2016 Volume 80 Issue 8 Pages 1773-1780
2016 Volume 80 Issue 8 Pages 1773-1780
Background: Depressive symptoms and memory impairment are prevalent in patients with chronic heart failure (CHF). Although the mechanisms remain to be elucidated, the hippocampus (an important brain area for emotion and memory) may be a possible neural substrate for these symptoms.
Methods and Results: We prospectively enrolled 40 Stage C patients, who had past or current CHF symptoms, and as controls 40 Stage B patients, who had structural heart disease but had never had CHF symptoms, in Brain Assessment and Investigation in Heart Failure Trial (B-HeFT) (UMIN000008584). As the primary index, we measured cerebral blood flow (CBF) in the 4 anterior-posterior segments of the hippocampus, using brain MRI analysis. Depressive symptoms, immediate memory (IM) and delayed memory (DM) were assessed using Geriatric Depression Scale (GDS), and Wechsler Memory Scale-revised (WMS-R), respectively. Hippocampus CBF in the most posterior segment was significantly lower in Stage C than in Stage B group (P=0.029 adjusted for Holm’s method). Multiple regression analysis identified significant association between hippocampus CBF and GDS or DM score in Stage C group (all P<0.05). GDS score was significantly higher, and IM and DM scores were lower in Stage C patients with hippocampus CBF below the median than those with hippocampus CBF above the median (all P<0.05).
Conclusions: Hippocampus abnormalities are associated with depressive symptoms and cognitive impairment in CHF patients. (Circ J 2016; 80: 1773–1780)
In patients with chronic heart failure (CHF), depression and cognitive impairment frequently coexist and have been reported to be associated with worse prognosis.1–5 The prevalence of clinically significant depression and of cognitive impairment is approximately 20% and up to 75% of CHF patients, respectively.1,2 CHF patients with depression and/or cognitive impairment have an increased risk of death, hospitalization and emergency room visits compared with those without the disorders.1–5 A possible mechanism of depression and/or cognitive impairment in CHF is abnormality of the hippocampus, which is the important brain area for emotion and memory.6,7 Abnormality of the hippocampus, such as reduction in gray matter and cerebral blood flow (CBF), is associated with depressive symptoms in patients with depression and with memory impairment in those with Alzheimer disease, respectively.6,7 Moreover, the hippocampus is one of the regions of the brain most vulnerable to cerebral hypoxia.8,9 The hippocampus is predominantly damaged by cardiac arrest and subsequent cardiopulmonary resuscitation,8,9 with resultant long-term neurological and cognitive deficits.10–12 Global CBF is also decreased in CHF patients.13 We have previously demonstrated that structural abnormalities of the hippocampus, such as reduction in gray matter, neurogenesis and neurite outgrowth and increase in the number of astrocytes, in a rat model of CHF.14 These lines of evidence imply an association between hippocampal abnormalities and depressive symptoms/cognitive impairment in CHF patients.
Editorial p 1702
In contrast to the result of our previous animal studies, hippocampal abnormality could not be detected in previous clinical studies. Woo et al examined brain magnetic resonance images (MRI) of 9 people with CHF and 27 controls, and showed a reduction in gray matter in the insula, basal ganglia, cingulate gyrus, parahippocampal gyrus, dorsal midbrain, ventral and superior frontal cortex, and bilateral parietal and lateral parietal-occipital cortex in the CHF patients compared with the controls.15 Because the results of Woo et al were hampered by lack of a control group with cardiovascular risks and diseases, which can affect brain structure,16 Almeida et al compared brain MRI images between 35 patients with CHF and 56 patients with ischemic heart disease and revealed loss of gray matter in the right middle temporal lobe, the right anterior cingulate and medial frontal lobe, the left lentiform nucleus and adjacent areas, the right precuneus, the right thalamus and lentiform nucleus, and other regions extending from the right and left middle and superior frontal cortex in the CHF patients than in the patients with ischemic heart disease.17 The probable reason for the undetectability of hippocampal abnormality is that both of those studies used whole-brain analysis, which can simultaneously analyze many different brain regions but fail to detect differences of small brain regions such as the hippocampus.18 However, automated region of interest (ROI)-based analysis can examine only a specific brain region, but has a stronger power compared with whole-brain analysis.18 In the present study, we used automated ROI-based analysis to examine whether CBF in the hippocampus is lower in CHF patients based on the a priori hypothesis of hippocampal abnormality that was observed in our previous animal study, and if so, whether the abnormalities in the hippocampus correlate with depressive symptoms and memory impairment in these patients. Following the ESC/ACC/AHA guidelines,19 we enrolled Stage C patients, who had past or current HF symptoms, and as controls, Stage B patients, who had structural heart disease but had never had HF symptoms, to minimize the confounding effects of risk factors of cardiovascular disease on brain structural and functional differences.16
The study protocol of the present study (known as the “Brain Assessment and Investigation in Heart Failure Trial (B-HeFT)”) was approved by the Ethics Committee of the Tohoku University Graduate School of Medicine and registered in the University Hospital Medical Information Network (UMIN000008584).
Study SubjectsWe initially enrolled a total of 116 patients, including 56 with asymptomatic Stage B and 60 with symptomatic Stage C CHF, aged 45–90 years, from August 2012 to April 2013 (Figure 1). Exclusion criteria were as follows: contraindication of cardiac MRI, unstable cardiac function, past history that could have significantly affected CBF and/or brain structure, and difficulties in evaluating the brain and/or MRI. The patients underwent psychological tests, blood sampling and brain MRI recordings within 6 months. Finally, 40 patients in each group were enrolled (Figure 1). The underlying structural heart diseases in the Stage B and C groups, respectively, included ischemic heart disease (n=22 and 21), primary cardiomyopathy (n=10 and 15), valvular heart disease (n=6 and 2), hypertensive heart disease (n=1 and 0), cardiac sarcoidosis (n=1 and 1), and congenital heart disease (n=0 and 1).

Patient enrollment in the present study of hippocampal blood flow.
Echocardiography was performed according to the American Society of Echocardiography guidelines.20 Left ventricular ejection fraction (LVEF) was assessed using the Simpson’s biplane method. LV end-diastolic diameter (LVDd) and LV end-systolic diameter (LVDs) were obtained from the parasternal long-axis view of the LV. Fractional shortening (FS) was calculated as the difference between LVDd and LVDs divided by LVDd.
Psychological TestsThe patients underwent the 15-item Geriatric Depression Scale (GDS) and Mini-Mental State Examination (MMSE) for assessment of depressive symptoms and global cognitive function, respectively.21,22 Memory function was evaluated using the logical memory subtest of the Wechsler Memory Scale-revised (WMS-R).23 Briefly, an examiner read 2 stories, and at the end of each story asked the patient to recall the story immediately (immediate memory (IM) task) and at 30 min after the first recall as a test of delayed memory (DM). All the psychological tests were performed by well-experienced examiners who were blinded to the patient’s clinical status.
Brain MRI RecordingAll MRI recordings were performed using a 1.5-T whole-body MRI system (Signa HDxt, GE Medical Systems, Milwaukee, WI, USA) and an 8-channel NVArray coil (coil selection using head-A). Structural MRI images were obtained from a 3D T1-weighted sequence (vascular time-of-flight spoiled gradient-recalled acquisition) with the following parameters: axial, repetition time (TR) 40 ms; echo time (TE) 2.2 ms; flip angle 45°; field of view (FOV) 220 mm; matrix 256×256; slice thickness 2 mm; 96 sections; and number of excitations 1. CBF images were constructed from pseudocontinuous arterial spin labeling perfusion images (3D fast spin-echo acquisition) WITH the following parameters: post-label delay 1.525 s; TR 4.587 s; TE 10.5 ms; spiral readout of 8 arms×512 samples; axial thickness 4.0 mm; 32 sections; effective resolution 3.49×3.49 mm; FOV 240 mm; reconstructed matrix 128×128; NEX 3; and acquisition time 4 min 26 s.
Brain MRI AnalysisBrain MRI analyses were performed using Statistical Parametric Mapping 8 (SPM8) (Wellcome, Department of Cognitive Neurology, London, UK) and custom-written software in Matlab (Math Works, Natick, MA, USA).8,14,17 First, THE CBF images were normalized to the standard Montreal Neurological Institute (MNI) space to perform statistical analysis of brain MRI images in the same space. Details of the CBF image normalization were written in the CBF image normalization. Next, CBF in the hippocampus was calculated in each normalized CBF image using automated ROI-based MRI analysis modified by the previous study to examine whether CBF in the hippocampus was lower in CHF patients.24 The ROI of the hippocampus was defined according to the Wake Forest University PickAtlas toolbox25 and was divided into 4 segments from anterior to posterior at each center of gravity (Figure 2). CBF was compared between the Stage B and C groups in the whole hippocampus and the most anterior (antero-anterior), second anterior (postero-anterior), second posterior (antero-posterior) and the most posterior (postero-posterior) regions of the hippocampus (Figure 2).
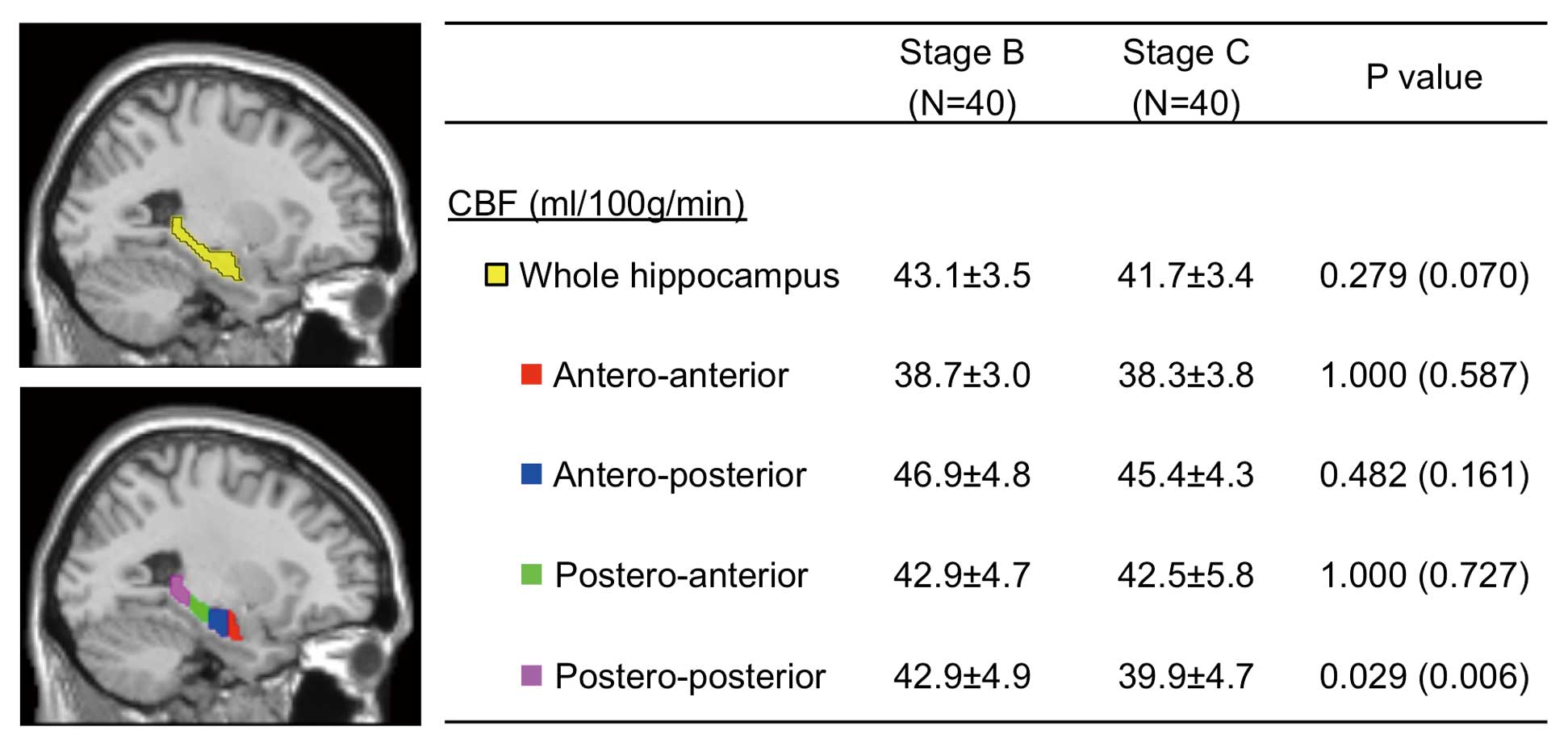
Cerebral blood flow (CBF) in the hippocampus in Stage B and C groups of chronic heart failure patients. The whole, antero-anterior, antero-posterior, postero-anterior and postero-posterior ROI of the hippocampus (yellow, red, blue, green and pink regions, respectively) are displayed on the selected slice of the MRI template available in the SPM8 system. The numbers in the parentheses are nominal P values without correction for multiple comparisons. MRI, magnetic resonance image; ROI, region of interest.
Before normalization of the CBF images, normalization of the structural MRI images was performed using voxel-based morphometry with diffeomorphic anatomical registration using exponentiated lie algebra (DARTEL),26 as we previously described in detail.14,27 First, structural MRI images were segmented into gray and white matter probability maps using the “new segmentation” algorithm. Second, these 80 segmented tissue maps were used to create a population-specific template using the DARTEL template creation tool. Third, the template space was matched to the standard MNI space using an affine-only registration. Finally, each patient’s gray matter map was warped using its corresponding smooth and reversible deformation parameters to transform it to the custom template space and then to the standard MNI space without modulation of the Jacobian determinants derived from the special normalization step.
Each CBF map was normalized to the standard MNI space as modified by the previous study.28 First, a binary mask of the segmented gray and white matter maps was made in each structural brain image. Second, extracerebral signals in each structural brain image were removed using the binary mask. Third, the structural brain image without extracerebral signals and its corresponding binary mask were co-registered to the CBF map. Fourth, extracerebral signals of each CBF map were removed using the co-registered binary mask. Fifth, the CBF maps without extracerebral signals were co-registered back to the space of the original structural brain MRI image. Finally, each co-registered CBF map was warped to the MNI standard space using the same parameters of each structural brain MRI image.
Statistical AnalysisAge, LVEF, FS, LVDd, LVDs, GDS, IM, DM, and CBF in the whole hippocampus, antero-anterior hippocampus, postero-anterior hippocampus, antero-posterior hippocampus and postero-posterior hippocampus were expressed as mean±standard deviation (Stage B vs. Stage C group) and were analyzed using Student’s t-test. All continuous variables except for MMSE showed normal distribution at P>0.05 with Kolmogorov-Smirnov test. MMSE was expressed as median (interquartile range) and analyzed using the Mann-Whitney test. The ratios of female sex, education ≥12 years, ischemic HF, hypertension, diabetes, smoking and administration of angiotensin-converting enzyme inhibitor (ACEI), angiotensin-receptor blocker (ARB) or β-blocker were analyzed using Fisher’s exact test. Because there was no previous report that measured CBF in the hippocampus in CHF patients, global CBF was alternatively used for sample size calculation. With a CBF difference of 5.0 ml/min/100 g between CHF patients and normal subjects, 40 samples for each group provided a power level of 98.0% with a type I error of 0.01 in the past study.13
To examine whether CBF in the hippocampus is associated with depressive symptoms and memory impairment in CHF patients, we first applied univariate and multivariate regression analyses, and then CBF in the postero-posterior hippocampus was regressed onto confounding factors, echocardiography data and psychological test scores. For multivariate analysis, stepwise variable selection was used to identify the best subset of covariates associated with CBF in the postero-posterior hippocampus (multivariate analysis 1). We also analyzed the model consisting of the variables that were identified in multivariate analysis 1 and major confounders for the hippocampus, including age, sex, and education (multivariate analysis 2). Parameter estimates (B) and standard errors (SE) were calculated and reported. Second, we performed a subgroup analysis with a median-split between Stage C patients with CBF in the most posterior segment of the hippocampus, where CBF was significantly lower in the Stage C group than in the Stage B group (Figure 2), above the median and those with CBF below the median. All hypothesis testing was 2-sided with a significance level of P<0.05, and was corrected for multiple comparisons using the Holm method.
Table 1 shows the clinical characteristics, including age, sex, education ≥12 years, ischemic origin, cardiovascular risk factors and medications, were comparable between the 2 groups. In contrast, for the echocardiographic data, in the Stage C group, as compared with Stage B, LVEF and FS were significantly lower and LVDd and LVDs were significantly larger (LVEF, 43.1±17.5 vs. 59.6±14.7%, P<0.001; FS, 22.3±10.0 vs. 32.6±9.9%, P<0.001; LVDd, 55.8±11.5 vs. 50.3±4.9 mm, P=0.007; LVDs, 44.1±13.5 vs. 34.2±7.6 mm, P<0.001).
| Stage B (n=40) |
Stage C (n=40) |
P value | |
|---|---|---|---|
| Clinical characteristics | |||
| Age (years) | 65.0±10.9 | 66.8±8.9 | 0.419 |
| Female (%) | 22.5 | 32.5 | 0.453 |
| Education ≥12 years (%) | 87.5 | 77.5 | 0.239 |
| Ischemic HF (%) | 55.0 | 52.5 | 1.000 |
| Hypertension (%) | 52.5 | 65.0 | 0.364 |
| Diabetes (%) | 35.0 | 20.0 | 0.210 |
| Smoking (%) | 70.0 | 57.5 | 0.352 |
| ACEI/ARB (%) | 92.5 | 95.0 | 1.000 |
| β-blocker (%) | 90.0 | 97.5 | 0.378 |
| Echocardiography | |||
| LVEF (%) | 59.6±14.7 | 43.1±17.5 | <0.001 |
| FS (%) | 32.6±9.9 | 22.3±10.0 | <0.001 |
| LVDd (mm) | 50.3±4.9 | 55.8±11.5 | 0.007 |
| LVDs (mm) | 34.2±7.6 | 44.1±13.5 | <0.001 |
| Psychological tests | |||
| MMSE | 29 (28, 29) | 29 (28, 29) | 0.745 |
| GDS | 3.2±2.4 | 4.4±2.5 | 0.025 |
| IM | 19.8±8.2 | 17.4±7.6 | 0.187 |
| DM | 15.6±8.0 | 14.9±7.4 | 0.663 |
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin-receptor blocker; CHF, chronic heart failure; DM, delayed memory; FS, fractional shortening; GDS, Geriatric Depression Scale; HF, heart failure; IM, immediate memory; LVDd, left ventricular diastolic diameter; LVDs, left ventricular systolic diameter; LVEF, left ventricular ejection fraction; MMSE, Mini-Mental State Examination.
In the psychological tests, GDS score was significantly higher in the Stage C group compared with the Stage B group (4.4±2.5 vs. 3.2±2.4, P=0.025). Scores of MMSE, IM and DM tended to be lower in the Stage C group compared with the Stage B group without statistical significance (MMSE, 29 (28, 29) vs. 29(28, 29), P=0.745; IM, 17.4±7.6 vs. 19.8±8.2, P=0.187; DM, 14.9±7.4 vs. 15.6±8.0, P=0.663). These results showed that depressive symptoms and memory impairment were more severe in the Stage C than in the Stage B group in general.
CBF in the HippocampusTo examine whether MRI-detectable differences in the hippocampus were noted in CHF patients, we performed automated ROI-based MRI analysis to compare CBF in the hippocampus between the Stage B and C groups (Figure 2). CBF in the whole hippocampus tended to be lower in the Stage C group than in the Stage B group without statistical significance (41.7±3.4 vs. 43.1±3.5 ml/100 g/min, P=0.279). However, CBF in the most posterior (postero-posterior) portion of the hippocampus was significantly lower in the Stage C group than in the Stage B group (39.9±4.7 vs. 42.9±4.9 ml/100 g/min, P=0.029) (Figure 2).
Associations Between Hippocampal CBF and Depressive Symptoms or Cognitive DysfunctionTo examine whether CBF in the hippocampus is associated with depressive symptoms and memory impairment in CHF patients, we first performed univariate and multivariate regression analyses of CBF in the postero-posterior hippocampus with confounding factors, echocardiographic data and psychological test scores in each group. In the Stage B group, neither univariate nor multivariate regression analysis showed a significant association between CBF in the postero-posterior hippocampus and psychological variables (Table S1). In the Stage C group, univariate regression analysis showed that CBF in the postero-posterior hippocampus was significantly associated with GDS, IM and DM scores (GDS, B=–0.84, P=0.048; IM, B=0.26, P=0.105; DM, B=0.30, P=0.034). Multivariate regression analysis demonstrated that stepwise variable selection identified GDS and IM scores as the best subset of the covariates associated with CBF in the postero-posterior hippocampus (GDS, B=–0.62, P=0.015; DM, B=0.22, P=0.013). The association between CBF in the postero-posterior hippocampus and GDS or DM score was also significant in the model including age, sex, and education (GDS, B=–0.60, P=0.031; DM, B=0.22, P=0.044) (Tables 2, S2).
| Univariate analysis | Multivariate analysis 1 | Multivariate analysis 2 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimate | SE | P value | Estimate | SE | P value | Estimate | SE | P value | |
| MMSE | 0.94 | 0.58 | 1.000 (0.222) | ||||||
| GDS | –0.84 | 0.27 | 0.048 (0.003) | –0.62 | 0.24 | 0.015 | –0.60 | 0.27 | 0.031 |
| IM | 0.26 | 0.09 | 0.105 (0.007) | ||||||
| DM | 0.30 | 0.09 | 0.034 (0.002) | 0.22 | 0.08 | 0.013 | 0.22 | 0.11 | 0.044 |
The model in multivariate analysis 2 was adjusted for age, sex and education. The numbers inside parentheses are nominal P values without correction for multiple comparisons. CBF, cerebral blood flow; SE, standard error. Other abbreviations as in Table 1.
We subsequently performed a subgroup analysis with a median-split between Stage C patients with CBF in the postero-posterior hippocampus above the median and those with CBF below the median. Clinical characteristics and echocardiographic data were comparable between these 2 groups (Table 3). As expected, CBF in the postero-posterior hippocampus was significantly lower in the low hippocampal CBF group than in the high hippocampal CBF group. Moreover, GDS score was significantly higher, and scores of IM and DM were significantly lower in the low hippocampal CBF group than in the high hippocampal CBF group (GDS, 5.7±2.1 vs. 3.2±2.4, P=0.001; IM, 14.5±7.2 vs. 20.4±6.9, P=0.012; DM, 11.8±6.0 vs. 18.0±7.5, P=0.006). MMSE score tended to be lower in the low hippocampal CBF group than in the high hippocampal CBF group (29 (29, 29) vs. 28(27.5, 29), P=0.077). Thus, the results of regression and subgroup analyses indicated that alterations in CBF in the posterior hippocampus were significantly associated with depressive symptoms and memory impairment in CHF patients.
| High CBF (n=20) |
Low CBF (n=20) |
P value | |
|---|---|---|---|
| Clinical characteristics | |||
| Age (years) | 65.7±7.9 | 67.8±9.8 | 0.461 |
| Female (%) | 35 | 30 | 1.000 |
| Education ≥12 years (%) | 85 | 70 | 0.451 |
| Ischemic HF (%) | 45 | 60 | 0.527 |
| Hypertension (%) | 60 | 70 | 0.741 |
| Diabetes (%) | 15 | 25 | 0.695 |
| Smoking (%) | 60 | 55 | 1.000 |
| ACEI/ARB (%) | 95 | 95 | 1.000 |
| β-blocker (%) | 100 | 95 | 1.000 |
| Echocardiography | |||
| LVEF (%) | 40.1±16.5 | 46.1±18.3 | 0.280 |
| FS (%) | 20.1±9.1 | 24.5±10.6 | 0.171 |
| LVDd (mm) | 58.1±12.7 | 53.4±10.0 | 0.196 |
| LVDs (mm) | 47.2±14.1 | 41.1±12.4 | 0.151 |
| Psychological tests | |||
| MMSE | 29 (29, 29) | 29 (27.5, 29) | 0.077 |
| GDS | 3.2±2.4 | 5.7±2.1 | 0.001 |
| IM | 20.4±6.9 | 14.5±7.2 | 0.012 |
| DM | 18.0±7.5 | 11.8±6.0 | 0.006 |
| CBF (ml/100 g/min) | |||
| Postero-posterior | 43.7±2.3 | 36.2±3.1 | <0.001 |
Postero-posterior, postero-posterior segment of the hippocampus. Other abbreviations as in Tables 1,2.
Major findings of the present study were that CBF in the posterior hippocampus was lower in CHF patients with Stage C as compared with those with Stage B, and that there was a significant association between CBF in the posterior hippocampus and the extent of depressive symptoms or cognitive impairment in CHF patients with Stage C. To the best of our knowledge, this is the first study demonstrating that hippocampal CBF abnormalities are associated with depressive symptoms and cognitive impairment in CHF patients.
We recently demonstrated structural abnormalities of the hippocampus in CHF rats,14 but hippocampal abnormalities have not been previously documented in clinical studies of CHF patients.15,17 This is probably because previous clinical studies used whole-brain analysis, which fails to detect differences of small brain regions such as the hippocampus.18 Automated ROI-based analysis, which has a stronger power compared with whole-brain analysis,18 was used in the present study and successfully detected CBF differences in the posterior hippocampus. Almeida et al also reported that cognitive function was impaired in CHF patients compared with both control subjects and patients with ischemic heart disease, but was not significantly associated with total brain gray matter.17 The association between psychological valuables and the posterior hippocampus, not total brain gray matter, was assessed and achieved significance in this study.
Mechanisms of Hippocampal CBF Abnormalities in CHFThe present study elucidated an abnormality of the posterior, but not anterior, hippocampal CBF in CHF patients. The hippocampus has functionally and neuroanatomically distinct subregions in the anterior-posterior axis.29 The posterior hippocampus is connected to the retrosplenial and anterior cingulate cortices and plays a major role in cognitive function.29 Lesioning the dorsal (posterior in humans) hippocampus in rodents leads to memory deficits.29 Licensed taxi drivers with extensive navigation experience have a larger volume of the posterior hippocampus as compared with control subjects.30 These lines of evidence support the present results that CBF in the posterior hippocampus was associated with depressive symptoms, which may represent a possible clinical continuum with cognitive dysfunction in the elderly,31 and memory impairment in CHF patients.
The decrease in CBF in the hippocampus might be based on histological changes in the region.8,32 Potential mechanisms of MRI-detectable and histological differences in the hippocampus in CHF patients include low cardiac output and resultant cerebral hypoxia. The vulnerability of the posterior hippocampus after cardiac arrest and following resuscitation has been reported.8 Low cardiac output is associated with reduction in total brain volume in the absence of cardiovascular disease.33 However, both acute and chronic cerebral hypoperfusion increase hippocampal neurogenesis,34,35 which is not consistent with a reduction in neurogenesis in HF rats.14 Thus, the effect of cardiac output on brain structural abnormality in CHF is still controversial. Another mechanism could be that stress and the resultant increase in blood glucocorticoid level in CHF inhibit neurogenesis in the hippocampus.36–38 This system can form a positive feedback loop because decreased neurogenesis leads to an increased glucocorticoid response to stress.39 Interestingly, we previously showed that aberrant activation of the hippocampus, as well as the brainstem and basal ganglia, is associated with takotsubo cardiomyopathy,40,41 which also indicates that the hippocampus is the possible neural substrate mediating the association between cardiac function and stress. These possible mechanisms remain be examined in future studies.
Clinical ImplicationsA growing proportion of CHF patients, such as those with HF with preserved EF (HFpEF), do not benefit from any therapies with improved outcome.42 Non-cardiac comorbidities may be a possible explanation for treatment failure of CHF patients.43 Thus, it is important to elucidate the mechanism of depression and cognitive impairment, which are the major brain comorbidities of CHF patients. The present study demonstrated that a novel neural substrate, the hippocampus, could explain the association between CHF and depression and cognitive dysfunction. The abnormal hippocampal CBF in CHF patients may be reversible. Reduction in gray matter has also been noted in patients with depression and those with obstructive sleep apnea, which can be improved by treatment with antidepressants and continuous positive airway pressure, respectively.44,45 Exercise training also increases hippocampal gray matter in healthy subjects24,46 and reduces depressive symptoms and the composite of death and hospitalization in CHF patients.47 Thus, the beneficial effects of exercise in CHF patients may be mediated, at least in part, through improvement of hippocampal CBF abnormality. In the present study, CBF in the hippocampus was significantly different between Stage B and C patients despite the fact that they were similarly treated with ACEI/ARB and β-blockers. Thus, an additional intervention such as exercise may be effective to improve CBF in the hippocampus of CHF patients. On the other hand, both Stage B and C patients have high nursing needs48 and lack of social support is associated with new onset of significant depressive symptoms in CHF patients.49 Thus, the preferable intervention for the brain comorbidities of CHF may be human support such as nursing and social care.
Study LimitationsSeveral limitations should be mentioned. First, the present study focused on abnormalities of the hippocampal CBF based on our previous study in CHF rats.14 Substantial anatomical differences in the prefrontal cortex exist between rats and humans.50 Thus, possible alterations in other brain region(s) including the prefrontal cortex remain to be examined. Second, CHF patients have cardiovascular risk factors and hypertensive drugs that could affect brain structural and functional differences.16 In the present study, there was a significant association between β-blocker administration and CBF in the posterior hippocampus. ACEI and β-blockers affect CBF during memory processing in hypertensive patients.51 Although these confounding factors were comparable between the Stage B and C groups in the present study, it remains to be examined how these confounding risk factors and antihypertensive drugs affect brain function and structure in CHF patients. Third, the present study was cross-sectional and thus un able to demonstrate a cause-result relationship between the hippocampal CBF abnormalities and depressive symptoms/cognitive impairment in CHF patients. Longitudinal studies are needed to address this important issue. Fourth, the present study had a possible selection bias, although age, ratio of female sex and ischemic origin among the excluded patients were not significantly different from those of the study sample. Fifth, CBF in the whole hippocampus tended to be lower in the Stage C group than in the Stage B group, although it did not reach a statistically significant level. Thus, the present findings remain to be confirmed in future studies with a large number of patients.
In the present study, we were able to demonstrate for the first time that abnormalities of blood flow in the posterior hippocampus were associated with depressive symptoms and cognitive impairment in CHF patients. The hippocampus may be a novel therapeutic target for these important disorders in CHF patients.
The authors thank Hidemitsu Miyazawa for his support with brain MRI acquisition, Mari Ootsuki and Yuka Kotozaki and their colleagues for their performance of the psychological tests, and Tomoyuki Suzuki for acquisition of echocardiography.
This study was supported in part by grants-in-aid from the Ministry of Health, Labor, and Welfare and those from the Ministry of Education, Culture, Sports, Science, and Technology, Japan (to H. Shimokawa) and the grant for young investigators of translational research from Tohoku University Hospital (to H. Suzuki).
All authors report no conflicts of interest to disclose.
Supplementary File 1
Table S1. Regression analysis of CBF in the posterior hippocampus of stage B CHF patients
Table S2. Regression analysis of CBF in the posterior hippocampus of stage C CHF patients
Please find supplementary file(s);
http://dx.doi.org/10.1253/circj.CJ-16-0367