2016 Volume 80 Issue 8 Pages 1710-1711
2016 Volume 80 Issue 8 Pages 1710-1711
Uric acid (UA) is the end product of purine metabolism in humans. Although UA acts as an antioxidant at physiological concentrations,1 it also acts as a pro-oxidant in the hyperuricemic state. Hyperuricemia refers to a serum UA (SUA) level >7 mg/dl in both adult men and women according to the Japanese guideline for the management of hyperuricemia and gout. Hyperuricemia has been reported to be associated with various diseases such as hypertension, metabolic syndrome, and diabetes mellitus, as well as chronic kidney disease (CKD).
Article p 1857
In patients with CKD, kidney dysfunction and albuminuria have been acknowledged as independent risk factors for cardiovascular and renal events. Hyperuricemia is also considered a risk factor for the progression of CKD. There are several epidemiological studies showing that higher SUA levels are associated with higher risk of CKD. Community-based studies enable the extrapolation of findings to general populations, and are of great benefit for establishing the management of patients in the real world. SUA levels >8.0 mg/dl indicated a higher risk of developing kidney dysfunction in Japanese community-based screening.2 Higher SUA levels were also associated with the development of kidney dysfunction in healthy American subjects.3 This issue of the Journal carries a study by Takae et al4 in which they assess the influence of high SUA levels on the development of kidney dysfunction and albuminuria as well as the rate of change in the estimated glomerular filtration rate (eGFR) in a general Japanese population (the Hisayama Study), during a 5-year follow-up. They found that the risk for CKD increased significantly and in a dose-dependent manner in those with higher SUA levels after adjustment for other risk factors, and this association was observed even in the normal range of SUA. Furthermore, the subjects with higher SUA levels showed a greater decline in eGFR. Interestingly and importantly, they also found that the higher SUA levels were a significant risk factor not only for developing kidney dysfunction, but also for albuminuria. Takae et al are the first to clearly demonstrate SUA and future risk of both kidney dysfunction and albuminuria in a community-based Asian population,4 but the underlying mechanism for the role of SUA in the development of kidney dysfunction still remains unknown.
At present, there are several hypotheses to explain how hyperuricemia could have harmful effects on kidney function. The “uric acid crystal-independent mechanism” has been attracting attention in experimental studies involving activation of the renin-angiotensin-aldosterone system (RAAS) under hyperuricemic conditions.5 Activation of the RAAS is a well-established mechanism leading to glomerular hyperfiltration through an imbalance of vasoconstriction between afferent and efferent arteries, eventually resulting in glomerular sclerosis. There is a large body of experimental evidence that UA could stimulate circulating and local RAAS in the renocardiovascular system. Yu et al reported that UA upregulated the cellular expression of angiotensinogen, angiotensin-converting enzyme (ACE) and angiotensin II receptors while increasing angiotensin II levels, all of which were ameliorated by ACE inhibitors, UA transporter (UAT) blockers and antioxidants.6 Corry et al, on the other hand, found that UA stimulated cell proliferation as well as angiotensin II production, which was inhibited by a MAP kinase inhibitor.7 UA also increased oxidative stress in vascular smooth muscle cells (VSMCs) through local RAAS.7 Taken together, these findings suggest that hyperuricemia could cause kidney disorders by stimulating vascular local RAAS mediated by the MAP kinase pathway at least partially. Hyperuricemia has been reported to increase mRNA expression of renin in the juxtaglomerular apparatus in rats and to play a role in the development of glomerular hypertension,5 suggesting involvement of the circulating RAAS system activated by UA. Takae et al report that elevation of the SUA level was accompanied by albuminuria, an index of hyperfiltration, associated with a decline in eGFR, suggesting the involvement of glomerular hyperfiltration caused by the circulating RAAS activated by UA,4 which may contribute to worsening kidney dysfunction (Figure). On the other hand, Kohagura et al reported that in patients with CKD, elevation of SUA significantly correlated with the severity of afferent arteriolar sclerosis.8 It is well-known that inflammation and endothelial dysfunction play an important role in the development of CKD. Several reports have indicated that hyperuricemia induces expression of inflammatory proteins such as C-reactive protein in the vascular cells, causing inflammation through activation of UATs. Kang reported that hyperuricemia caused inflammation of human VSMCs, as well as in human endothelial cells, which could be blocked by UAT inhibitors.9 We recently found that human endothelial cells (HUVEC) expressed UATs and that under hyperuricemic conditions nitric oxide production was impaired through activation of UATs, which could be attenuated by certain UAT inhibitors.10 Taken together, it is possible that hyperuricemia induces sclerosis of afferent arterioles through activation of vascular UATs, resulting in renal arteriolar damage (Figure).
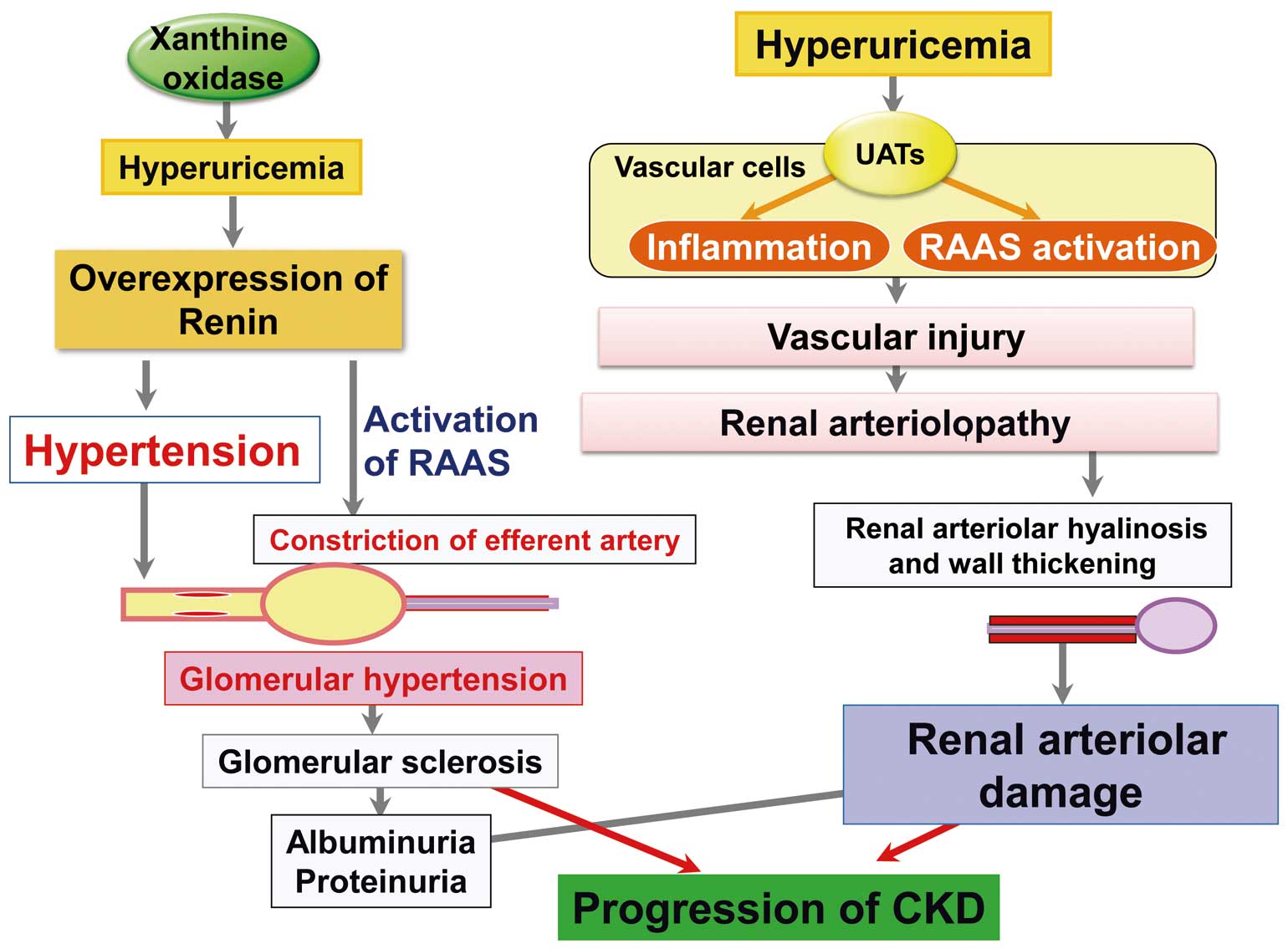
Uric acid crystal-independent mechanism of kidney disease. Hyperuricemia could cause kidney dysfunction through 2 pathways: glomerular hyperfiltration via activation of the renin-angiotensin-aldosterone system RAAS or renal arteriolar damage via inflammation.
Takae et al conclude that at the present time it remains unknown to which extent SUA levels should be reduced to attenuate the risk of albuminuria and kidney disease. So far, reports on whether or not lowering the SUA level could slow the decline in kidney function in patients with CKD are controversial. There are several randomized control clinical trials studying whether allopurinol improves kidney function in patients with CKD. Siu et al report that UA-lowering therapy reduced the risk of kidney dysfunction,11 and Goicoechea et al report that allopurinol slowed the rate of kidney disease progression in patients with CKD and reduced cardiovascular risk,12 although the number of the patients was relatively small in these clinical trials. Thus, a large-scale clinical trial to test reduction of SUA levels is needed to clarify whether lower SUA levels will reduce the risk of developing kidney disease, not only in patients with CKD but also in subjects with SUA levels within the normal range.