2016 Volume 80 Issue 7 Pages 1583-1589
2016 Volume 80 Issue 7 Pages 1583-1589
Background: The functional significance of an intermediate coronary lesion is crucial for determining the treatment strategy, but age-related changes in cardiovascular function could affect the functional significance of an epicardial stenosis. The aim of this study was therefore to investigate the impact of age on fractional flow reserve (FFR) measurements in patients with intermediate coronary artery disease (CAD).
Methods and Results: Intracoronary pressure measurements and intravascular ultrasound (IVUS) were performed in 178 left anterior descending coronary arteries with intermediate stenosis. The morphological characteristics and FFR of 91 lesions in patients <65 years old were compared with those of 87 patients ≥65 years old. There was no difference in lesion location, diameter stenosis, minimum lumen area, plaque burden, or lesion length between the 2 age groups. Elderly patients had higher FFR (0.81±0.06 vs. 0.79±0.06, P=0.004) and lower ∆FFR, defined as the difference between resting Pd/Pa and FFR (0.13±0.05 vs. 0.15±0.05, P=0.014). Age, along with the location and degree of stenosis, was independently associated with FFR and ∆FFR (β=0.162, P=0.008; β=−0.131, P=0.043, respectively).
Conclusions: Elderly patients with intermediate CAD are more likely to have higher FFR and lower ∆FFR, despite a similar degree of epicardial stenosis, compared with younger patients. (Circ J 2016; 80: 1583–1589)
Arevascularization strategy based on the presence or absence of myocardial ischemia improves outcome by avoiding potential risks related to unnecessary procedures, even in the current era of advanced percutaneous coronary intervention (PCI) techniques and use of drug-eluting stents.1,2 Given that the majority of PCI patients are elderly, and generally have complex coronary artery disease (CAD) and more procedure-related complications, an ischemia-based strategy with objective evidence is relatively more important for this patient population.3–7
Editorial p 1527
Fractional flow reserve (FFR) is considered the most reliable method to determine the functional significance of coronary stenosis in the cardiac catheterization laboratory, and it provides useful guidance for determining treatment strategy in patients with CAD.1,2,8 There is a concern, however, that age-related changes in the vascular system and myocardium may be related to the causes of ischemia and the functional significance of coronary narrowing because impaired microvascular function and/or diastolic dysfunction might decrease the translesional pressure gradient and increase FFR.9 In addition, several conditions, such as diabetes, hypertension, diffuseness of coronary atherosclerotic lesions, and left ventricular (LV) hypertrophy, which are more prevalent in elderly patients, may affect vascular compensatory capacity and the functional relevance of epicardial stenosis.10–12 Therefore, assessing the impact of age on FFR is important for determining treatment in this patient group, particularly in cases of intermediate stenosis, in which adjunctive, invasive evaluations are proposed frequently and accurate measurements are critical. The aim of this study was therefore to investigate the impact of age on FFR measurements in patients with homogenous intermediate coronary stenosis, assessed using intravascular ultrasound (IVUS).
We consecutively enrolled 178 patients from the Ajou University Hospital coronary registry database, who underwent FFR and IVUS to evaluate de novo intermediate 40–70% diameter stenosis (DS) by visual estimation on diagnostic coronary angiography of the proximal or middle segment of the left anterior descending artery (LAD) between January 2010 and December 2013. Patients with 2 or more stenotic segments (>40% DS on visual estimate) in the same vessel, left main (LM) stenosis, LV ejection fraction <40%, previous myocardial infarction, presence of a collateral vessel, or contraindication to adenosine were excluded. The patients were stratified into older (≥65 years old) and younger (<65 years old) groups for age-specific analysis. This cross-sectional study was approved by the Institutional Review Board.
Quantitative Coronary Angiography (QCA)QCA was performed using the cardiovascular angiography analysis system II (CAAS II, pie Medical, Maastricht, the Netherlands). Minimum lumen diameter, reference vessel diameter (RVD), and %DS were measured. To evaluate the proportion of the myocardium perfused by the LAD, the Alberta Provincial Project for Outcome Assessment in Coronary Heart Disease (APPROACH) score, which is based on the division of the LV into regions in accordance with human pathologic studies, and with correlation with cardiac catheterization results of the LAD, was evaluated using the standard method previously described.13
IVUSIVUS was performed after intracoronary injection of 200 µg nitroglycerin using the Galaxy2TM System (Boston Scientific, Natick, MA, USA). A 40-MHz coronary imaging catheter (Atlantis SR Pro, Natick, MA, USA) was pulled back from the distal LAD through the target stenosis to the ostium of the LM coronary artery with a motorized pullback device at a speed of 0.5 mm/s. Quantitative IVUS was performed using planimetry software (EchoPlaque 3.0; Indec Systems, Santa Clara, CA, USA), according to the American College of Cardiology Clinical Expert Consensus Document on Standards for acquisition, measurement, and reporting of IVUS Studies.14 Minimum lumen area (MLA) and the external elastic membrane (EEM) were measured at the narrowest lumen cross-section of the target lesion. Plaque burden at the narrowest site was calculated as: [100×(EEM area−MLA)/EEM area]. Lesion length was defined as the region around the MLA where lumen area was <50% of the reference lumen area.14
FFRFFR was measured using a 0.014-in intracoronary pressure guidewire (St. Jude Medical, St. Paul, MN, USA) according to routine clinical practice. After equalizing was performed with the pressure sensor positioned at the guiding catheter tip, a pressure sensor was advanced distally to the index lesion. After recording baseline distal intracoronary pressure, maximum hyperemia was induced using a continuous intracoronary infusion of adenosine (>360 µg/min) via a microcatheter to induce reliable, sustained maximum hyperemia, as described previously.15 FFR was calculated as the ratio of distal coronary pressure (Pd) to aortic pressure (Pa) during maximum hyperemia. ∆FFR was calculated by subtracting FFR during maximum hyperemia from resting Pd/Pa.16 FFR ≤0.8 was considered functionally significant.
Statistical AnalysisData are expressed as mean±SD for continuous variables and as n (%) for categorical variables. Student’s t-test was used to compare continuous variables. Multivariate linear and binary logistic regression analyses were performed to identify the parameters independently associated with FFR, ∆FFR, and functional significance (FFR ≤0.80). All statistical analysis was carried out using SPSS ver. 22 for Windows (SPSS, Chicago, IL, USA). P<0.05 was considered significant.
Baseline clinical features are listed in Table 1. Mean age was 71±5 years in patients ≥65 years old and 55±6 years in patients <65 years old. Older patients were less likely to be male (54% vs. 75%, P=0.004), to be dyslipidemic (16% vs. 31%, P=0.021), and to smoke (19% vs. 42%, P=0.002), as compared with their younger counterparts. The prevalences of hypertension and diabetes were not different, and LV mass index was similar for the 2 age groups. Among all of the patients, mean APPROACH score of the LAD was 35.1±7.8, and there was no difference between younger and older patients (35.2±7.9 vs. 34.9±7.6, P=0.343; Table 1). The angiographic and IVUS characteristics were similar for the 2 age groups, with no differences in lesion location, RVD, %DS, lesion length, MLA, or plaque burden (Table 1).
| All (n=178) |
<65 years (n=91) |
≥65years (n=87) |
P-value† | |
|---|---|---|---|---|
| Age (years) | 63±10 | 55±6 | 71±5 | <0.001 |
| Sex (male) | 115 (65) | 68 (75) | 47 (54) | 0.004 |
| Hypertension | 107 (60) | 54 (59) | 53 (61) | 0.830 |
| Diabetes | 53 (30) | 28 (31) | 25 (29) | 0.767 |
| Hyperlipidemia | 42 (24) | 28 (31) | 14 (16) | 0.021 |
| Smoking | 54 (30) | 37 (42) | 17 (19) | 0.002 |
| Previous PCI or CABG | 6 (3) | 4 (4) | 2 (2) | 0.438 |
| Clinical presentation | 0.742 | |||
| Stable angina | 84 (47) | 42 (46) | 42 (48) | |
| Unstable angina | 74 (42) | 40 (44) | 34 (39) | |
| Others | 20 (11) | 9 (10) | 11 (13) | |
| LVMI (g/mm2) | 102.0±24.4 | 102.6±24.6 | 101.4±24.5 | 0.746 |
| Angiography | ||||
| Location of the stenosis | 0.747 | |||
| Proximal | 56 (32) | 30 (33) | 26 (30) | |
| Mid | 122 (68) | 60 (67) | 62 (70) | |
| Multivessel disease | 64 (36) | 32 (35) | 32 (37) | 0.822 |
| Reference vessel diameter (mm) | 3.2±0.2 | 3.2±0.2 | 3.2±0.2 | 0.614 |
| Minimum lumen diameter (mm) | 1.5±0.3 | 1.5±0.3 | 1.5±0.3 | 0.163 |
| Diameter stenosis (%) | 54.6±6.8 | 55.0±7.0 | 53.9±7.4 | 0.287 |
| Lesion length (mm) | 18.4±5.7 | 18.4±5.7 | 18.3±5.7 | 0.935 |
| APPROACH score of LAD | 35.1±7.8 | 35.2±7.9 | 34.9±7.6 | 0.343 |
| IVUS | ||||
| Minimum lumen area (mm2) | 2.94±0.84 | 2.97±0.82 | 2.90±0.87 | 0.601 |
| Plaque burden (%) | 71.7±8.8 | 72.1±9.0 | 71.4±8.7 | 0.614 |
| Lesion length (mm) | 19.8±6.3 | 20.1±6.2 | 19.6±6.4 | 0.642 |
Data given as mean±SD or n (%). †Older vs. younger. APPROACH, Alberta Provincial Project for Outcome Assessment in Coronary Heart Disease; CABG, coronary artery bypass grafting; IVUS, intravascular ultrasound; LAD, left anterior descending artery; LVMI, left ventricular mass index; PCI, percutaneous coronary intervention.
Age was positively correlated with FFR (r=0.202, P=0.007) and negatively correlated with ∆FFR (r=−0.168, P=0.025). Resting Pd/Pa, however, was not correlated with age (Figure 1A). No correlation was observed between age and MLA or plaque burden or lesion length on IVUS (Figure 1B). The FFR vs. age and MLA results are presented in Figure 2. FFR had positive and graded associations with MLA and age within every tertile of age and MLA, respectively.

Age vs. intracoronary pressure and intravascular ultrasound (IVUS) parameters. Age was positively correlated with fractional flow reserve (FFR) and negatively correlated with ∆FFR. No correlations were seen between age and resting ratio of mean blood pressure measured distal to the stenosis to mean aortic blood pressure (Pd/Pa), minimum lumen area (MLA) and plaque burden, or lesion length on IVUS.

Fractional flow reserve (FFR) stratified by age and minimum lumen area (MLA) tertiles.
Pa, Pd, resting Pd/Pa, and ∆Pa (Pa at baseline minus Pa during maximum hyperemia) remained similar for older and younger patients (Table 2; Figure 3). In contrast, younger patients had significantly lower Pd during hyperemia and higher ∆Pd (Pd at baseline minus Pd during hyperemia) than older patients. As a consequence, FFR was significantly higher with lower ∆FFR in older patients, as compared with younger patients (0.81±0.06 vs. 0.79±0.06, P=0.004; 0.13±0.05 vs. 0.15±0.05, P=0.014, respectively). In addition, the percent change between resting Pd/Pa and FFR was lower in older patients than in younger patients (13.6±5.4% vs. 15.7±5.2%, P=0.009; Table 2).
| All (n=178) |
<65 years (n=91) |
≥65years (n=87) |
P-value† | |
|---|---|---|---|---|
| Baseline Pa (mmHg) | 99.0±11.7 | 98.5±11.6 | 99.6±11.9 | 0.528 |
| Baseline Pd (mmHg) | 92.6±11.2 | 91.8±10.7 | 93.5±11.8 | 0.302 |
| Resting Pd/Pa | 0.94±0.03 | 0.93±0.03 | 0.94±0.02 | 0.115 |
| Pa at maximum hyperemia (mmHg) | 94.5±11.6 | 93.9±11.7 | 95.1±11.6 | 0.495 |
| Pd at maximum hyperemia (mmHg) | 75.5±10.2 | 74.1±9.9 | 77.4±10.7 | 0.032 |
| FFR | 0.80±0.06 | 0.79±0.06 | 0.81±0.06 | 0.004 |
| ΔPa | 4.5±4.3 | 4.6±4.2 | 4.5±4.4 | 0.905 |
| ΔPd | 17.1±5.9 | 17.9±5.9 | 16.2±5.9 | 0.052 |
| ΔFFR | 0.14±0.05 | 0.15±0.05 | 0.13±0.05 | 0.014 |
| % change in Pd/Pa (%) | 14.8±5.4 | 15.7±5.2 | 13.6±5.4 | 0.009 |
Data given as mean±SD. †Older vs. younger. ΔFFR, difference between resting Pd/Pa and FFR. FFR, fractional flow reserve; Pa, mean aortic blood pressure; Pd, mean blood pressure measured distal to the stenosis.
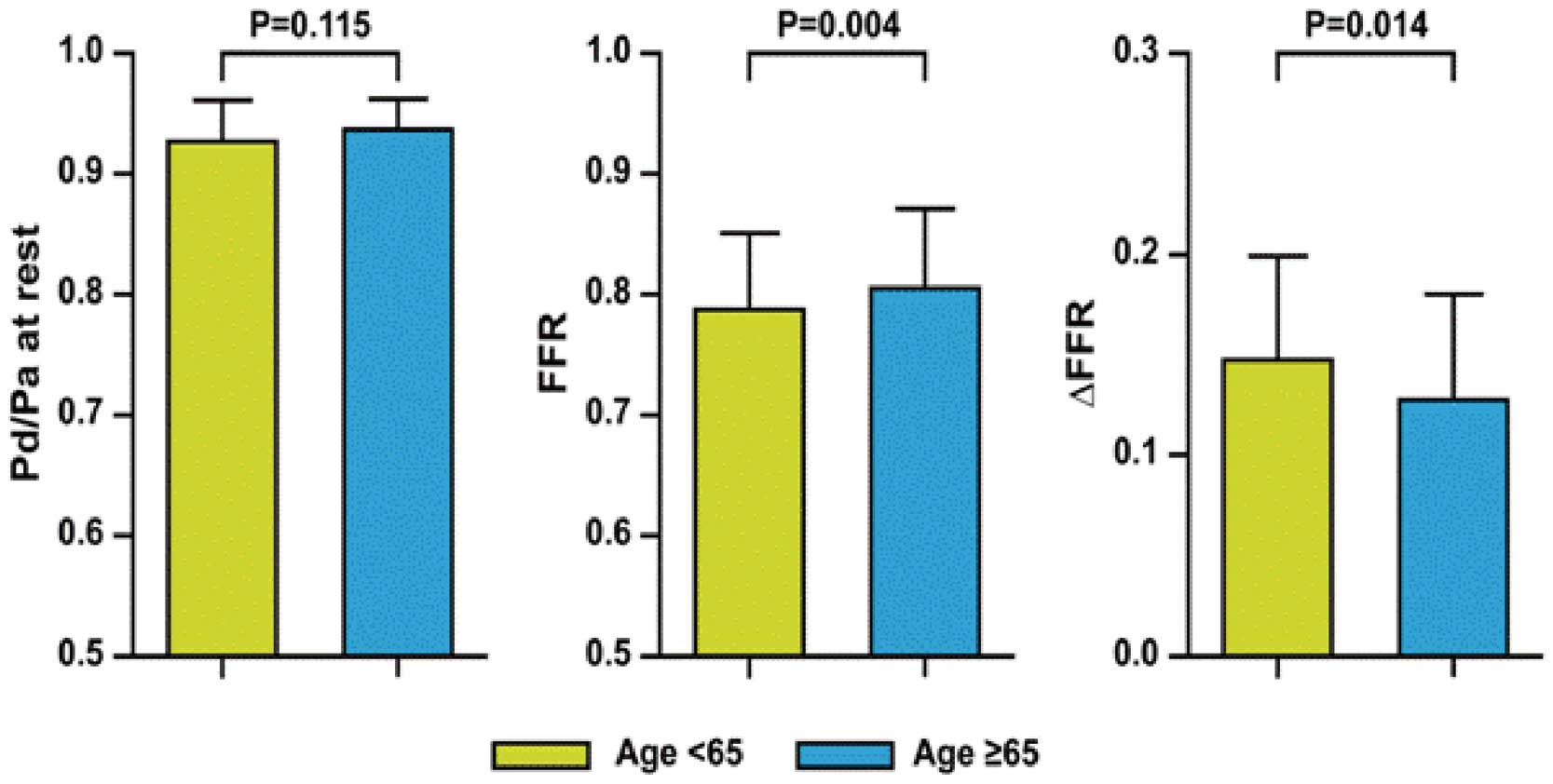
Ratio of mean blood pressure measured distal to the stenosis to mean aortic blood pressure (Pd/Pa) at rest and at maximum hyperemia, fractional flow reserve (FFR), and ∆FFR vs. age. FFR was higher with lower ∆FFR in patients ≥65 years. ∆FFR, difference between resting Pd/Pa and FFR.
On multivariate analysis, age was an independent predictor of FFR, ∆FFR, and functional significance (FFR ≤0.80; β=0.162, P=0.008; β=−0.131, P=0.043; OR, 0.955; P=0.015, respectively) along with MLA, %DS, lesion length, and lesion location. Female sex was also independently associated with FFR (Table 3).
| Variable | FFR | ΔFFR | FFR ≤0.80 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| β | B | P-value | 95% CI | β | B | P-value | 95% CI | OR | P-value | 95% CI | |
| Age | 0.162 | 0.001 | 0.008 | 0.000– 0.002 |
−0.131 | −0.001 | 0.043 | −0.001 to 0.000 |
0.955 | 0.015 | 0.920– 0.991 |
| Sex | 0.007 | 0.017 | 0.019 | 0.003– 0.032 |
– | – | – | – | – | – | – |
| Proximal lesion location |
−0.239 | −0.029 | <0.001 | −0.044 to −0.015 |
0.137 | 0.015 | 0.032 | 0.001– 0.028 |
2.739 | 0.019 | 1.177– 6.374 |
| % diameter stenosis |
−0.199 | −0.002 | 0.002 | −0.003 to −0.001 |
0.206 | 0.001 | 0.003 | 0.000– 0.002 |
1.096 | 0.005 | 1.028– 1.168 |
| Minimum lumen area |
0.383 | 0.026 | <0.001 | 0.017– 0.035 |
−0.393 | −0.024 | <0.001 | −0.032 to −0.015 |
0.358 | 0.001 | 0.200– 0.643 |
| Lesion length | −0.198 | −0.002 | 0.002 | −0.003 to −0.001 |
0.144 | 0.001 | 0.037 | 0.000– 0.002 |
1.095 | 0.009 | 1.023– 1.172 |
Multivariate analysis included: age, sex, diabetes, hypertension, dyslipidemia, smoking, LVMI, lesion location, minimum lumen diameter, %diameter stenosis, minimum lumen area, plaque burden, lesion length. ΔFFR, difference between resting Pd/Pa and FFR. Other abbreviations as in Tables 1,2.
In the present study involving patients evaluated using FFR and IVUS, age, lesion location, lesion length, and degree of stenosis were independently associated with FFR and ∆FFR. Elderly patients had higher FFR and lower ∆FFR, despite similar anatomical characteristics, compared with younger patients. The distinguishing feature of this study is that the impact of age on FFR was evaluated in the homogenously proximal or mid-LAD where the diagnostic accuracy of MLA for functional significance is the highest,17 and in intermediate lesions where anatomical characteristics were confirmed to be similar between the 2 age groups on IVUS.
Because the ultimate goal of coronary revascularization is to relieve myocardial ischemia, PCI for only ischemia-producing lesions is essential to improve outcome and avoid unnecessary procedures.1,2 An ischemia-based strategy with objective evidence is even more important in elderly patients because of the higher rates of adverse outcome and procedure-related complications in this population.4–7 Moreover, the majority of PCI are currently performed in older patients.3 FFR has been used successfully to determine the functional significance of intermediate coronary stenoses and to identify those with inducible ischemia.18,19 Based on the verified clinical usefulness, it is currently recommended in daily practice in East Asian patients as well.20–22
Given, however, that myocardial ischemia is not solely caused by epicardial narrowing, the contribution of epicardial stenosis to ischemic potential of the subtended myocardium may be affected by age-related changes in the vascular system and myocardium itself,9 and by other factors, such as diabetes, hypertension, hyperlipidemia, diffuseness of coronary artery stenosis, and LV hypertrophy that impair the maximum flow rate across a stenotic lesion.10–12,23
Aging is a well-known major risk factor for the pathogenesis of vascular disease, and it has profound effects on the structure and function of the vascular system. Both macrovascular and microvascular abnormalities increase with age.24 Age is also associated with impaired microcirculatory vasodilatation and altered endothelial function.25
In the present study, age was independently associated with FFR along with lesion location, %DS, MLA, and lesion length, which are obvious determinants of functional significance of a coronary lesion. Severity of stenosis itself was found to be a major determinant of FFR, but age affects the ischemic potential of stenosis in the epicardial artery and is manifested by higher FFR.
We also found that elderly patients were more likely to have lower ∆FFR, and that age was an independent determinant of ∆FFR. Kocaman et al reported that ∆FFR, which is the difference between resting Pd/Pa and FFR, reflects the microvascular compensatory function in response to epicardial coronary stenosis, particularly significant stenotic lesions.16 An inadequate drop in Pd/Pa related to impaired vasodilatation capacity in the microcirculation may appear as increased FFR, but ∆FFR is affected by not only microvascular dysfunction but also by several factors such as diffuse coronary artery disease, small vessel disease, vessel size, and hypertension, which could change the pressure distal to the lesion and become more prevalent with aging. Also, ∆FFR may represent endothelial function related to vascular response to a vasodilator as well. Aging may have impact on such factor(s) already noted, including microvascular resistance, and thus ∆FFR could be affected by these various factors related to age.
Even though ∆FFR is not a specific indicator of microvascular dysfunction, ∆FFR was useful to predict severity of coronary lesions and clinical outcome in the patients with gray zone FFR. Otherwise, ∆FFR has no value in patients with insignificant stenotic lesion (FFR >0.80).16 That is, the patients with FFR >0.8 would be more likely to have preserved vascular compensatory capacity, hence the added diagnostic value of ∆FFR to FFR seems to be limited, and clinical outcome is favorable regardless of ∆FFR in that case. In contrast, low ∆FFR in the case of FFR ≤0.8 may represent impaired ischemic compensatory capacity despite significant epicardial stenosis, which has been reported to be related to poor clinical outcome.16
It is possible that these age-related changes could lead to underestimation of lesion severity by falsely increasing FFR, but FFR provides exact information on the extent to which successful PCI would restore maximum myocardial blood flow, regardless of resistant vessel dysfunction, because it specifically represents the functional severity of epicardial narrowing. Thus, non-ischemic FFR does not mean the absence of myocardial ischemia related to a vessel, but indicates that no benefit to epicardial stenosis should be expected by PCI. This was confirmed by recent studies showing that age does not modify the usefulness of FFR-guided revascularization decision in patients with multi-vessel CAD,26 and that microvascular resistance assessed on index of microcirculatory resistance had no meaningful correlation with FFR in real-world clinical settings.27 As a result, as age increases, ∆FFR tends to decrease but the usefulness of FFR is maintained in evaluating functional significance of epicardial stenosis and determining PCI or medical treatment.
The present results suggest that coronary microvascular dysfunction needs to be actively evaluated and treated in elderly patients, particularly when there is anatomical-functional discordance. This strategy is supported by a study reporting a 21% incidence of microvascular dysfunction in patients without obstructive coronary disease and a direct correlation between age and microvascular dysfunction.28 Therefore, evaluation of coronary disease in older patients using anatomy alone would lead to an incomplete picture.
In addition, the current recommended FFR cut-off ≤0.8 for determining the need for revascularization should not be modified solely based on the present findings. Although the usefulness of revascularization for a coronary lesion with gray zone FFR is controversial,20,29,30 a FAME trial age-specific subanalysis confirmed the benefit of the current cut-off FFR ≤0.8 to guide revascularization decisions, regardless of age.26
Study LimitationsThe present study has several limitations. First, the cross-sectional nature of the IVUS parameters means that we could not completely account for other lesion characteristics, such as diffuseness and eccentricity, which may affect FFR. This is consistent, however, with contemporary practice that uses %DS for visual or QCA assessment of lesion severity and MLA or plaque burden for the IVUS assessment of lesion severity. Second, if the invasive measurements of microvascular function (ie, index of microcirculatory resistance, coronary flow reserve) were available, we could provide more informative findings on the role of microcirculatory dysfunction in the discordance between anatomy and FFR. Third, the cut-off age of 65 years was arbitrary, but we believe it is reasonable, given that the majority of PCI occur in patients ≥65 years old.3 This value is very close to the mean and median age of 63 years in the present study.
It is important to use a more comprehensive physiologic assessment, including integration of macro- and microvascular measurements to determine the need for PCI, as opposed to QCA or IVUS, particularly in older patients because they are more likely to have higher FFR and lower ∆FFR, presumably because of age-related changes in cardiovascular function, despite a similar degree of epicardial stenosis in those with intermediate CAD, compared with younger patients.
This research was supported by Ajou University School of Medicine (M-2015-C0460-00109), Suwon, Republic of Korea.
W.F.F. received research support from St. Jude Medical. The other authors declare no conflicts of interest.