2016 Volume 80 Issue 8 Pages 1684-1688
2016 Volume 80 Issue 8 Pages 1684-1688
Peripartum cardiomyopathy (PPCM) is a rare, but life-threatening condition that occurs during the peripartum period in previously healthy women. Although its etiology remains unknown, potential risk factors include hypertensive disorders during pregnancy, such as preeclampsia, advanced maternal age, multiparity, multiple gestation, and African descent. Several cohort studies of PPCM revealed that the prevalence of these risk factors was quite similar. Clinically, approximately 40% of PPCM patients are complicated with hypertensive disorders during pregnancy. Because PPCM is a diagnosis of exclusion, heterogeneity is a common element in its pathogenesis. Recent genetic research has given us new aspects of the disease. PPCM and dilated cardiomyopathy (DCM) share genetic predisposition: 15% of PPCM patients were found to have genetic mutations that have been associated with DCM, and they showed a lower recovery rate. Other basic research using PPCM model mice suggests that predisposition genes related to both hypertensive and cardiac disorders via angiogenic imbalance may explain common elements of hypertensive disorders and PPCM. Furthermore, hypertensive disorders during pregnancy are now found to be a risk factor of not only PPCM, but also cardiomyopathy in the future. Understanding genetic variations allows us to stratify PPCM patients and to guide therapy. (Circ J 2016; 80: 1684–1688)
Peripartum cardiomyopathy (PPCM) causes unexplained left ventricular systolic dysfunction (ie, left ventricular ejection fraction (LVEF) <45%) and heart failure towards the end of pregnancy or in the months following delivery, in previously healthy women.1,2 PPCM is a rare but leading cause of pregnancy-related morbidity and mortality.3 The pathogenesis of PPCM has been unclear, but many new findings from both clinical and basic research have been reported recently. The dilated left ventricle and decreased cardiac contraction in PPCM are similar to dilated cardiomyopathy (DCM). Some PPCM patients also have a family history of DCM. These facts suggest that an overlap of these conditions. An American National Institute of Health Working Group, however, concurred that PPCM is a distinct entity, rather than a clinically silent underlying cardiomyopathy unmasked by the hemodynamic stresses of pregnancy, because the reported incidence is much higher than the incidence of DCM or myocarditis.4 In addition, PPCM is often present in the postpartum period, which is when pregnancy-induced volume load is already reduced.
PPCM is a diagnosis of exclusion and thus considered a heterogeneous disease group. The precise mechanisms of PPCM remain unexplained, but several theories have been proposed, including autoimmune disorder,5 viral myocarditis,6 and antiangiogenic factors such as cleaved prolactin and solubleFLT1 (sFLT1).7,8 All these factors may contribute to the deterioration of PPCM in each individual patient, because it is such a heterogeneous disease.
Lately, more genetic research about PPCM has been performed. Therefore, we review the literature about PPCM from a genetic perspective, because it may contribute to understanding of the disease and suggest further investigations of its pathogenesis.
The incidence of PPCM is known to be approximately 1 in 1,000–4,000 in the USA,9–11 1 in 1,000 in South Africa,12 and 1 in 300 in Haiti.13 In contrast, the incidence in Japan and some European countries is less, approximately 1 in 10,000–15,000.14 Such differences are mainly explained by ethnicity, including genetic diversity, and the increased diagnostic yield. A large population-based study of PPCM highlighted important racial differences in the USA; the incidence of PPCM was greatest in African-Americans, in which it was 7-fold higher compared with the lowest in Hispanics.11
Several risk factors are associated with PPCM: advanced maternal age, multiparity, multiple gestations, hypertensive disorders during pregnancy, tocolytic agents such as β2 adrenergic agonists, as well as African descent.1,2,4,15 The prevalence of these risk factors among PPCM patients is almost same in many countries (Table),14,16–19 which indicates that we share the same concept of the disease and diagnosis.
| Japan14 (n=102) |
Germany16 (n=115) |
USA17 (n=100) |
South Africa18,19 (n=100) |
|
|---|---|---|---|---|
| Age (years) | 32.7 | 34 | 30 | 31.6 |
| Parity | 1.7 | 2 | 2.2 | 3 |
| Primipara (%) | 55 | – | – | 20 |
| African descent (%) | 0 | – | 30 | 100 |
| Risk factors | ||||
| Hypertension disorders during pregnancy (%) | 42 | 45 | 45 | 2 |
| Tocolytic therapy (%) | 14 | 4 | – | 9 |
| Twin pregnancy (%) | 15 | 15 | – | 6 |
| Mortality (%) | 4 | 2 | 4 | 15 |
Symptoms of heart failure, such as dyspnea, edema and weight gain, resemble those complained about by normal peripartum women, which makes it very difficult to diagnose PPCM. At the same time, a low level of awareness and failure to routinely think of a cardiac cause for these symptoms are related to delayed diagnosis in many cases. In Japan, more than 60% of patients were initially seen by an obstetrician when they complained of heart failure symptoms, otherwise less than 10% were primarily managed by a cardiology specialist.14 Goland et al reported that diagnosis delay was significantly associated with worse prognoses such as death or heart transplantation.20 Increased awareness of PPCM is required for early diagnosis and better outcomes.
In Japan, diagnosis of PPCM was established antepartum in 31% of cases and intra- to postpartum in 69%. One-third of patients were diagnosed within 1 week after delivery.14 The Registry On Pregnancy And Cardiac disease (ROPAC) study reported that timing of heart failure was dependent on the underlying cardiac diagnosis, with heart failure in the second trimester occurring mainly in patients with structural heart disease, such as shunt lesions or valvular heart disease. By contrast, patients with cardiomyopathy and ischemic heart disease developed heart failure shortly after delivery.21 This finding suggests that, besides pregnancy-induced volume overload, other pathophysiological mechanisms underlie the development of PPCM.
The clinical course of PPCM is distinguishing; some patients show rapid progression to end-stage heart failure and others recover ventricular function spontaneously and completely. Although early improvement in cardiac function predicts a good outcome, some women will have slow, gradual improvement over years. The most severe cases (≈5–10% of PPCM patients) result in maternal death or heart transplant. Approximately 50–70% of patients show clinically normalized cardiac function within a year. Overall, the patients with PPCM had better survival, as compared with the patients with idiopathic cardiomyopathy.22
The Japanese retrospective nationwide survey of PPCM revealed that, although cardiac parameters at diagnosis were similar in patients with and without hypertensive disorders, patients with hypertensive disorders were hospitalized for a shorter period and had better cardiac function in the long-term.14 The same tendency has been observed in other studies.16 It is partly explained by the fact that PPCM patients without hypertensive disorders tend to include women with a DCM background, as we will discuss later. Otherwise, if PPCM and hypertensive disorders, such as preeclampsia, share the same pathogenesis, cardiomyopathy is recovered within months, whereas preeclampsia dramatically improves after delivery. Other prognostic factors are known, such as the LVEF at diagnosis and after 2 months, the LV diameter, LV thrombus and the ethnicity.23
Several cohort studies revealed that a positive family history of DCM among PPCM patients was approximately 10%.16,17 There also have been reports in which some cases of PPCM may actually be part of familial DCM. van Spaendonck-Zwarts et al24 studied 90 families with familial DCM in the Netherlands and the presence of PPCM was found in 5 (6%) of them. A DCM-related genetic mutation was found in 1 family. In a reverse approach, cardiac screening of 1st-degree relatives of 3 PPCM patients, who did not show full recovery, revealed undiagnosed DCM in all 3 families. After these findings, the same group collected 18 more families with PPCM and DCM from various countries and found that titin gene (TTN) mutations were the most common variant among the families.25 In another study from the USA, Morales et al made similar observations in a large cohort study. Of 4,110 women from 520 pedigrees in the familial DCM database, 45 cases of PPCM were identified. Of these, 19 had been resequenced for known DCM-related genetic mutations, and 6 carried mutations.26 These findings suggest that a proportion of PPCM cases has a genetic cause and therefore, cardiologic screening of such families is important. Furthermore, cardiologic screening during pregnancy and puerperium should be considered for 1st-degree relatives or relatives carrying the underlying mutation of familial DCM patients.
As for DCM, more than 50 different genetic mutations have been linked to the disease.27 Current research28 was performed to sequence the 43 genes with variants that had been associated with DCM in 172 women with PPCM. The prevalence of truncating variants (26 of 172: 15%) was significantly higher than that in a reference population (4.7%), but was similar to that in a cohort of patients with DCM (17%).28 Interestingly, the prevalence of variants was the same in the USA, Germany and Japan, and even among a cohort of patients who underwent either cardiac transplantation or placement of a LV assist device. Two-thirds of the identified truncating variants were in TTN. TTN encodes a protein, titin, which comprises 1 of the 3 major filaments of the cardiac sarcomere, and its truncated variants are responsible for approximately 20% of DCM cases.29 In a clinically well-characterized cohort of 83 women with PPCM, the presence of TTN truncating variants was significantly correlated with a lower LVEF at 1-year follow-up.28
In many reports, PPCM patients with a family history of DCM and/or positive genetic background of DCM showed a lower recovery rate.25,29 However, the German cohort results showed that the recovery rate of patients with a positive family history of cardiomyopathy (PPCM, DCM, sudden death, and arrhythmias in 1st-degree relatives) was similar to patients without such a history. Considering the result, a family history of sudden death or arrhythmias may not be related to poor outcome of PPCM. However, strong consideration should be given to screening family members of PPCM patients and peripartum women with a family history of PPCM and/or DCM, because manifestation of PPCM may indicate a genetic predisposition for cardiomyopathy.
One of the most challenging issues in the investigation of DCM-related genetic mutations among PPCM cases is how to identify whether a mutation actually causes cardiomyopathy.30 Even 5% of the general population has DCM-related genetic mutations. The disease phenotype should segregate with the mutation in a large family containing members that have both normal and affected phenotypes. We must very carefully assess any genetic diagnosis.
Another big issue is whether a PPCM patient with a positive genetic background of DCM has to be considered as overt DCM and whether it should be distinguished from PPCM or not. The answer is still controversial. Palojoki et al found no major adverse effects or worsening of cardiac condition during or after pregnancy in 11 pregnancies in 5 women who were carriers of the lamin A/C gene mutation known to cause DCM.31 An analysis of adverse outcomes during pregnancy in women with DCM revealed that there were no adverse events in women with none of these 3 clinical parameters (moderate or severe LV dysfunction, NYHA functional class III or IV, and/or a previous cardiac event).32 In clinical practice, asymptomatic women with mild DCM mostly tolerated pregnancy well. Therefore, it is reasonable to surmise PPCM-specific factors, such as angiogenic imbalance, also affect many women with a positive genetic background of DCM and aggravate the condition. Furthermore, the latest review suggested the possibility that truncations of TTN may have an interaction with late gestational antivascular insults.33 Because the current concept of “PPCM” probably includes both overt DCM and PPCM with a genetic background of DCM, as well as other non-specified cardiac dysfunction, we should continue to investigate PPCM-specific factors or genetic interactions to distinguish between these entities (Figure). Differentiating these entities will enable tailor-made treatment in the long-term and subsequent pregnancies.
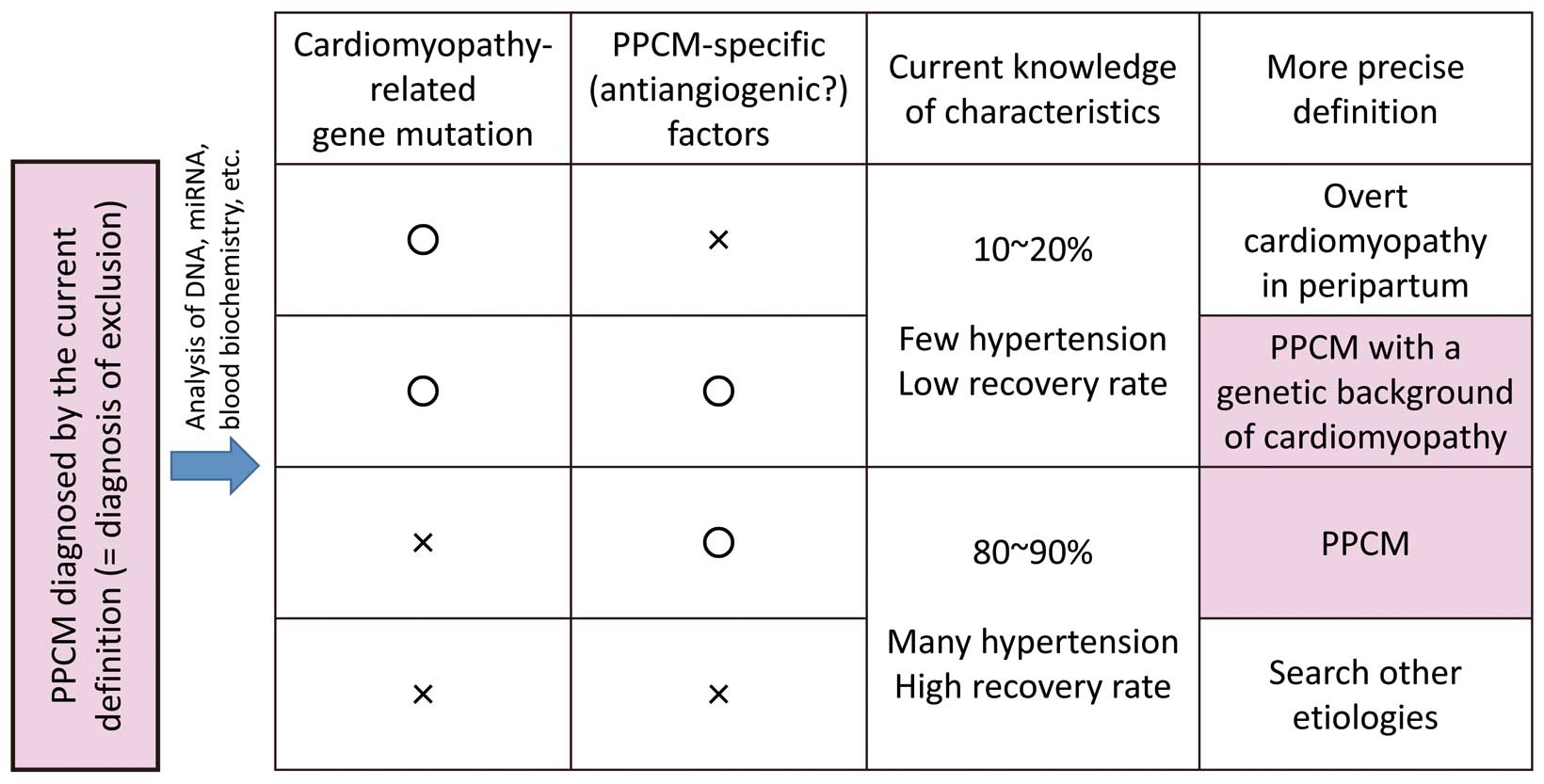
Hypothesis for a more precise definition of PPCM, which is currently a diagnosis of exclusion and includes both overt DCM and PPCM with a genetic background of DCM, as well as other entities. PPCM, peripartum cardiomyopathy; DCM, dilated cardiomyopathy; HF, heart failure.
Hypertensive disorders during pregnancy are categorized as gestational hypertension, preeclampsia, eclampsia, preeclampsia superimposed on chronic hypertension and chronic hypertension; which occur in 6–8% of pregnancies.34 Even though the etiology of preeclampsia remains unknown, as well as that of PPCM, a 2-stage theory has become widely known as a possible mechanism of preeclampsia: poor placentation in early pregnancy (stage 1) can cause hypoxic placenta, which produces an excessive amount of antiangiogenic factors during the later pregnancy (stage 2). The angiogenic imbalance leads to a systemic inflammatory response and endothelial dysfunction, which result in preeclampsia. For instance, the placenta in late gestation secretes vascular endothelial growth factor (VEGF) inhibitors such as sFLT1, and this is accentuated by multiple gestation and preeclampsia.
Hypertensive disorders during pregnancy are the most major risk factors of PPCM. A systematic review of 22 PPCM studies (n=979) revealed that the pooled prevalence of 22% was more than the average worldwide background rate of preeclampsia in pregnancy.35 The rates of hypertensive disorders during pregnancy (37%) and multiple gestations (9%) were also elevated. There were no geographic or racial differences detected in the prevalence of hypertensive disorders in women with PPCM.
Patten et al8 reported that model mice under an antiangiogenic environment in the heart developed PPCM. That is, exogenous sFLT1 caused only diastolic dysfunction in wild-type mice, and profound systolic dysfunction in mice lacking cardiac PGC-1α, a powerful regulator of angiogenesis. In addition, proangiogenic therapies rescued model mice from PPCM. These results indicate that PPCM is mainly a vascular disease, and explain why preeclampsia and multiple gestations are important risk factors for the development of PPCM.
Interestingly, the DCM-related genome sequencing in PPCM, as referred to earlier, showed that the prevalence of hypertensive disorders was very low among patients with TTN truncating variants (1/11, 9%), compared with those without (35/68, 51%, P=0.009).29 The burden of TTN truncating variants among the women without hypertension (10/43, 23%) was significantly higher than that among those with hypertension (1/40, 2%, P=0.005). From these results, PPCM with hypertensive disorders (and probably multiple gestations) and PPCM with a genetic background of DCM are unique subsets in the heterogeneous disease group.
Many genetic factors involved in the development of preeclampsia have been reported.36,37 Focusing on angiogenic imbalance, specific VEGF receptor genotype (VEGF C−460T SNP and VEGFG 405C SNP) in the placenta was reported to have an increased risk to develop a HELLP syndrome (a variant or complication of preeclampsia with 3 main features such as hemolysis, elevated liver enzymes, and low platelet counts).38 Preeclampsia is considered as a multiplex genetic disease. Only a few patients develop PPCM among women with a hypertensive disorder during pregnancy. They may have predisposition genes related to both hypertensive and cardiac disorders, like the model mice lacking cardiac PGC-1α.
It is well established that women who have had a pregnancy complicated by preeclampsia are at increased risk of hypertension, ischemic heart disease, and premature cardiovascular death, compared with women with normotensive pregnancies.39,40 Remarkably, Behrens et al reported that women with a history of hypertensive disorders during pregnancy, compared with those without such a history, had a small but statistically significant increased risk of cardiomyopathy more than 5 months after delivery, in a Danish nationwide cohort.41 In the study, compared with women with normotensive pregnancies (7.7/100,000 person-years), women with a history of hypertensive disorders during pregnancy had significantly increased rates of cardiomyopathy (cardiomyopathy events among women with severe preeclampsia; 15.6/100,000 person-years, among women with mild preeclampsia; 14.6/100,000 person-years, among women with gestational hypertension; 17.3/100,000 person-years). Only half of them were complicated with hypertension when they were diagnosed with cardiomyopathy. These increases persisted more than 5 years after the latest pregnancy. Furthermore, in that cohort 11% of all cardiomyopathy events occurred in women with a history of hypertensive disorders during pregnancy. Although whether there is a causal mechanism behind this association needs further investigation, endothelial dysfunction has been reported to have an association with heart failure and cardiomyopathy.42,43
Pregnancy is a stress test for life – we have to understand that pregnancy can temporarily unmask subclinical disease, which may return in later life when the effects of ageing diminish the limited reserves of a vulnerable organ.44 Even though PPCM patients recover their cardiac contraction, long-term follow-up may be necessary.
Nowadays, the technique of genetic testing is advancing amazingly. Next-generation sequencing is beginning to be introduced into clinical practice. Although analysis of genome sequencing remains challenging because of the complexity of the human genome, understanding genetic variation may help us to predict prognosis and to decide treatment plans for PPCM patients. Further, it will enable us to detect vulnerability for PPCM pre-pregnancy and give us a new therapeutic strategy, such as gene therapy.45
The heterogeneity of PPCM makes it more difficult to investigate pathophysiology in human patients. Categorizing into subsets by genetic assessment, such as DCM and hypertensive disorders, may allow us to research them individually and to find specific mechanisms.
Several cohort studies of PPCM revealed that the prevalence of risk factors among PPCM patients was quite similar in the USA, some European countries and Japan, which indicates that we share the same concept of the disease. Clinically, approximately 40% of PPCM patients are complicated with hypertensive disorders during pregnancy, and approximately 10% of them have a family history of PPCM and/or DCM. The recent genetic research has given us many new aspects concerning PPCM; 15% of PPCM patients had truncating variants of DCM-related genes, and they showed a lower recovery rate. Basic research revealed that angiogenic imbalance was a common element in hypertensive disorders and PPCM. Furthermore, hypertensive disorders during pregnancy are now proven to be a risk factor of not only PPCM, but also cardiomyopathy in the future.
None.