement of ISA was also correlated with that of LVEDVI and LVSVI. There was a significant correlation between changes in RVEF and that in LVEF, although LVEF was not significantly increased after BPA. These findings suggest that BPA reduces PAP and improves RV functions first, followed by resultant improvement of LV functions. Given that increase in LVEDV is a strong predictor of better survival in IPAH,18 it is highly possible that BPA may also improve the prognosis of inoperable CTEPH.10–12
BPA Improves Pulmonary Flow in CTEPHAnother novel finding of the present study is that BPA improves PA flow in inoperable CTEPH. Eysmann et al reported that AT, as measured on echocardiography, correlated with poor prognosis in PAH.31 Indeed, in PAH patients, peak and average velocity decrease and AT becomes shorter with the progression of the disorder.32,33 Furthermore, blood flow in the main PA forms a vortex in patients with PH,34 and we have previously reported on a CTEPH patient in whom the vortex flow was improved after BPA.35 After BPA, the increase in maximum peak velocity was also noted in the present study, as was the case in the PEA study.17,22 BPA, however, caused no change in AT/ET, which was different to the effect of PEA.22 This could be explained by the different location of organized thrombi between operable and inoperable CTEPH. Other explanations for the different results could be that PEA removes the artery lining with thrombi, while BPA only dilates thrombotic obstructions. Further study is needed to clarify the difference in the mechanisms involved between PEA and BPA.
Study LimitationsSeveral limitations should be mentioned for the present study. First, the present study was retrospective, and thus the present findings remain to be confirmed in future studies in a prospective manner. Second, given that CMR was not performed after optimal medication but before BPA, the present results include the effects of both optimal medication and BPA. But, given that the use of pulmonary vasodilators is usually reduced or even stopped in most CTEPH patients after BPA, the impact of pulmonary vasodilators might be smaller than that of BPA. Third, the number of patients was relatively small and 2-D phase contrast imaging was not acquired in any patients. Fourth, it has been reported that RV and LV remodeling are important prognostic determinants of PH.18 In the present study, all patients treated with BPA had good long-term prognosis, and none died. Thus, we were unable to examine the relationship between improvement of ventricular function and prognosis in CTEPH patients treated with BPA. Future studies with a large number of patients are therefore warranted.
BPA improves biventricular functions and pulmonary flow in patients with inoperable CTEPH.
We thank the radiology technologists at Tohoku University Hospital who carried out the CMR.
None.