2016 Volume 80 Issue 8 Pages 1787-1794
2016 Volume 80 Issue 8 Pages 1787-1794
Background: Recent studies have shown that visit-to-visit blood pressure variability (BPV) is an independent risk factor for cardiovascular disease. However, it has not been clarified whether obstructive sleep apnea (OSA) is associated with visit-to-visit BPV.
Methods and Results: The 56 subjects with OSA and 26 control subjects without OSA were examined. Office BP was measured on 5 separate consecutive occasions prior to a polysomnography examination. The visit-to-visit BPV was expressed as the standard deviation and the coefficient of variation of the 5 systolic BP measurements. In subjects with an apnea-hypopnea index (AHI) of more than 20 episodes per hour, the visit-to-visit BPV was also measured after the start of continuous positive airway pressure (CPAP) therapy. Overall, the AHI positively correlated with the standard deviation and the coefficient of variation of systolic BP. In a multivariate analysis, the plasma noradrenaline level and the AHI were independently and positively correlated with the standard deviation and the coefficient of variation of the systolic BP. Among the patients who underwent CPAP therapy, good adherence with CPAP therapy significantly reduced the visit-to-visit BPV.
Conclusions: OSA is associated with abnormal visit-to-visit BPV and sympathetic activation seems to be related in some way. (Circ J 2016; 80: 1787–1794)
Hypertension is acknowledged as one of the greatest and established risk factors for cardiovascular disease. However, treatment and control rates of hypertension are unacceptable.1 Blood pressure variability (BPV) is assessed at different time intervals, namely, very short-term interval (beat-to-beat), short-term interval (24 h), or long-term interval (day-by-day or visit-to-visit). All BPV abnormalities are reportedly associated with progression of cardiovascular damage or cardiovascular events.2–7 To date, the underlying mechanisms of BPV abnormalities have not been fully clarified. Even so, several factors, such as clinical characteristics (ie, age, sex, and obesity),6,8,9 pathophysiological abnormalities (ie, diabetes mellitus, chronic kidney disease, and increased arterial stiffness),10–13 and antihypertensive medication (ie, calcium-channel blockers [CCBs])14 are known to be associated with the visit-to-visit BPV (vvBPV).
Obstructive sleep apnea (OSA) is a risk factor for atherosclerosis, coronary artery disease and stroke,15–19 and is associated with short-term BPV abnormalities.20,21 However, to the best of our knowledge, it has not been fully clarified whether OSA is associated with vvBPV. To clarify this association, the present study was conducted in subjects who had visited our sleep clinic for an assessment of their sleep disorders. The office BP of each subject was measured on 5 separate consecutive occasions to obtain the vvBPV prior to a sleep disorder assessment. For subjects treated with continuous positive airway pressure (CPAP) therapy, their BP was also measured on 5 separate consecutive occasions after the start of CPAP therapy. The plasma noradrenaline (NAD) levels of the patients were also measured, because OSA activates the sympathetic nervous system.22,23 We further evaluated whether vvBPV was associated with OSA severity and the plasma NAD level independently of factors known to be associated with the vvBPV.6,8–14 We also examined the effects of CPAP therapy on the vvBPV.
Among the outpatients followed for the management of risk factors for cardiovascular disease at the Department of Internal Medicine at Tokyo Medical University Hospital, 455 who were suspected of having OSA based on clinical findings were referred to the Sleep Laboratory at the Hospital between December 2004 and September 2011. The patients were analyzed using polysomnography (PSG) (Figure 1). The interval between each of the BP measurements performed at each visit to the Hospital before PSG and after CPAP therapy (if performed) was 1 month (Figure 2). Subjects with hypertension were already taking antihypertensive medication prior to the 5 consecutive visits for BP measurement.
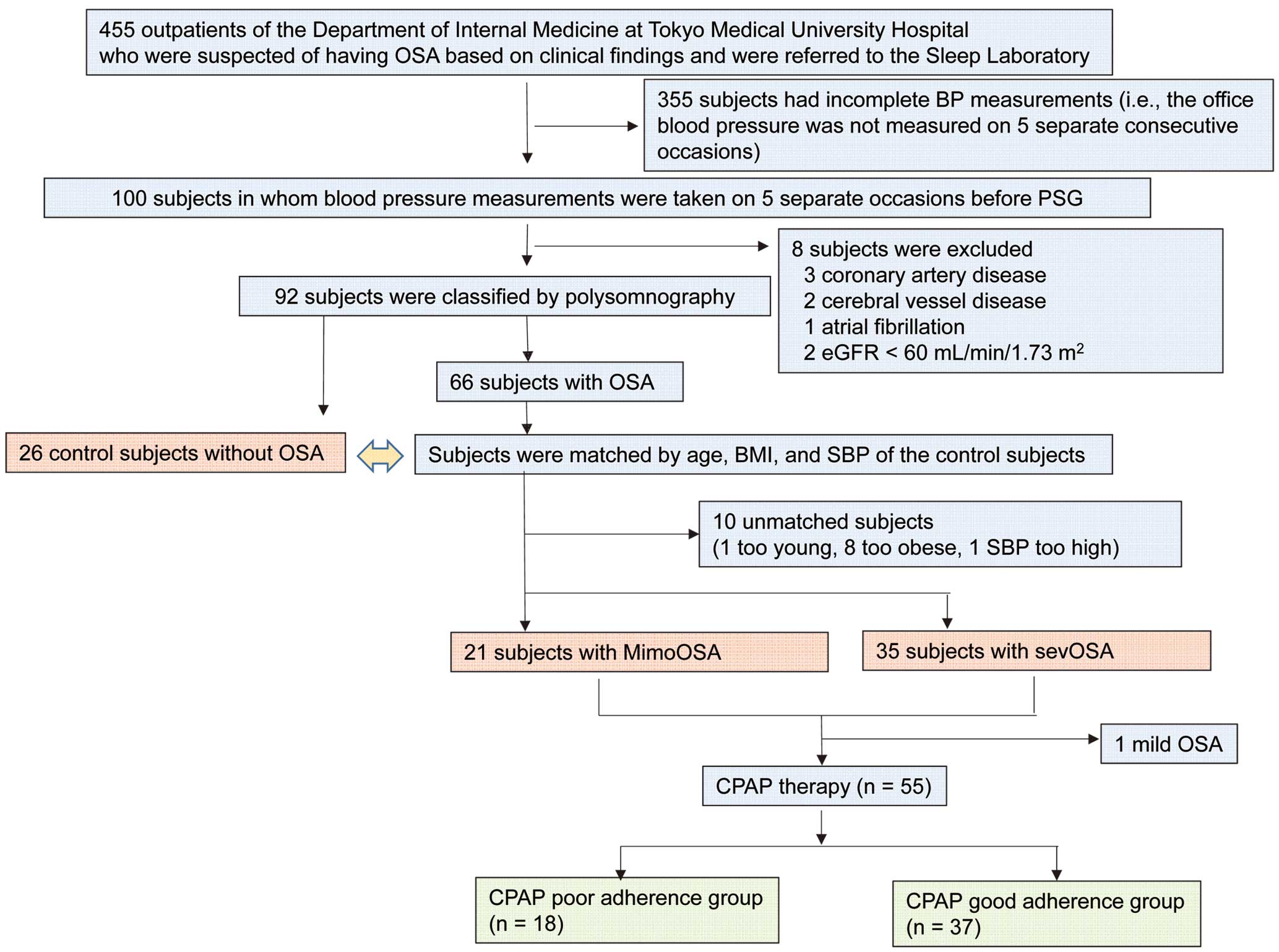
Flow chart of study subjects selection. BMI, body mass index; BP, blood pressure; CPAP, continuous positive airway pressure; eGFR, estimated serum creatinine-based glomerular filtration rate; MimoOSA, subjects with mild-to-moderate OSA; OSA, obstructive sleep apnea; PSG, polysomnography; SBP, systolic blood pressure; sevOSA, subjects with severe OSA.
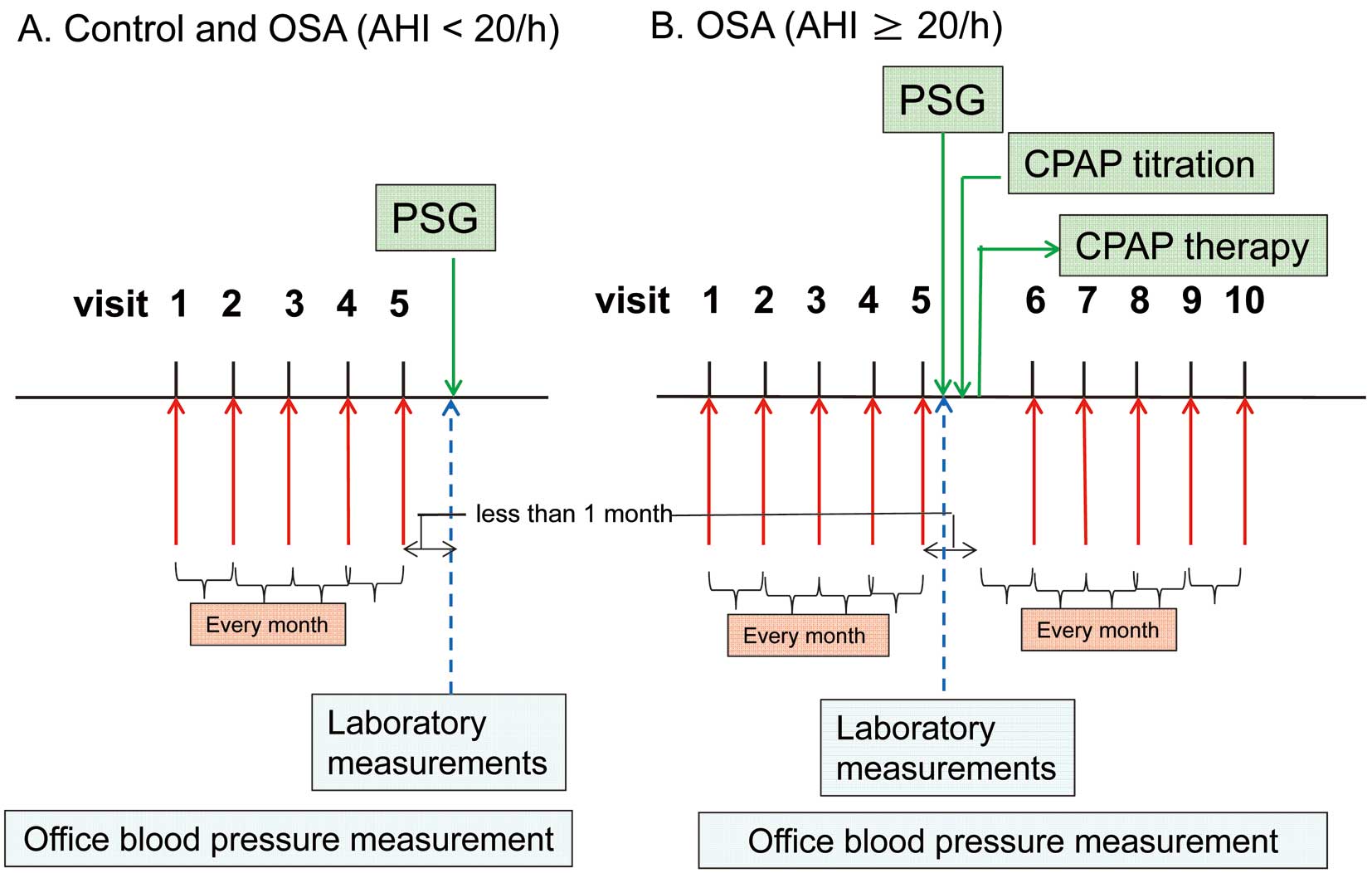
(A,B) Schematic diagram of the study protocol: BP measurements were obtained on at least 5 separate consecutive occasions both prior to the PSG and after CPAP therapy (if performed) at 1-month intervals. AHI, apnea-hypopnea index; CPAP, continuous positive airway pressure; OSA, obstructive sleep apnea; PSG, polysomnography.
The subject inclusion criteria were: (1) BP measurement on at least 5 separate consecutive occasions both prior to the PSG and after CPAP therapy (if performed), at 1-month intervals (Figure 2); and (2) antihypertensive medication unchanged at any time during the study period (as confirmed by the medical records). In addition, the subjects were also confirmed not to be taking any drugs other than those prescribed for their sleep and that might have affected their sleep (ie, steroids, interferon, anti-Parkinson disease drugs, anti-allergy drugs, non-steroidal anti-inflammatory drugs, or opioids),24 based on their medical records. The exclusion criterion was the presence of any disease associated with BP variability,8,11,14,25,26 which were defined as follows: (1) coronary artery disease (ie, a history of prior myocardial infarction, prior percutaneous coronary intervention, or prior coronary artery bypass grafting) and stroke, which were based on both clinical findings and brain magnetic resonance imaging and confirmed by the medical records; (2) atrial fibrillation, based on the results of ECG; and (3) estimated serum creatinine-based glomerular filtration rate (eGFR) <60 ml/min/1.73 m2.
Based on the inclusion criteria, we initially included 100 subjects for whom BP measurements had been obtained on at least 5 separate consecutive occasions prior to the PSG (ie, BP measurements from 5 separate consecutive occasions were not available for 355 patients) (Figure 1). Based on the exclusion criterion, a total of 92 subjects were enrolled before case matching (Figure 1).
The study was conducted with the approval of the Ethics Committee of Tokyo Medical University. Written informed consent was given by each subject before enrollment in the study.
Case Matching (Figure 1)Based on the results of the PSG, 26 subjects were diagnosed as not having OSA (apnea-hypopnea index (AHI) <5 episodes/h) and 66 subjects were diagnosed as having OSA (AHI ≥5 episodes/h). The 26 subjects without OSA were included in this study as control subjects (Figure 1). Case matching was conducted using a nested case-control design. Because the number of subjects without OSA (control subjects) was relatively small (n=26), the case-matching procedure was conducted for the subjects with OSA based on the clinical characteristics of the control subjects. Among the 66 subjects with OSA, the study subjects were selected according to matching for age (mean±2 SD: range, 29–81 years old), body mass index (BMI) (mean±2 SD: range, 19–31 kg/m2), and systolic BP (SBP) (mean±2 SD: range, 114–154 mmHg). A total of 10 subjects were not matched (1 subject was too young [27 years], 8 subjects were obese [BMI=32–36 kg/m2], and 1 subject’s BP was too high [SBP=158 mmHg]). Finally, 26 control subjects without OSA, 21 subjects with mild-to-moderate OSA (MimoOSA) (5≤AHI<30 episodes/h) and 35 subjects with severe OSA (SevOSA) (AHI ≥30 episodes/h) were included in this study (Figure 1).
BP Measurements and Assessment of BPVPhysicians measured the office BP of each subject using an automated oscillometric device while the subject was seated and had rested for more than 5 min. The equipment (Omron HEM-907; OMRON Healthcare, Kyoto, Japan), measurement time (1–3 h after taking antihypertensive medication in the morning), room temperature (controlled at ≈24–26℃), and food intake/drinking (≥60 min of fasting) were the same for each of the measurements. As mentioned for the inclusion criteria, all BP measurements performed at each visit, both before the PSG and after CPAP therapy (for subjects with an AHI ≥20 episodes/h), were obtained at 1-month intervals (Figure 2). The duration from the 5th visit until the PSG (Figure 2A) and, in OSA subjects (AHI ≥20/h), the duration from the 5th visit until the start of CPAP therapy were both less than 1 month (Figure 2B). The office BP was measured once at every visit. The BP indices and vvBPV before the PSG and after CPAP therapy were calculated as follows. The BP values measured on 5 visits were used to determine the mean SBP, the mean diastolic BP (DBP), the maximum SBP, and the vvBPV of the SBP; the mean SBP and DBP were defined as the averages of the SBP and the DBP values measured on each of the 5 visits; the maximum SBP was defined as the highest SBP value among the BP measurements obtained on 5 separate consecutive occasions; the vvBPV of the SBP was defined using the standard deviation (SD) and the coefficient of variation (CV, defined as SD/mean SBP).
Sleep StudyFully attended PSG monitoring was performed overnight in the Sleep Laboratory using the Alice 4 Sleep SystemTM (Respironics, Inc, Murrysville, PA, USA). Apnea, hypopnea, sleep stages, and electroencephalographic arousal were scored according to standard criteria.27 The AHI was defined as the number of apnea-hypopnea episodes per hour and calculated as the total number of apnea-hypopnea episodes per hour of sleep. Patients were diagnosed as having OSA when an obstructive component accounted for more than 50% of the events and the AHI was ≥5/h. OSA severity was classified according to the criteria of the American Academy of Sleep Medicine (ie, AHI of 5–14 episodes/h=mild; 15–29 episodes/h=moderate; ≥30 episodes/h=severe).27
CPAP TitrationAfter the diagnostic PSG, OSA subjects with an AHI ≥20 episodes/h were brought back repeatedly for additional overnight CPAP titration studies (REMStar Auto) performed at the Sleep Laboratory. The CPAP level was increased until respiratory events, snoring, and oxygen desaturation were eliminated during all stages of sleep in the supine position. The optimal pressure was determined by 2 expert researchers based on visual evaluation of the raw data from the night studies. Thereafter, subjects requiring CPAP therapy were followed monthly in the Outpatient Department of Tokyo Medical University Hospital.
Each patient visited the outpatient clinic monthly and was encouraged to use the CPAP device every night. At each outpatient visit, the average CPAP usage time and the AHI under CPAP treatment were calculated using the software package included with the CPAP device (Encore Pro; Respironics, Inc). Good adherence to CPAP was defined as meeting the following criteria: mean usage time of the CPAP ≥4 h/night, and adherence rate ≥0.70 regarding CPAP usage at ≥4 h/night in the previous 1 month.28
Brachial-Ankle Pulse Wave Velocity (baPWV) MeasurementbaPWV was measured using a volume-plethysmographic apparatus (Form/ABI; Colin Co, Ltd, Komaki, Japan), as previously described.29 Briefly, occlusion cuffs that were connected to both plethysmographic and oscillometric sensors were attached to both the upper arms and the ankles of each subject while the subject was lying supine. The brachial and post-tibial arterial pressures were then measured using the oscillometric sensors. The measurements were conducted after the subjects had rested for at least 5 min while supine in a temperature-controlled room (24–26℃) designated exclusively for this purpose.
ElectrocardiographyElectrocardiographic left ventricular hypertrophy was assessed by 12-lead ECG and applying the Sokolow-Lyon voltage (RV5+SV1) criteria.30
Laboratory MeasurementsThe serum levels of total cholesterol, high-density lipoprotein cholesterol, triglycerides, creatinine, glycohemoglobin A1c (HbA1c) and the fasting plasma glucose level were measured enzymatically using blood samples obtained from the subjects after an overnight fast. BP was not measured on the same day. Blood samples were drawn from the antecubital vein. The plasma NAD level was measured using high-performance liquid chromatography31 (HPLC kit: CA-testTM; TOSOH Laboratories, Tokyo, Japan). The eGFR was calculated using the Modification of Diet in Renal Disease equation for Japanese subjects:32 eGFR=194×CRN–1.094 ×age–0.287 (×0.739 for women). Diabetes mellitus was defined as a HbA1c level ≥6.5% or the use of antidiabetic medication.
Statistical AnalysisAll the data are expressed as the mean±SD, and categorical variables are expressed as proportions. Continuous variables that did not show a normal distribution are expressed as the median value (25–75th percentile range). The χ2 test was used for group comparisons of categorical variables. Continuous variables were compared using analysis of variance or the Mann-Whitney test, as appropriate. Bonferroni corrections were used for multiple pairwise comparisons of proportions or means among groups. Linear relationships between key variables were determined using multicollinearity analysis. Variables with non-normal distribution were transformed logarithmically before multiple logistic regression analysis to evaluate clinical variables that were significantly associated with vvBPV. To assess the significance of the correlations between vvBPV, AHI, plasma NAD and variables (age, sex, BMI, diabetes mellitus, and baPWV) that were previously shown to be associated with vvBPV,6,8–10,12 univariate linear regression analyses were performed. Next, significant variables in the univariate analyses were entered into a multivariate linear regression analysis. The intragroup differences before and after CPAP therapy were evaluated using paired t-tests, and intergroup comparisons of the change in BP indices were assessed using an unpaired t-test. P<0.05 was considered to indicate a statistically significant difference. Statistical analysis was performed using the SPSS software package (J22.0, SPSS Inc, Chicago, IL, USA).
The characteristics of the subjects (control subjects, MimoOSA and SevOSA) are shown in Table 1. Among these study subjects, 8 subjects had electrocardiographic left ventricular hypertrophy, but none of the study subjects had marked proteinuria (urine dipstick proteinuria ≥2+). Age, sex, BMI, SBP, DBP, heart rate, dosage of antihypertensive drugs, and baPWV did not differ significantly among the 3 groups. The plasma NAD level was significantly higher in the SevOSA group than in the control group or the MimoOSA group. The SD and CV of the SBP measurements were significantly higher in the SevOSA group than in the MimoOSA group or the controls (Figure 3).
| Control (AHI <5.0/h) |
MimoOSA (5.0≤AHI<30/h) |
SevOSA (AHI ≥30/h) |
|
|---|---|---|---|
| Mean AHI (n/h) | 4±1 | 25±5 | 59±18 |
| Variable | n=26 | n=21 | n=35 |
| Age (years) | 55±13 | 56±11 | 56±9 |
| Sex (M/F) | 22/4 | 17/4 | 32/3 |
| BMI (kg/m2) | 25±3 | 27±4 | 27±3 |
| Diabetes mellitus (%) | 23 | 20 | 26 |
| Smoking (%) | 36 | 35 | 34 |
| Mean SBP (mmHg) | 134±10 | 135±10 | 136±11 |
| Mean DBP (mmHg) | 77±10 | 87±10 | 85±9 |
| Heart rate (beats/min) | 77±10 | 76±10 | 81±13 |
| LDL-C (mg/dl) | 123±43 | 119±33 | 122±26 |
| TG (mg/dl) | 148±75 | 146±84 | 173±131 |
| HbA1c (%) | 5.8±0.5 | 5.9±0.6 | 6.1±0.8 |
| CRN (mg/dl) | 0.8±0.3 | 0.8±0.2 | 0.8±0.2 |
| eGFR (ml/min/1.73 m2) | 101±34 | 111±38 | 110±34 |
| CRP (mg/dl) | 0.3±0.8 | 0.1±0.1 | 0.2±0.4 |
| ESS | 9±4 | 9±4 | 8±4 |
| Plasma NAD (pg/ml) | 208 (176–283) | 237 (186–292) | 317 (243–402)*,† |
| Antihypertensive drugs | 0.9±0.9 | 1.1±1.0 | 1.3±1.0 |
| CCB (%) | 33 | 38 | 53 |
| ACEI/ARB (%) | 39 | 38 | 55† |
| β-blocker (%) | 15 | 19 | 12 |
| Diuretics (%) | 8 | 14 | 13 |
| Statin (%) | 35 | 38 | 23 |
| Antidiabetic drugs (%) | 20 | 14 | 14 |
| Sedatives (%) | 4 | 5 | 6 |
| Sleep study | |||
| Mean SpO2 (%) | 97±1 | 95±1* | 93±2*,† |
| Min. SpO2 (%) | 91±4 | 81±6* | 71±12*,† |
| Time SpO2 <90% (%) | 0.1±0.2 | 2±2 | 13±13*,† |
| CTR (%) | 48±5 | 47±3 | 47±13 |
| LVH on ECG (%) | 8 | 10 | 11 |
| baPWV (cm/s) | 1,565±322 | 1,518±316 | 1,459±263 |
Data are mean±standard deviation, median (25–75th percentiles), or number (%) of subjects. *P<0.05 vs. Control; †P<0.05 vs. MimoOSA. ACEI, angiotensin-converting enzyme inhibitor; AHI, apnea-hypopnea index; ARB, angiotensin II receptor blocker; baPWV, brachial-ankle pulse wave velocity; BMI, body mass index; CCB, calcium-channel blocker; CRN, serum creatinine concentration; CRP, serum C-reactive protein; CTR, cardiothoracic ratio; DBP, diastolic blood pressure; eGFR, estimated serum creatinine-based glomerular filtration rate; ESS, Epworth sleepiness scale; FPG, fasting plasma glucose; HbA1c, glycohemoglobin A1c; LDL-C, serum low-density lipoprotein cholesterol; LVH, left ventricular hypertrophy; NAD, noradrenaline; SBP, systolic blood pressure; SpO2, blood oxyhemoglobin saturation; mean SpO2, mean nocturnal oxyhemoglobin saturation; min. SpO2, minimal nocturnal oxyhemoglobin saturation; time SpO2 <90%, recording time spent at nocturnal oxyhemoglobin saturation <90%; TG, serum triglycerides.
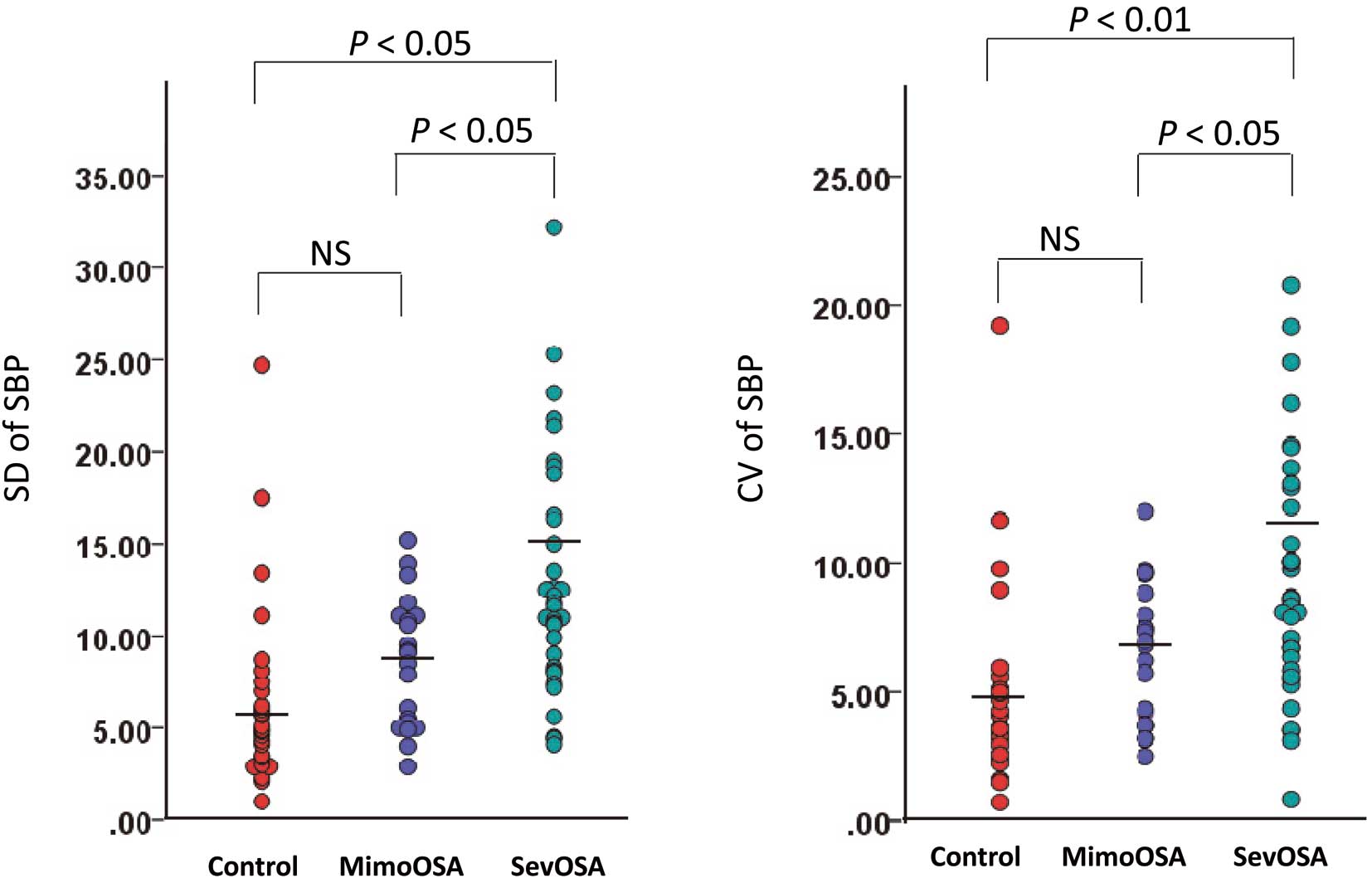
Standard deviations and coefficients of variation of the systolic blood pressure (SBP) measurements in the 3 subject groups. The horizontal lines indicate the mean value. CV, coefficient of variation; MimoOSA, subjects with mild-to-moderate OSA; NS, not significant; OSA, obstructive sleep apnea; SD, standard deviation; sevOSA, subjects with severe OSA.
To assess the significance of the association between the vvBPV and other variables, the AHI, plasma NAD level, and other variables that have already been reported to be significantly associated with vvBPV (ie, age, sex, BMI, diabetes mellitus, CCB use, and PWV)6,8–12,14 were included in the univariate linear regression analyses. As shown in Table 2, the plasma NAD level, AHI, and CCB use were significantly associated with the SD and CV of the SBP. Multivariate linear regression analysis demonstrated that the AHI and the logarithm (log) plasma NAD level significantly correlated with the SD and CV of the SBP (Table 2). In the multicollinearity analysis, the variance inflation factor of CCB, log plasma NAD and AHI was ≤2.0.
| A | |||||||
|---|---|---|---|---|---|---|---|
| Variable | Univariate | Multivariate (R-square=0.505) | |||||
| β | SE | P value | β | SE | P value | VIF | |
| Age (years) | 0.103 | 0.061 | 0.094 | – | – | – | |
| Sex (male) | −1.819 | 2.064 | 0.380 | – | – | – | |
| BMI (kg/m2) | 0.360 | 0.181 | 0.050 | – | – | – | |
| Diabetes mellitus | 2.329 | 1.683 | 0.170 | – | – | – | |
| CCB use | 3.051 | 1.417 | 0.034 | −0.022 | 1.177 | 0.821 | 1.125 |
| baPWV (cm/s) | −0.002 | 0.002 | 0.515 | – | – | – | |
| Log plasma NAD (pg/ml) | 0.523 | 4.023 | <0.0001 | 0.208 | 4.032 | 0.048 | 1.332 |
| AHI (n/h) | 0.120 | 0.023 | <0.0001 | 0.592 | 0.027 | <0.0001 | 1.400 |
| B | |||||||
| Variable | Univariate | Multivariate (R-square=0.478) | |||||
| β | SE | P value | β | SE | P value | VIF | |
| Age (years) | 0.068 | 0.043 | 0.114 | – | – | – | |
| Sex (male) | −1.460 | 1.438 | 0.313 | – | – | – | |
| BMI (kg/m2) | 0.239 | 0.127 | 0.063 | – | – | – | |
| Diabetes mellitus | 1.533 | 1.228 | 0.216 | – | – | – | |
| CCB use | 2.112 | 0.985 | 0.035 | −0.044 | 0.829 | 0.657 | 1.104 |
| baPWV (cm/s) | −0.002 | 0.002 | 0.266 | – | – | – | |
| Log plasma NAD (pg/ml) | 0.532 | 2.853 | <0.0001 | 0.268 | 2.911 | 0.018 | 1.411 |
| AHI (n/h) | 0.008 | 0.016 | <0.0001 | 0.524 | 0.020 | <0.0001 | 1.439 |
CV, coefficient of variation; log, logarithm; SD, standard deviation; VIF, variance inflation factor. Other abbreviations as in Table 1.
Based on the results of the PSG (AHI ≥20 episodes/h), 55 subjects were assigned to CPAP therapy. The average use of CPAP was 5.5±2.8 h per night, and the subjects were divided into CPAP groups with good or poor adherence. In the former group (n=37), CPAP significantly decreased the SBP, DBP, maximum SBP, SD of the SBP, and CV of the SBP (Table 3). No significant changes in these variables were observed in the latter group (n=18) (Table 3). In addition, there was a significant difference between the CPAP group with good adherence and the group with poor adherence regarding the changes in SBP, maximum SBP, SD of the SBP, and CV of the SBP (Table 3).
| CPAP poor adherence group (n=18) | CPAP good adherence group (n=37) | |||||
|---|---|---|---|---|---|---|
| Baseline | After CPAP | P value | Baseline | After CPAP | P value | |
| Intragroup difference | ||||||
| Mean SBP | 135±8 | 135±9 | 0.946 | 138±13 | 128±10 | <0.05 |
| Mean DBP | 86±9 | 84±9 | 0.399 | 85±9 | 79±10 | <0.05 |
| Max. SBP | 148±14 | 148±14 | 1.000 | 153±20 | 133±11 | <0.05 |
| SD of SBP | 12±5 | 9±6 | 0.589 | 12±6 | 6±5 | <0.05 |
| CV of SBP | 8±4 | 7±5 | 0.599 | 8±4 | 4±2 | <0.05 |
| Intergroup difference | ||||||
| ΔSBP | −0.2±10.3 | −9.8±15.1 | <0.05 | |||
| ΔDBP | −2.0±9.8 | −5.0±9.6 | 0.283 | |||
| ΔMaxSBP | −0.0±20.1 | −19.7±19.7 | <0.05 | |||
| ΔSD of SBP | −3.0±8.4 | −6.8±6.4 | <0.05 | |||
| ΔCV of SBP | −0.8±6.3 | −4.3±4.7 | <0.05 | |||
Mean SBP and mean DBP defined as the averages of the SBP and DBP values measured on each of 5 visits; maximum SBP defined as the highest SBP value among the BP measurements obtained on 5 separate consecutive occasions; vvBPV of the SBP using the SD and the CV (defined as SD/mean SBP); Δ, value after CPAP minus the value before CPAP. CPAP, continuous positive airway pressure; vvBPV, visit-to-visit blood pressure variability. Other abbreviations as in Tables 1,2.
The present study attempted to clarify the association between OSA and vvBPV abnormalities. Sleep abnormalities (ie, insomnia and abnormal sleep duration) have been shown to be associated with BPV abnormalities.33 Among sleep disorders, OSA is significantly associated with future cardiovascular events.15–17 Steinhorst et al demonstrated that OSA increases nighttime BPV, as assessed using 24-h ambulatory BP monitoring.21 CPAP therapy is an established method for OSA treatment. Long-term CPAP therapy reduces BP34 and is highly effective in patients with good CPAP compliance.35 However, to the best of our knowledge, the association between OSA and vvBPV abnormalities and the effect of CPAP therapy on vvBPV have not previously been examined.
Impaired arterial compliance decreases baroreceptor sensitivity because of the impairment of carotid arterial wall distension.36 This decrease augments BP fluctuations. Mancia et al reported that carotid arterial remodeling is associated with vvBPV abnormalities.37 Renal dysfunction induces fluid/sodium retention, which increases nighttime BP. Nakano et al reported that eGFR is associated with vvBPV abnormalities in patients with chronic kidney disease.11 In the present study, factors known to be associated with vvBPV (age, sex, BMI, diabetes mellitus, and CCB) and PWV were added as covariates for analysis.6,8–12,14 In addition, subjects with eGFR <60 ml/min/1.73 m2 were excluded. The present study demonstrated 2 important findings, as follows: (1) AHI showed a significant correlation with the SD and the CV of visit-to-visit SBP before CPAP therapy, independently of the factors associated with vvBPV; and (2) an improvement in vvBPV abnormalities was observed in the CPAP group with good adherence, but not in the CPAP group with poor adherence. Despite this investigation being a cross-sectional analysis and a retrospective study, the present work is the first study, to our knowledge, to suggest a significant association between OSA and vvBPV abnormalities.
Several studies have demonstrated that sympathetic activation affects BPV abnormalities.38,39 In subjects with OSA, intermittent hypoxemia is one of the major factors that activate sympathetic activity,22,23 which in turn elevates BP.40 In this study, the AHI and the plasma NAD level significantly correlated with the SD and CV of the SBP. Many factors (ie, emotional factors, sleep condition, and environment such as room temperature) affect visit-to-visit fluctuations of BP, and neural BP regulation counteracts such BP fluctuation.41 The autonomic nervous system affects baroreceptor regulation of BP,42,43 and therefore, the autonomic imbalance related with increased plasma NAD level might attenuate the counteracting effect of neural BP regulation.
The clinical implications of this study are as follows. (1) To our knowledge, this study is the first to report that OSA is associated with vvBPV abnormalities. High vvBPV has been reported in subjects with a history of stroke or a history of myocardial infarction.6,8,24,25 On the other hand, OSA is associated with coronary artery disease and stroke.15–17 The presence of OSA should be carefully taken into account in patients with vvBPV abnormalities. (2) Previous studies have reported that OSA-related hypertension is characterized by resistant hypertension and morning hypertension, and the present study suggests that the prevalence of vvBPV abnormalities should be taken into account in subjects with OSA.
Study LimitationsThis study was a small, retrospective cohort study, so although an association between vvBPV and OSA was identified, we cannot speculate on the causality of the association. Furthermore, because of the weakness and inconsistency of the association between NAD and vvBPV, whether sympathetic activation contributes to vvBPV is highly speculative. We failed to examine adherence to antihypertensive treatment. In addition, office BP was measured only once at each visit. Because the subjects were recruited from a cross-sectional study cohort, we did not measure the plasma NAD levels after the start of CPAP therapy. In the MimoOSA group, 55 of the 56 patients were assigned to CPAP therapy, and only 1 patient had an AHI <20 episodes/h (7.2/h). Thus, the MimoOSA group was predominantly composed of subjects with moderate OSA. We did not take into account the effects of the menstruation cycle; however, women in this study were all older than 50 years of age and were probably postmenopausal.
In conclusion, a significant independent correlation was observed between AHI and the SD and CV of the office SBP. CPAP therapy reduced the SD and CV of the office SBP, and these reductions were prominent in patients with good CPAP adherence. Therefore, OSA should be carefully taken into account in patients with vvBPV abnormalities. Sympathetic activation seems to be related to OSA-associated abnormal BPV in some way. However, further studies with prospective design and large sample size are needed to provide evidence of causality.
The authors thank the sleep technicians at Tokyo Medical University for technical assistance.
The authors also thank the medical editors of the Department of International Communications of Tokyo Medical University for their assistance with the manuscript.
None declared.