2017 Volume 81 Issue 10 Pages 1522-1527
2017 Volume 81 Issue 10 Pages 1522-1527
Background: Acute kidney injury (AKI) is the most common and most serious complication following heart surgery. We aimed to determine the prevalence of, and risk factors for, AKI following pediatric cardiac surgery.
Methods and Results: We retrospectively analyzed 135 patients aged ≤18 years who underwent cardiac surgery for congenital heart defects; by RACHS-1 category, 58 patients (43%) had an operative risk score ≥3. AKI was defined and classified using the pediatric pRIFLE criteria (Pediatric Risk, Injury, Failure, Loss, and End-stage Kidney Disease); 19 patients (14.1%) developed AKI: 17 had AKI with a severity classified as risk (R) and 2 had AKI classified as injury (I). Body weight, height, body surface area, and preoperative mechanical ventilation were all independently associated with AKI development (P=0.038, 0.040, 0.033 and 0.008, respectively). Preoperative ventilation strongly correlated with AKI severity. Higher pRIFLE classification positively correlated with increased incidence of peritoneal dialysis, increased postoperative mechanical ventilation duration, and longer hospital stay (P=0.009, 0.039 and 0.042, respectively).
Conclusions: In this study, we found a low prevalence of postoperative AKI in pediatric patients undergoing severe cardiac surgery. AKI was associated with worse early postoperative outcomes. Early prediction and appropriate treatment of AKI during the postoperative period are emphasized.
Acute kidney injury (AKI) remains the most common and severe complication following pediatric cardiac surgery. Although AKI is a reversible type of kidney damage, it is associated with worse clinical outcomes, such as longer hospital stay, increased incidence of infection, and higher risk of morbidity and mortality.1–3 Several studies have evaluated the numerous risk factors associated with the development of AKI following surgery for congenital heart conditions.1–7
There are various diagnostic criteria for pediatric AKI that are based on serum creatinine (SCr) and urine output (UO), but the validity of AKI diagnosis remains unclear.2,8,9 The RIFLE criteria are the most commonly used for the diagnosis of AKI.1,3–7,9 RIFLE (an acronym for Risk of renal dysfunction, Injury to the kidney, Failure of kidney, Loss of kidney function, and End-stage renal disease) was introduced in 2004 when the Acute Dialysis Quality Initiative working group developed the first consensus definition of AKI.10 In 2007, modified RIFLE criteria for critically ill pediatric patients were developed (pRIFLE).4
The primary purpose of the present study was to determine the prevalence of AKI in pediatric cardiac surgery patients using the pRIFLE criteria. Our secondary objectives were to identify the risk factors for the development of postoperative AKI and to analyze the association between AKI and poor clinical outcomes. We also evaluated clinical factors that may contribute to more severe AKI, according to the pRIFLE criteria. Finally, we determined whether mild AKI following congenital cardiac surgery is associated with any prolonged renal dysfunction.
We conducted a single-center, retrospective study of 135 patients with congenital heart disease (CHD) who underwent cardiac surgery with cardiopulmonary bypass (CPB) at Konkuk University Medical Center between April 2011 and December 2011. We excluded patients >18 years of age at the time of surgery, patients who had multiple operations within 14 days, patients with preexisting renal insufficiency, and patients with a history of dialysis requirement.
We obtained demographic data, preoperative SCr, blood urea nitrogen, CPB time, aortic cross-clamping (ACC) time, and postoperative data (UO, dose of diuretics, dose of inotropic medication, pRIFLE classification, and need for peritoneal dialysis (PD) within the week following cardiac surgery). The need for PD was determined by the attending physicians. We analyzed all medical records, from the time of patient discharge to November 2016, for survival, subsequent unplanned cardiac surgery, and/or renal complications.
Pediatric AKI was defined using the pRIFLE criteria (Table 1) based on the patient’s estimated creatinine clearance (eCCl) and UO.4 If patients were assigned to a different category based on their eCCl vs. UO, we chose the higher pRIFLE score.
| eCCl | Urine output | |
|---|---|---|
| Risk | eCCl decrease by 25% | <0.5 mL/kg/h for 8 h |
| Injury | eCCl decrease by 50% | <0.5 mL/kg/h for 16 h |
| Failure | eCCl decrease by 75% or eCCl <35 mL/min/1.73 m2 | <0.3 mL/kg/h for 24 h or anuria for 12 h |
| Loss | Persistent failure >4 weeks | |
| End-stage | Persistent failure >3 months |
eCCl, estimated creatinine clearance, according to the Schwartz formula.
eCCl was determined using the Schwartz formula according to following equation:11
eCCl=κ×body length (cm)/SCr value (mg/dL),
where the coefficient κ is 0.33 in preterm infants <1 year old, 0.45 in full-term infants <1 year old, 0.55 in children 2–12 years old, and 0.55 and 0.70 in children >12 years old.
The individual operative risk was classified into 6 categories using the Risk Adjustment for Congenital Heart Surgery-1 (RACHS-1) scale.6 Dosage of inotropic drug was quantified using the vasoactive inotropic score (VIS) according to the following equation:12
VIS=dopamine dose (µg/kg/min)+dobutamine dose (µg/kg/min)+100×epinephrine dose (µg/kg/min)+10×milrinone dose (µg/kg/min)+10,000×vasopressin dose (U/kg/min)+100×norepinephrine dose (µg/kg/min).
Data for duration of mechanical ventilation and length of intensive care unit (ICU) stay were also collected.
Statistical AnalysisWe first performed tests for descriptive statistics. Continuous variables are described as the mean±standard deviation (SD), as the median value (interquartile range [IQR]), or as a frequency (N) with proportion (%). When comparing an AKI group with a non-AKI group, the Wilcoxon rank-sum test was performed to determine the statistical significance of continuous variables and the Chi-square test was performed to compare 2 categorical variables. A simple logistic regression analysis was performed to evaluate risk factors for AKI. A correlation analysis was then performed between the 2 variables. A multiple logistic regression analysis was included when independent variables were P<0.1 in the univariate analysis. AKI severity was analyzed using a Kruskal-Wallis test. In all analyses, P<0.05 was considered statistically significant. The data were analyzed using SPSS software version 23.0 (IBM SPSS Software for Predictive Analytics; SPSS, Chicago, IL, USA).
This study enrolled 135 pediatric patients (≤18 years old): 13 (9.6%) were neonates and 77 (57%) were infants. Palliative surgery was performed in 48 patients (35.6%) and corrective surgery was performed in 87 patients (64.4%). A total of 58 patients (43%) had a RACHS-1 operative risk score ≥ 3. The demographic and physiological data are presented in Table 2 and Table 3.
| Diagnosis | n (%) |
|---|---|
| ASD | 12 (8.9) |
| VSD | 44 (32.6) |
| AVSD | 5 (3.7) |
| CoA | 5 (3.7) |
| TOF | 13 (9.6) |
| PA-IVS | 3 (2.2) |
| PA-VSD | 5 (3.7) |
| DORV | 6 (4.4) |
| HLHS | 1 (0.7) |
| UVH | 18 (13.3) |
| TGA | 2 (1.5) |
| TGA-VSD | 5 (3.7) |
| Other | 16 (11.9) |
| Total | 135 (100) |
ASD, atrial septal defect; AVSD, atrioventircular septal defect; CHD, congenital heart disease; CoA, coarctation of aorta; HLHS, hypoplastic left heart syndrome; IVS, intact ventricular septum; PA, pulmonary atresia; TGA, transposition of the great arteries; TOF, tetralogy of Fallot; UVH, univentricular heart; VSD, ventricular septal defect.
| Characteristic | All patients (n=135) |
|---|---|
| Preoperative clinical status | |
| Age (days) | 480.01±903.86 |
| Age by group | |
| ≤28 days | 13 (9.6) |
| >28 days and ≤1 year old | 77 (57) |
| >1 and <18 years old | 45 (33.3) |
| Sex | |
| Female | 66 (48.9) |
| Male | 69 (51.1) |
| Weight (kg) | 8.40±7.35 |
| Height (cm) | 69.48±19.87 |
| BSA (m2) | 0.39±0.21 |
| Palliative operation | 48 (35.6) |
| UVH | 18 (13.3) |
| RACHS-1 ≥3 | 58 (43) |
| Intraoperative factors | |
| Use of CPB | 117 (86.7) |
| CPB time (min) | 64.63±50.75 |
| ACC time (min) | 26.22±30.08 |
| Outcome | |
| MV (days) | 2.26±3.75 |
| ICU stay (days) | 5.71±9.44 |
| PD | 8 (5.9) |
| In-hospital death | 1 (0.7) |
Data are n (%) or mean±SD. ACC, aortic cross-clamping; BSA, body surface area; CPB, cardiopulmonary bypass; ICU, intensive care unit; MV, mechanical ventilation; PD, peritoneal dialysis; RACHS-1, Risk Adjustment for Congenital Heart Surgery-1. Other abbreviations as in Table 2.
According to the pRIFLE criteria, 19 patients (14.1%) developed AKI and 116 (85.9%) did not. The incidence of AKI in neonates, infants, and children was 30.8% (4/13), 14.3% (11/77) and 8.9% (4/45), respectively. Among patients who developed AKI, 17 developed AKI with a severity classified as risk (R) and 2 patients developed AKI with a classification of injury (I); none developed AKI classified as failure (F) or worse (Figure 1). The pRIFLE classification was applied using UO in 1 patient (5.3%), eCCl in 18 patients (94.7%), and both in 2 patients with AKI. A total of 14 patients developed AKI on the first postoperative day and 5 patients developed AKI within 7 days after cardiac surgery (Figure 2). Only one patient, who had AKI classified as “I”, experienced AKI for 7 full days; this patient’s AKI changed from “I” to “R” over time. All patients diagnosed with AKI within 1 week following cardiac surgery fully recovered before discharge. There were no postoperative deaths in the AKI group. One patient in the non-AKI group died from brain injury during the postoperative period.
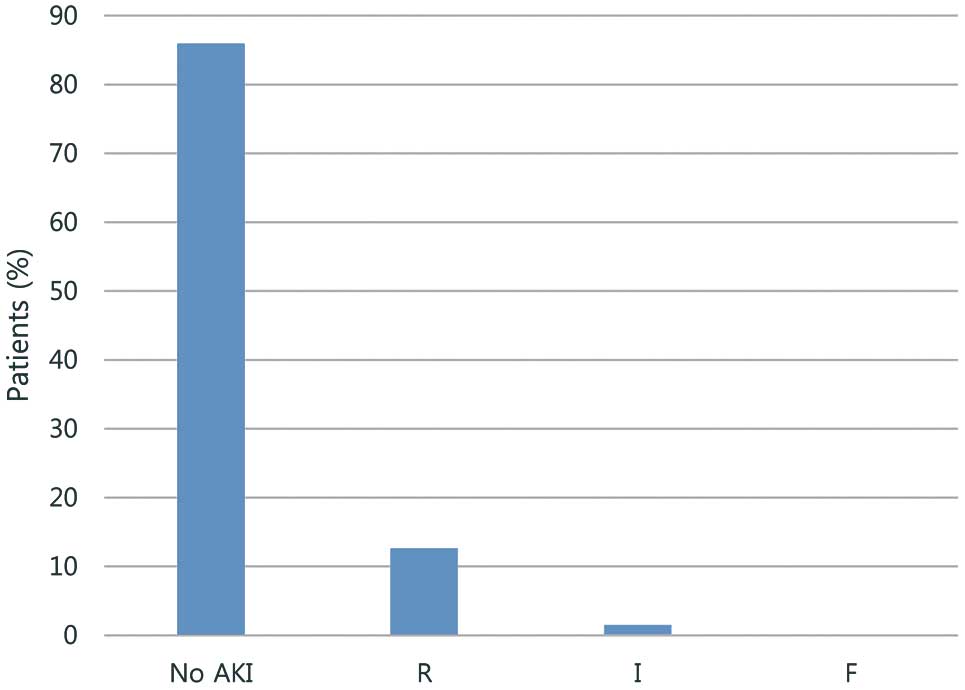
Acute kidney injury (AKI) incidence and distribution within pediatric RIFLE classes R (risk of renal dysfunction), I (injury to the kidney) and F (failure of kidney). [0 cases in classes L (loss of kidney function) and E (end-stage renal disease).]

Day vs. percentage of patients diagnosed with acute kidney injury (AKI) following surgery for congenital heart disease.
There was no difference in median age (P=0.068) or sex (P=0.397) between the AKI and non-AKI patients. Median weight, height, and body surface area (BSA) were lower in the AKI patients than in those without AKI (P=0.024, 0.046 and 0.030, respectively). The preoperative mechanical ventilation rate was higher in patients with AKI than in those without AKI (P=0.011). Preoperative SCr and VIS did not differ between groups (0.564 and 0.308, respectively). There was no difference in the incidence of palliative operations, RACHS-1 score ≥3, univentricular heart (UVH), or use of CPB (P=0.246, 0.935, 0.284 and 0.719, respectively). The median CPB time and ACC time did not differ between groups (P=0.834 and 0.078, respectively). There was a higher incidence of PD use in the AKI group (P=0.014). The duration of both postoperative mechanical ventilation and ICU stay was increased for AKI patients compared with non-AKI patients (P=0.011 and 0.012, respectively; Table 4).
| Characteristic | AKI (n=19) |
Non-AKI (n=116) |
P value |
|---|---|---|---|
| Preoperative clinical status | |||
| Age (days) | 198.33 (20–1,088) | 210.50 (8–6,565) | 0.068 |
| Age by group | 0.135 | ||
| ≤28 days (%) | 4 (21.1) | 9 (7.8) | |
| >28 days and ≤1year old | 11 (57.9) | 66 (56.9) | |
| >1 and <18 years old | 4 (21.1) | 41 (35.3) | |
| Female | 11 (57.9) | 55 (47.4) | 0.397 |
| Weight (kg) | 5.2 (1.1–14.1) | 7.65 (1.0–65.4) | 0.024 |
| Height (cm) | 59 (37–91.5) | 68.45 (38–160) | 0.046 |
| BSA (m2) | 0.29 (0.11–0.6) | 0.38 (0.1–1.6) | 0.030 |
| Palliative operation | 9 (47.4) | 39 (33.6) | 0.246 |
| RACHS-1 ≥3 | 8 (42.1) | 50 (43.1) | 0.935 |
| UVH | 4 (21.1) | 14 (12.1) | 0.284 |
| Preoperative MV | 6 (31.6) | 10 (8.6) | 0.011 |
| Preoperative SCr | 0.31 (0.2–1.1) | 0.32 (0.2–1.1) | 0.564 |
| Preoperative VIS | 0 (0–10) | 0 (0–20.3) | 0.308 |
| Intraoperative factors | |||
| Use of CPB | 16 (84.2) | 101 (87.1) | 0.719 |
| CPB time (min) | 53 (0–182) | 55 (0–253) | 0.834 |
| ACC time (min) | 8 (0–75) | 23 (0–185) | 0.078 |
| Outcome | |||
| MV (days) | 2 (0–28) | 1 (0–23) | 0.011 |
| ICU stay (days) | 4 (1–47) | 3 (1–60) | 0.012 |
| PD (%) | 4 (3.3) | 4 (21) | 0.014 |
Data are n (%) or median (IQR). AKI, acute kidney injury; IQR, interquartile range; SCr, serum creatinine; VIS, vasoactive inotropic score. Other abbreviations as in Tables 2,3.
We determined the risk factors for AKI development using a simple logistic regression. Body weight (odds ratio [OR] 0.841, 95% confidence interval [CI] 0.714–0.99, P=0.038), height (OR 0.841, 95% CI 0.928–0.998, P=0.040), BSA (OR 0.01, 95% CI 0–0.692, P=0.033), and a higher proportion of preoperative mechanical ventilation (OR 4.892, 95% CI 1.527–15.677, P=0.008) were all independently associated with the development of AKI. Age (OR 0.999, 95% CI 0.997–1.001, P=0.212), female sex (OR 1.525, 95% CI 0.572–4.067, P=0.399), palliative surgery (OR 0.563, 95% CI 0.211–1.499, P=0.250), RACHS-1 score ≥3 (OR 0.96, 95% CI 0.36–2.563, P=0.935), and UVH (OR 1.943, 95% CI 0.564–6.689, P=0.292) were not associated with AKI development. Furthermore, preoperative VIS (OR 1.065, 95% CI 0.925–1.225, P=0.381), CPB time (OR 0.999, 95% CI 0.989–1.009, P=0.791) and ACC time (OR 0.98, 95% CI 0.957–1.003, P=0.087) were not associated with AKI. However, the multivariate analysis did not show any risk factors for AKI. Correlation analysis was performed between each variable and AKI development. BSA, weight, height, and preoperative ventilation no longer predicted AKI following correlational analysis.
Variables Affecting AKI SeverityA Kruskal-Wallis test was performed between AKI severity and each variable. Age, low body weight, height, and lower BSA were not related to a higher pRIFLE class of AKI (P=0.173, 0.067, 0.118, 0.080, respectively), nor were RACHS-1 ≥3 (P=0.969) and preoperative VIS (P=0.428). There was a higher proportion of preoperative mechanical ventilation in severe compared with moderate cases of AKI (P=0.011). CPB time (P=0.229) and ACC time (P=0.185) did not differ in severe cases. A higher pRIFLE class was associated with the outcome data including a higher proportion of postoperative mechanical ventilation (P=0.039), a higher need for PD (P=0.009), and longer ICU stay (P=0.042) (Table 5).
| Characteristic | None (n=116) |
Risk (n=17) |
Injury (n=2) |
P value |
|---|---|---|---|---|
| Preoperative clinical status | ||||
| Age (days) | 210.5 (8–6,565) | 130 (20–1,088) | 121 (24–218) | 0.173 |
| Weight (kg) | 7.65 (1–65.4) | 5.2 (1.1–14.1) | 4.87 (2.94–6.8) | 0.067 |
| Height (cm) | 68.45 (38–160) | 59 (37–91.5) | 55.35 (43.5–67.2) | 0.118 |
| BSA (m2) | 0.38 (0.1–1.6) | 0.28 (0.11–0.6) | 0.27 (0.19–0.4) | 0.080 |
| RACHS-1 ≥3 | 50 (43.1) | 7 (41.2) | 1 (50) | 0.969 |
| Preoperative MV | 10 (8.6) | 5 (29.4) | 1 (50) | 0.011 |
| Preoperative VIS | 0 (0–20.3) | 0 (0–10) | 0 | 0.428 |
| Intraoperative factor | ||||
| CPB time (min) | 55 (0–253) | 58 (0–182) | 18 (0–36) | 0.229 |
| ACC time (min) | 23 (0–185) | 8 (0–75) | 7 (0–14) | 0.185 |
| Outcome | ||||
| MV (days) | 1 (0–23) | 2 (0–28) | 2.5 (1–4) | 0.039 |
| ICU stay (days) | 3 (1–60) | 4 (1–47) | 9 (2–16) | 0.042 |
| PD | 4 (3.4) | 3 (17.6) | 1 (50) | 0.009 |
Data are n (%) or median (IQR). Abbreviations as in Tables 2–4.
A total of 97 patients were followed for 5 years following surgery. There was 1 hospital death from thrombosis and 4 late deaths unrelated to kidney injury. During follow-up, only one patient (14.3%) had persistent proteinuria (>3.5 g/day), which was determined by 3 consecutive urine analyses.13
There were 27 patients who underwent cardiac reoperation for various reasons, and 14 of them (51.9%) developed AKI following reoperation. Of the 15 patients who had AKI following their first operation, 7 had cardiac reoperation and 5 of them (71.4%) redeveloped AKI. All 5 patients recovered prior to discharge. However, of the 82 patients who did not develop AKI following their first operation, 20 patients had cardiac reoperation and of them 9 patients (45%) developed AKI following reoperation; all fully recovered prior to discharge (Table 6).
| Variable | All patients (n=134, %) |
|---|---|
| Lost to follow-up | 33 (24.6) |
| Death | 4 (3.0) |
| Related to kidney problems | 0 |
| Other reasons | 4 |
| Follow-up (5 years) | 97 (72.4) |
| Patients with AKI in 2011 | 15 (15/19, 78.9%) |
| No reoperation | 8 |
| Reoperation (planned, unplanned) | 7 |
| AKI | 5 (71.4) |
| Non-AKI | 2 (28.6) |
| Other renal problems | 1 |
| Patients without AKI in 2011 | 82 (82/116, 70.7%) |
| No reoperation | 62 |
| Reoperation (planned, unplanned) | 20 |
| AKI | 9 (45.0) |
| Non-AKI | 11 (55.0) |
| Other renal problems | 0 |
Abbreviations as in Tables 2,4.
Several studies have shown that AKI is a risk factor for a more complicated clinical course, higher mortality, and longer hospitalization following cardiac surgery.1,3,7 Clinical manifestations of AKI vary from asymptomatic to fluid retention, oliguria, azotemia, and renal failure. Characteristics of cardiac surgery, including the use of CPB, CPB time, ACC time and high levels of vasopressors, increase the risk of AKI development.14 These factors alter renal perfusion, induce cycles of ischemia and reperfusion, increase oxidative damage, increase renal and systemic inflammation, and contribute to the development of AKI.14,15
To date, there are many diagnostic criteria and biomarkers for AKI diagnosis, because the incidence of AKI varies according to age and other criteria. Based on previous studies, the incidence of AKI following pediatric cardiac surgery varies from 3% to 52% and dialysis is required in up to 53% of children with postoperative AKI.1,8,14,16–20 In the present study, we found a relatively low incidence of AKI following pediatric cardiac surgery (14.1%), despite the high number of severe cardiac procedures (43% of surgeries had a RACHS-1 risk score ≥3). However, the incidence of AKI following reoperation was much higher (51.9%) and was particularly high in patients who had developed AKI following their first cardiac surgery (71.4%).
Although the use of eCCl can lead to overestimation of AKI, the pRIFLE criteria are useful for diagnosing lower severity AKI with higher sensitivity.8 However, pRIFLE may have lower specificity in the case of severe kidney injury. Nevertheless, most AKI patients in the present study developed only mild kidney injury and all patients had nearly fully recovered prior to discharge. There were zero AKI-related deaths. Only one person developed proteinuria (>3.5 g/day) during the 5-year follow-up. There is a higher prevalence of proteinuria in CHD patients who remain cyanotic despite cardiac surgery compared with the general population.21 Therefore, our single case of proteinuria could be related to more than just the patient’s cardiac surgery. It can be concluded that kidney injury during the postoperative period after surgery for CHD is mild and patients tend to fully recover.
Our findings showed that low body weight, small height, lower BSA, and preoperative mechanical ventilation were risk factors for AKI. This suggests that patients with a smaller body size are more vulnerable to developing AKI because of fluid overload, electrolyte imbalance, inflammatory response to surgery, or CPB. Requirement for preoperative mechanical ventilation suggested relatively poor preoperative condition. Younger age showed a tendency to be a risk factor for AKI development, though there was no significant correlation. This suggests that lower body indices may have been related to younger age and/or to poor general condition.
Surprisingly, we found that the severity of the cardiac procedure according to RACHS did not influence AKI incidence, which differed from some studies’ findings,3,17 but was similar to that of others.1,5,22 The reasons for this finding are unclear, but may relate to the fact that patients with UVH would account for much of the variance in requiring operations in terms of RACHS-1 category.23 A small AKI group may be another reason. Similarly, preoperative VIS, use of CPB, the CPB time, and ACC time were not associated with AKI development. There was no difference in AKI prevalence between patients with a univentricular vs. a biventricular heart. This is consistent with findings from Ricci et al.24 Furthermore, low body weight, small height, and lower BSA were not associated with a higher grade of AKI.
During the postoperative period, pediatric cardiac surgery patients are vulnerable to developing AKI. Cardiac surgery patients often require renal replacement therapy (RRT) for conditions such as hypoxemia, volume overload, presence of cytokines related to CPB, inflammatory response to surgery, the use of nephrotoxic drugs, and high levels of inotropic medication. Patients with RRT show particularly higher incidences of volume overload, high doses of diuretics, and high levels of inotropic agents.
We show here that the development of more severe AKI, according to pRIFLE criteria, was associated with early postoperative outcomes such as frequent use of PD, prolonged postoperative mechanical ventilation, and prolonged ICU stay. Thus, clinical risk factors such as those identified in this study should be used to predict the development of severe AKI. pRIFLE is a useful tool for diagnosing postoperative AKI and subsequently determining RRT requirements. There remains a need to identify a rapidly diagnostic biomarker for AKI in the future.
Study LimitationsSeveral limitations should be considered. First, this study was a retrospective review study with a relatively short clinical follow-up period. Biomarkers of renal damage, including NGAL, KIM 1, IL-18, NAG, and GST and the biomarker of renal function cystatin C, have advantages over SCr.15 Although various studies used these renal function and damage biomarkers to predict AKI, we were unable to collect data for these biomarkers in our retrospective study. However, we were still able to identify clinical risk factors that predicted AKI severity. Moreover, this was a single-center study with a relatively small number of AKI patients with heterogeneous CHD. A larger study group could have revealed other risk factors for AKI. Furthermore, although AKI is typically diagnosed by assessing SCr and UO, UO is relatively nonspecific. Oliguria is common following cardiac surgery as a response to intravascular hypovolemia. AKI is thus most often diagnosed using SCr levels; however, using SCr endpoints requires many hours or days to diagnose AKI following renal insult. Finally, the Schwartz formula was used to calculate eCCl and is known to overestimate the glomerular filtration rate.
We found that the incidence of AKI development following pediatric cardiac surgery was low despite a high number of severe cardiac procedures. The incidence and severity of AKI were related to certain preoperative clinical factors and patients with AKI had poorer early postoperative outcomes. Therefore, early prediction of AKI and appropriate subsequent treatment following pediatric cardiac surgery are emphasized.