2016 Volume 80 Issue 9 Pages 1931-1936
2016 Volume 80 Issue 9 Pages 1931-1936
Background: The HeartMate II (HMII) continuous-flow LVAD was approved for Japanese health insurance coverage in April 2013 as a bridge to transplantation (BTT). We report on post-approval Japanese multicenter outcomes, and a comparison between patients with low and high body surface area (BSA).
Methods and Results: HMII LVAD was implanted in 104 consecutive patients at 15 Japanese centers between April 2013 and July 2014. Perioperative data were submitted to the Japanese Registry for Mechanically Assisted Circulatory Support. Patients were divided into 2 groups on the basis of BSA less or greater than 1.5 m2. Survival outcomes, New York Heart Association functional class, and adverse event rates were compared between the 2 groups. Preoperative hemodynamics and INTERMACS profiles were similar between groups. There were more females and younger patients in the low BSA group. The respective 6-month and 1-year death- or pump exchange-free survival rates were excellent: 90% and 90% in the BSA <1.5 group vs. 90% and 85% in the BSA ≥1.5 group. In the BSA <1.5 group, occurrence of hemorrhagic stroke was 10% and occurrence of embolic stroke was 0%, vs. 12% and 8% in BSA ≥1.5 group. Driveline infection was encountered more frequently in the BSA <1.5 group.
Conclusions: Results for HMII LVAD as BTT in the post-approval era showed excellent survival and functional capacity improvement. Of particular interest to the Japanese patient population are the excellent results in patients with small BSA. (Circ J 2016; 80: 1931–1936)
Implantable left ventricular assist devices (LVADs) are an important treatment option for the growing worldwide population of patients with advanced heart failure. Implanting, and then safely supporting the circulation of patients with devices containing electric motors, pumping mechanisms, and conduits attached to the heart and major blood vessels, is not trivial. The 1st-generation implantable pulsatile LVADs were designed to mimic the natural heart by providing a stroke volume and pump rate that could generate a cardiac output up to 10 L/min; accordingly, these devices were large and could only be accommodated by patients with a body surface area (BSA) >1.5 m2. Children and adult populations with a smaller BSA were disadvantaged by the size of these LVADs and were usually excluded as candidates for implantation.
Editorial p 1901
The introduction of the smaller continuous-flow LVAD offered the prospect of treating a broader range of patient size, to a BSA as small as 1.3 m2. The HeartMate II (HMII) LVAD (Thoratec Corp, Pleasanton, CA, USA) has been implanted in more than 20,000 patients worldwide, with the majority being adults with an average BSA of 2.0 m2.1–3 Experience with implanting durable LVADs in patients with a BSA <1.5 m2 is limited mostly to the pediatric population. Paracorporeal pneumatically powered pulsatile ventricular assist devices (VADs) have been most commonly used in small BSA patients; however, restricted mobility and higher complication rates lead to less favorable outcomes when compared with larger patients supported by continuous-flow LVADs.4–9
To date, the largest clinical experience with the HMII has been in the USA and Europe, where the average BSA is larger than in the Japanese population. With the increased use of this device in Japan as a bridge to transplant (BTT), there is now greater experience with implantation in patients with a BSA <1.5 m2. This study was conducted to assess any differences in outcomes and adverse events (AE) in patients within the range of smaller BSA.
This study was a retrospective analysis of patients implanted with the HMII LVAD as BTT therapy at 15 institutions in Japan. The data presented represents follow-up data in all patients who underwent HMII implantation between April 2013 and July 2014. The inclusion and exclusion criteria for implantation of the HMII LVAD have been previously published.10 Before LVAD implantation, all patients provided informed consent. All data used in this analysis were obtained from the Japanese Registry for Mechanically Assisted Circulatory Support (J-MACS).
Patients were divided into 2 groups based on a pre-LVAD implant BSA <1.5 m2 (n=30) or ≥1.5 m2 (n=74). Baseline characteristics, duration of LVAD support, clinical outcomes, survival, and AEs were compared between the 2 groups. Any cases of malposition of the device or issues related to the size of the device or the patient were examined. Data regarding pump speed and estimated flow rate was compared between groups.
Device ImplantationThe HMII LVAD was implanted by median sternotomy in all patients except in 5 cases in which pump exchange from another type of VAD was performed via subcostal incision. Standard cardiopulmonary bypass was established by aortic perfusion with bicaval or single atrial drainage, according to whether the patient required additional procedures or to follow each institutional policy. The majority of implants were performed under beating-heart conditions. Before starting cardiopulmonary bypass, a pump pocket was created in the left subcostal area. The pump pocket was created inferior and laterally enough to place the pump with an ideal inflow angle in small BSA patients. The pump pocket was further extended toward the right beyond the midline in some patients with a small rib cage or short body width in order to lay the pump to avoid compression of the right ventricle.
Heparin bridging in transition to warfarin after LVAD implantation was not routinely implemented. Anticoagulation management included warfarin, with a target prothrombin time-international normalized ratio (PT-INR) range of 2.0–2.5 and 100 mg of aspirin (Figure 1). Platelet function tests, such as thromboelastography, were not routinely used.
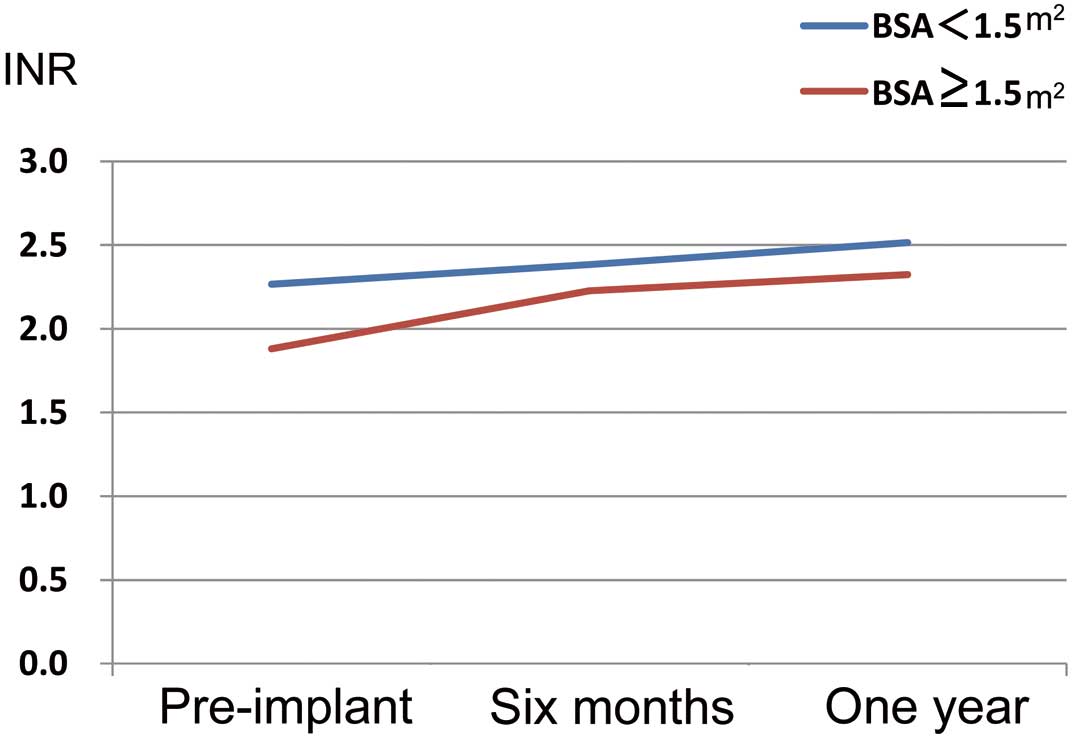
Trend in PT-INR among Japanese recipients of the HeartMate II left ventricular assist device. BSA, body surface area; PT-INR, prothrombin time-international normalized ratio.
Differences in categorical variables between groups were evaluated using the t-test and the Chi-square test, as appropriate. Statistical comparisons were 2-sided, and significance was set as P<0.05. Survival analysis was performed using the Kaplan-Meier method, with censoring for ongoing device support, transplantation, and recovery of heart function with device explant. Comparison of survival rates between the 2 groups was performed using the log-rank test. AEs are presented as percentage of patients and event rates (events per patient-year (EPPY)). All statistical analyses were done using JMP 11.0 (SAS Institute, Cary, NC, USA).
The 104 patients implanted with the HMII LVAD between April 2013 and July 2014 were included in the analysis. There were 30 patients in BSA <1.5 group and 74 patients in BSA ≥1.5 group. The baseline characteristics of the 2 study groups are provided in Table 1. Patients in the BSA <1.5 group were younger (mean age 38.5±15.4 vs. 45.3±12.7; P=0.04) and there was a larger proportion of females (53.4% vs. 13.5%; P<0.001) than in the BSA ≥1.5 group. The mean BSA for each group was 1.41±0.07 and 1.69±0.11, respectively (P<0.0001). Ischemic heart disease was present in 12 (16.2%) of the BSA ≥1.5 m2 group compared with none in the BSA ≥1.5 group (P=0.017). In the BSA <1.5 m2 group, there were 13 patients (43.3%) who had been supported by another type of VAD (Nipro paracorporeal pneumatically driven VAD (Nipro, Osaka, Japan)), which was significantly more frequent than the BSA ≥1.5 m2 group (18.9%, P=0.02). Left ventricular end-diastolic dimension before LVAD implantation was significantly smaller in the BSA <1.5 m2 group (P<0.001).
| BSA <1.5 m2 (n=30) |
BSA ≥1.5 m2 (n=74) |
P value | |
|---|---|---|---|
| Age | 38.5±19.3 | 45.3±23.4 | 0.04 |
| Male (%) | 46.7 | 86.5 | <0.001 |
| BSA (m2) | 1.41±0.64 | 1.69±0.78 | <0.001 |
| BSA range (m2) | 1.23–1.49 | 1.50–1.95 | |
| Etiology: ischemic (%) | 0.0 | 16.2 | 0.02 |
| Systolic blood pressure (mmHg) | 87.5±40.4 | 89.5±42.0 | 0.41 |
| Prior LVAD (%) | 43.3 | 18.9 | 0.02 |
| Inotropic support (%) | 56.7 | 77.0 | 0.37 |
| Indexed cardiac output (L·min−1·m−2) | 2.16±0.93 | 2.04±0.96 | 0.35 |
| LVEDD (mm) | 59.9±12.3 | 72.1±13.7 | <0.001 |
| INTERMACS profile (%) | 0.08 | ||
| I | 23.3 | 5.4 | |
| II | 26.7 | 37.8 | |
| III | 43.3 | 50.0 | |
| IV | 6.7 | 4.1 | |
| V+ | 0.0 | 2.8 |
BSA, body surface area; INTERMACS, Interagency Registry for Mechanically Assisted Circulatory Support; LVAD, left ventricular assist device.
Initial pump speed was set at 8,200–8,600 rpm in the majority of patients with small BSA, and appropriate pump speed was adjusted before discharge and at the time of outpatient clinic visit according to echocardiographic measurement or estimated flow on the monitor. During support, the average pump speed at 6 months was 8,524 rpm in the small BSA group, which was slower than that of the larger BSA group (8,657 rpm), but not statistically significant (P=0.08). Estimated pump flow rate at 6 months was similar in both groups (Table 2).
| BSA <1.5 m2 (n=30) | BSA ≥1.5 m2 (n=74) | P value | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Outcome | 0.93 | ||||
| Ongoing support | 26 | 86.7 | 64 | 86.5 | |
| Transplanted | 1 | 3.3 | 1 | 1.4 | |
| Weaned | 0 | 0.0 | 0 | 0.0 | |
| Pump exchange | 2 | 6.7 | 4 | 5.4 | |
| Died on device | 2 | 6.7 | 5 | 6.8 | |
| Support duration (days) | 347±178 (36–553) | 296±185 (8–551) | 0.12 | ||
| Patient status at 6 months | (n=22) | (n=35) | |||
| Systolic blood pressure (mmHg) | 84.8±42.4 | 86.3±43.3 | 0.79 | ||
| Estimated pump flow (L/min) | 4.6±2.4 | 4.5±2.3 | 0.73 | ||
| Pump speed (rpm) | 8,524±4,166 | 8,657±4,238 | 0.08 | ||
The average support duration of support for the BSA <1.5 m2 group and BSA ≥1.5 m2 group was 347±178 days and 296±185 days, respectively. The cumulative duration of support was 28.5 years for the BSA <1.5 m2 group and 60.0 years for BSA ≥1.5 m2 group. One patient in each group underwent heart transplantation. There were 7 deaths: 2 in the BSA <1.5 m2 group and 5 in the BSA ≥1.5 m2 group (Table 2). The causes of death were a cerebrovascular event and infection in the BSA <1.5 m2 group and all were cerebrovascular events in the BSA ≥1.5 m2 group. The Kaplan-Meier survival rate at 1 year was 92.6% and 90.9% (P=NS) for the BSA <1.5 m2 and BSA ≥1.5 m2 groups, respectively (Figure 2). If the patients were limited to those who underwent primary HMII implantation without prior paracorporeal VAD, 1 year survival rate was 93.8% and 92.5%, respectively (P=NS). The New York Heart Association (NYHA) functional class had improved dramatically at 6 months in both groups (Figure 3). As of October 2014, 28 of 30 (93.3%) patients in the BSA <1.5 m2 group and 70 of 74 (94.5%) in the BSA ≥1.5 m2 group had continued on LVAD support.

Freedom from all causes of death among Japanese recipients of the HeartMate II left ventricular assist device. BSA, body surface area.

Trend in New York Heart Association functional class among Japanese recipients of the HeartMate II left ventricular assist device. BSA, body surface area.
Driveline infection was the most frequent AE and was significantly higher in the group with BSA <1.5 m2 (Table 3). Embolic stroke occurred more frequently in the BSA ≥1.5 m2 group (P=0.001), whereas hemorrhagic stroke was nearly equal in both groups. Ventricular arrhythmia that required electrical or pharmacological conversion was documented more frequently in the BSA ≥1.5 m2 group (P=0.001). There was a trend toward a higher rate of occurrence of right heart failure in the BSA <1.5 m2 group, but was not significant. Pump thrombosis occurred similarly in both groups, with respective rates of 0.08 and 0.07 EPPY. During the 1-year follow-up period, there were no differences in hematologic or biochemistry laboratory values (Table S1). The trend in lactate dehydrogenase (LDH) levels was the same in both groups (Figure 4).
| BSA <1.5 m2 (n=30) |
EPPY | BSA ≥1.5 m2 (n=74) |
EPPY | P value | |
|---|---|---|---|---|---|
| Infection | |||||
| Driveline | 13 | 0.46 | 12 | 0.20 | 0.003 |
| Pump pocket | 0 | 0 | |||
| Pump | 0 | 0 | |||
| Mediastinum | 0 | 2 | 0.03 | NS | |
| Cerebrovascular event | |||||
| Embolic | 0 | 6 | 0.10 | 0.001 | |
| Hemorrhagic | 3 | 0.11 | 9 | 0.15 | NS |
| Other events | |||||
| Gastrointestinal bleeding | 0 | 1 | 0.02 | NS | |
| Ventricular arrhythmia | 0 | 6 | 0.10 | 0.001 | |
| Right heart failure | 4 | 0.14 | 3 | 0.05 | 0.07 |
| Myocardial infarction | 1 | 0.04 | 0 | NS | |
| Pump thrombosis | 2 | 0.08 | 4 | 0.07 | NS |
EPPY, events per patient-year; NS, not significant; VAD, ventricular assist device.

Trend in lactate dehydrogenase (LDH) among Japanese recipients of the HeartMate II left ventricular assist device. BSA, body surface area.
There were 6 pump exchanges, 2 of which were performed in 1 patient in the BSA <1.5 group for pump thrombosis, and 4 in the BSA ≥1.5 group (3 for pump thrombosis, 1 for pump pocket infection). Another type of VAD was used in 1 of 4 pump exchanges in the BSA >1.5 group. Death or pump exchange-free survival at 1 year was 90% in the BSA <1.5 group and 85% in the BSA ≥1.5 group (P=NS; Figure 5). If the patients were limited to those who underwent primary HMII implantation, the death or pump exchange-free survival was 88.2% and 88.3%, respectively (P=NS).

Freedom from death or pump exchange among Japanese recipients of the HeartMate II left ventricular assist device. BSA, body surface area.
The use of continuous-flow LVADs for supporting patients with endstage heart failure has increased greatly in recent years because of the improved outcomes and better acceptance of the technology.1–3,10–12 The smaller LVAD can be implanted with less surgical trauma, shorter cardiopulmonary bypass time, shorter recovery time, and overall fewer AEs related to implantation.13,14 The smaller devices should allow implantation in a much larger population of heart failure patients, such as children and small adults. This study indicates that the HMII can safely and effectively support the Japanese population of adults with heart failure and lower BSA.
Data from INTERMACS in the USA show that the average size of patients implanted with the earlier generation of pulsatile devices was 2.27 m2, and now those implanted with the current continuous-flow LVAD have an average BSA of 2.04 m2.15 To date, experience with HMII implantation in patients with a BSA <1.5 m2 has been limited. The HMII trials for both BTT and destination therapy (DT) had a lower limit of BSA at 1.3 m2, but these trials also limited enrollment to patients older than 18 years of age, which excluded smaller, younger children. However, the US clinical trial did have a cohort of small BSA <1.5 m2 patients, with results that were equivalent to the study as a whole.16,17 Even in the post-trial studies with fewer restrictions on patient selection, the average size of patients did not decline.2,3 In a recent study of pediatric and young adult patients implanted with the HMII LVAD, the average BSA was 1.91 m2 (1.47–2.65 m2) and 2.08 m2 (1.12–3.10 m2) in the 2 cohorts, which is still not representative of a small-size population.18 This is one of the first published reports of HMII LVAD use in a population of patients with a BSA <1.5 m2.
There has been discussion about possible increased risk of pump thrombosis in cases of lower pump speed and flow. In this multicenter study, we carefully checked the pump speed at 6 months; speeds were 8,524±4,166 rpm in the BSA <1.5 m2 group and 8,657±4,238 rpm in the BSA ≥1.5 m2 group. PT-INR levels were strictly controlled between 2.0 and 2.5, as shown in Figure 1. Pump thrombosis rates were lower than those reported in some studies from the USA. Thus, we believe the HMII LVAD can be implanted and managed safely in patients with small body size when PT-INR is strictly controlled. Higher pump speed, such as 9,400 rpm, is usually recommended in the US patient population to avoid pump thrombosis. In our experience, when the pump speed was increased as high as 9,400 rpm in a small patient, the interventricular septum was easily shifted toward the left ventricle and the chance of sucking events increased. We judged an optimal flow by observing the end-diastolic left ventricular dimension on echocardiography, by which means a sufficient assist flow was obtained while preserving a safe lv dimension.
The implant centers exchanged ideas on how to safely and effectively create a pump pocket in a patient with small body size. This exchange of ideas may have contributed to a uniform pump pocket creation technique and a lower pump thrombosis rate in the BSA <1.5 m2 group despite the smaller LV.
An interesting finding of this study was that very few gastrointestinal bleeding events occurred. However, our patients were younger than the DT patients in the USA, which may be an important factor. As already described, there was no significant difference in anticoagulation and antiplatelet therapies in Japan relative to that used in the USA. Whether the reduced occurrence of gastrointestinal bleeding is the result of any difference in intestinal arteriovenous fistula formation or stems from other genetic characteristics is to be elucidated.
Post-approval use of the HMII for BTT in Japan demonstrated excellent survival with improvement in functional capacity in the whole population. Of particular interest is that patients with a smaller BSA have equivalent outcomes and similar management to patients with a larger BSA.
None of the authors has a conflict of interest to disclose. The authors acknowledge all participants of the J-MACS Registry and all clinical institutions, surgeons and medical staff who contributed to this project.
Supplementary File 1
Table S1. Laboratory values before implantation of the HeartMate II LVAD, and at 6 and 12 months after
Please find supplementary file(s);
http://dx.doi.org/10.1253/circj.CJ-16-0203