Abstract
Background:
We investigated the effect of anemia on cardiovascular hemodynamics, therapeutic strategies and clinical outcomes in heart failure (HF) patients.
Methods and Results:
We divided 198 consecutive HF patients who underwent right heart catheterization before in-hospital HF treatment into 2 groups according to the presence or absence of hemodynamic congestion (HC: mean pulmonary capillary wedge pressure ≥15 mmHg and/or mean right atrial pressure ≥10 mmHg). The hemoglobin level correlated with the cardiac index (CI) and systemic vascular resistance index (SVRI) (r=−0.34 and 0.42, P<0.05, respectively), and was the strongest contributor of SVRI only in the HC group. Anemic patients more frequently required intravenous inotropic support despite having higher CI and lower SVRI than non-anemic patients in the HC group. The novel hemodynamic subsets based on mean right atrial pressure and estimated left ventricular stroke work index but not Forrester subsets appropriately predicted the need for intravenous inotropic support. The probability of hospitalization for worsening HF during 2-year follow-up period was significantly higher in anemic patients than in non-anemic patients in the HC group.
Conclusions:
Anemia had a direct effect on cardiovascular hemodynamics and thus can confound therapeutic planning in HF patients with HC. The novel hemodynamic subsets can be applied in daily clinical practice regardless of the presence or absence of anemia.
Anemia is a common comorbidity in patients with chronic heart failure (HF) and is associated with worse long-term outcomes.1–3
The mechanisms by which anemia worsens HF outcomes are unclear but may be related to increased myocardial workload.4
In chronic severe anemia, a low hemoglobin (Hb) concentration reduces systemic vascular resistance (SVR) as the result of decreases in blood viscosity and enhanced nitric oxide-mediated vasodilation.5–7
Low SVR reduces blood pressure (BP) and causes baroreceptor-mediated neurohormonal activation,5
identical to that seen in low-output HF. The increased sympathetic and renin-angiotensin activity decreases the renal blood flow and glomerular filtration rate (GFR), resulting in salt and water retention by the kidneys and expansion of the extracellular and plasma volumes. The combined effect of volume expansion and vasodilation increases cardiac output,5
which may help to increase oxygen transport. Although these hemodynamic and neurohormonal responses are observed in patients with severe anemia, it is unclear whether these mechanisms are also operative in HF patients with less severe anemia. Anemia of varying severity can coexist with HF in the clinical setting. However, it remains unknown whether and how anemia contributes to cardiac hemodynamic status in the HF population. The aim of our study was to determine whether and how anemia regulates cardiac hemodynamics and, if it does, to elucidate the best hemodynamic parameter(s) to evaluate hemodynamic status in anemic patients with HF.
Methods
Study Population
We retrospectively reviewed all 493 hospitalized HF patients with stages B–D who underwent right heart catheterization (RHC) during hospitalization in Mie University Hospital between January 2011 and March 2015. From among them, we selected patients who had RHC in order to evaluate their hemodynamic status before in-hospital HF treatment was initiated. Patients were excluded from the present study foe the following reasons: if they required urgent intravenous medical therapy for acute HF before RHC, or if they had been treated with intravenous inotropic agents and/or vasodilators, respirators, intra-aortic balloon pumping, extracorporeal membrane oxygenation or hemodialysis prior to RHC; if they had clinical and/or hemodynamic evidence of cardiac shunt or constrictive pericarditis; scheduled to undergo major cardiac surgery or with acute coronary syndrome. Ultimately, we selected 198 consecutive patients (mean age 67±16 years, range 14–88 years, 133 males) who underwent RHC after hospital admission (median: 2 days, interquartile range 1–2 days). This study was approved by the Mie University Hospital Institutional Review Board (reference no. 2413) and all patients gave “opt-out” informed consent.
Clinical Evaluation
Among the 198 patients, there were 70 who underwent elective RHC during routine cardiac catheterization. All patients underwent baseline blood tests including neurohumoral assessment in the early morning before breakfast on the day of RHC (n=180) or immediately after hospital admission (n=18) just before RHC. Anemia was defined using the World Health Organization definition: blood Hb level <13.0 g/dL for men and <12.0 g/dL for women.8
The estimated GFR of each patient was calculated from their serum creatinine (SCr) value and their age using the following equation: eGFR (mL/min/1.73 m2)=194×Age−0.287×SCr−1.094(if female×0.739).9
Echocardiography-derived left ventricular (LV) end-diastolic dimension (LVDd) was assessed from the parasternal long-axis view, and the LV ejection fraction (LVEF) was assessed using the biplane Simpson’s rule. All patients underwent RHC using pulmonary artery catheters and simultaneous arm-cuff BP measurement. The mean pulmonary capillary wedge pressure (PCWP), mean pulmonary arterial pressure (PAP), and mean right atrial pressure (RAP) were measured, and the stroke volume index (SVI) and cardiac index (CI) were estimated by the thermodilution method. Pulmonary vascular resistance index (PVRI), systemic vascular resistance index (SVRI) and estimated LV stroke work index (eLVSWI)10
were calculated using the following formulas:
PVRI=(mean PAP−mean PCWP)×80/CI, dyne/s/cm−5/m2
SVRI=(mean BP−mean RAP)×80/CI, dyne/s/cm−5/m2
eLVSWI=(mean BP−mean PCWP)×SVI×0.0136, g-m/m2
The patients were divided into 2 groups according to their hemodynamic parameters: hemodynamic congestion group (HC group), mean PCWP ≥15 mmHg and/or mean RAP ≥10 mmHg; Non-HC group, mean PCWP <15 mmHg11,12
and mean RAP <10 mmHg.13,14
Patients in each group were further divided into 2 subgroups according to the presence or absence of anemia.
Definition of Clinical Events
Clinical events were independently examined during hospitalization and during the 2-year follow-up after hospital discharge. Clinical events during hospitalization comprised in-hospital death and the need for intravenous inotropic support to relieve symptoms or improve the signs of HF. Clinical events during follow-up after discharge comprised death from any cause and unplanned hospitalization for worsening HF. All therapy during the study period was at the discretion of the treating physicians.
Statistical Analysis
All continuous variables are presented as the mean±standard deviation or median (25–75th percentile). In order to assess differences among the 4 subgroups, Pearson’s chi-squared test for nominal scales and a one-way analysis of variance (ANOVA) with Bonferroni posthoc analysis for other scales were performed for clinical and laboratory data. A two-way ANOVA with Tukey posthoc analysis was used to evaluate 2 group effects (HC and anemia) and their interaction effect for hemodynamic parameters. The linear correlations between the variables were parametrically evaluated using Pearson’s product moment correlation coefficient. We performed multivariate linear regression analysis using a forced-entry method to determine independent predictors of SVRI. Receiver-operating characteristic (ROC) analysis and stepwise logistic regression analysis were performed to determine the best combination of hemodynamic parameters for predicting the need for intravenous inotropic support. Cumulative event rates were assessed using the Kaplan-Meier method and compared using the log-rank test. For all tests, a P value <0.05 was considered statistically significant. Data were analyzed using SPSS Statistics for Windows, Version 23.0 (IBM Corp., Armonk, NY, USA).
Results
Patients’ Characteristics
Among the 198 patients, blood Hb levels were 12.4±2.4 g/dL (12.9±2.5 g/dL in males, 11.5±2.0 g/dL in females) with a range of 6.9–18.7 g/dL, and anemia was present in 50% of all patients (45% of men, 58% of women).
Table 1
shows the characteristics of the 4 subgroups. Statistical differences among them were observed for age, male sex, New York Heart Association (NYHA) functional class, the prevalence of dilated cardiomyopathy, valvular heart disease, atrial fibrillation and the prescription rates of calcium-channel blockers (CCBs) and diuretics. Anemic patients were older than non-anemic patients in both the non-HC and HC groups. Anemic patients had significantly higher NYHA functional class than non-anemic patients in the HC group only. The prescription rates of CCBs were higher for anemic patients only in the HC group. The Hb level in anemic patients in the HC group was lower than that in those in the non-HC group. Serum albumin levels and eGFR were significantly different among the 4 subgroups, and eGFR was lower in anemic patients than in non-anemic patients in both the non-HC and HC groups. In addition, eGFR in anemic patients in the HC group was lower than in those in the non-HC group. Plasma B-type natriuretic peptide levels, LVDd and LVEF were also was significantly different among the 4 subgroups.
Table 1.
HF Patients’ Characteristics and Comparison of Groups According to Filling Pressures and Prevalence of Anemia
| |
All
(n=198) |
Non-HC group (n=101) |
HC group (n=97) |
ANOVA
P value |
Non-anemia
(n=56) |
Anemia
(n=45) |
Non-anemia
(n=44) |
Anemia
(n=53) |
| Age, years |
67±16 |
63±15 |
73±11* |
57±18 |
76±10* |
<0.01 |
| Male sex, n (%) |
133 (67) |
36 (64) |
23 (51) |
37 (84)† |
37 (70) |
<0.01 |
| NYHA class III or IV, n (%) |
70 (35) |
5 (9) |
10 (22) |
18 (41)† |
37 (70)*,† |
<0.01 |
| Etiology of HF, n (%) |
| DCM |
70 (35) |
29 (52) |
10 (22)* |
21 (48) |
10 (19)* |
<0.01 |
| VHD |
50 (25) |
11 (20) |
16 (36) |
7 (16) |
17 (32) |
0.048 |
| IHD |
45 (23) |
10 (18) |
10 (22) |
7 (16) |
18 (34) |
0.13 |
| Other |
33 (17) |
6 (11) |
9 (20) |
10 (23) |
8 (15) |
0.39 |
| Clinical history, n (%) |
| Atrial fibrillation |
52 (26) |
5 (9) |
9 (20) |
14 (32)† |
24 (45)† |
<0.01 |
| Diabetes mellitus |
66 (33) |
17 (30) |
9 (20) |
16 (36) |
24 (45) |
0.06 |
| Hypertension |
103 (52) |
30 (54) |
28 (62) |
16 (36) |
29 (55) |
0.09 |
| Medications, n (%) |
| Vasodilators |
157 (79) |
45 (80) |
35 (78) |
30 (68) |
47 (89) |
0.10 |
| RAS inhibitors |
142 (72) |
39 (70) |
31 (69) |
27 (63) |
45 (85) |
0.09 |
| CCBs |
60 (29) |
21 (38) |
17 (38) |
2 (5)† |
17 (32)* |
<0.01 |
| Nitrovasodilators |
11 (6) |
2 (4) |
2 (4) |
1 (2) |
6 (11) |
0.19 |
| Others |
14 (7) |
3 (6) |
4 (9) |
3 (7) |
4 (8) |
0.93 |
| β-blockers |
92 (47) |
22 (39) |
18 (40) |
22 (51) |
30 (57) |
0.21 |
| Diuretics |
132 (67) |
33 (59) |
24 (53) |
31 (72) |
44 (83)† |
<0.01 |
| Laboratory data |
| Hemoglobin, g/dL |
12.4±2.4 |
14.1±1.3 |
10.9±1.3* |
14.7±1.3 |
10.0±1.5*,† |
<0.01 |
| Total protein, mg/dL |
6.9±0.7 |
7.0±0.5 |
7.0±0.7 |
6.9±0.7 |
6.8±0.7 |
0.23 |
| Albumin, mg/dL |
4.0±0.5 |
4.1±0.4 |
3.9±0.4 |
4.0±0.5 |
3.8±0.5 |
<0.01 |
| eGFR, mL/min/1.73 m2 |
55±26 |
69±30 |
51±20* |
63±23 |
38±16*,† |
<0.01 |
| BNP, pg/mL |
221 (78–523) |
99 (42–208) |
149 (50–395) |
305 (138–902)† |
425 (226–696) |
<0.01 |
| Echocardiographic data |
| LVDd, mm |
55±12 |
55±11 |
50±9 |
59±13 |
56±13 |
<0.01 |
| LVEF, % |
46±19 |
42±18 |
55±18* |
40±19 |
47±19 |
<0.01 |
*P<0.05, vs. non-anemic patients in each HC group; †P<0.05, vs. non-HC group in each anemic/non-anemic patient population. ANOVA, analysis of variance; BNP, B-type natriuretic peptide; CCB, calcium-channel blocker; DCM, dilated cardiomyopathy; eGFR, estimated glomerular filtration rate; HC, hemodynamic congestion; HF, heart failure; IHD, ischemic heart disease; LVDd, left ventricular diastolic diameter; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; RAS, renin-angiotensin-aldosterone system; VHD, valvular heart disease.
Table 2
shows comparisons of the hemodynamic parameters among the 4 subgroups. Systolic BP was greater in the non-HC group than in the HC group (HC effect P<0.05), but no difference was observed between non-anemic and anemic patients. Diastolic BP was lower and pulse pressure was greater in anemic patients compared with non-anemic patients in both the non-HC and HC groups with no significant interaction effects (P=NS). Heart rate was similar in the 4 subgroups. The HC group had higher mean PCWP, mean PAP and mean RAP than the non-HC group, but these parameters were similar between non-anemic and anemic patients in each group (anemic effect P=NS) with no significant interaction effects (P=NS). In contrast, CI was higher and SVRI was lower in anemic patients compared with non-anemic patients in the HC group only. Interestingly, estimated LVSWI was significantly lower in the HC group than in the non-HC group (HC effect P<0.05) but was not different between the non-anemic and anemic patients (anemic effect P=NS) with no significant interaction effects (P=NS).
Table 2.
Hemodynamic Parameters in HF Patients
| |
All |
Non-HC group |
HC group |
HC
Effect
P value |
Anemia
Effect
P value |
Interaction
Effect
P value |
| Non-anemia |
Anemia |
Non-anemia |
Anemia |
| SBP, mmHg |
125±26 |
126±26 |
132±24 |
120±24 |
122±27 |
0.03 |
0.26 |
0.55 |
| DBP, mmHg |
71±15 |
75±16 |
68±12* |
76±16 |
67±14* |
0.98 |
<0.01 |
0.70 |
| Mean BP, mmHg |
89±16 |
92±17 |
89±14 |
90±17 |
85±16 |
0.26 |
0.08 |
0.58 |
| PP, mmHg |
54±22 |
51±20 |
64±21* |
44±19 |
55±23*,† |
<0.01 |
<0.01 |
0.64 |
| HR, beats/min |
70±14 |
68±12 |
69±14 |
71±16 |
72±16 |
0.10 |
0.54 |
0.92 |
| Mean PCWP, mmHg |
14.8±8.6 |
8.1±3.4 |
8.1±3.4 |
22.1±8.0† |
21.3±5.1† |
<0.01 |
0.63 |
0.56 |
| Mean PAP, mmHg |
22.8±10.5 |
15.4±3.2 |
15.4±4.3 |
31.2±11.2† |
29.8±8.3† |
<0.01 |
0.52 |
0.50 |
| Mean RAP, mmHg |
7.7±5.3 |
4.0±2.6 |
4.0±2.6 |
11.7±4.9† |
11.5±4.4† |
<0.01 |
0.82 |
0.92 |
| SVI, mL/m2 |
39±13 |
42±13 |
42±13 |
34±11† |
38±13 |
<0.01 |
0.17 |
0.18 |
| CI, L/min/m2 |
2.6±0.6 |
2.7±0.6 |
2.7±0.6 |
2.3±0.6† |
2.6±0.7* |
<0.01 |
0.02 |
0.15 |
| eLVSWI, g-m/m2 |
40±18 |
48±20 |
46±15 |
32±16† |
34±15† |
<0.01 |
0.84 |
0.39 |
| PVRI, dyne/s/cm−5/m2 |
267±178 |
228±83 |
223±104 |
340±244† |
286±216 |
<0.01 |
0.24 |
0.32 |
| SVRI, dyne/s/cm−5/m2 |
2,641±748 |
2,747±717 |
2,566±603 |
2,875±761 |
2,402±817* |
0.87 |
<0.01 |
0.16 |
*P<0.05, vs. non-anemic patients in each HC group; †P<0.05, vs. non-HC group in each anemic/non-anemic patient population. CI, cardiac index; DBP, diastolic blood pressure; eLVSWI, estimated left ventricular stroke work index; HR, heart rate; PAP, pulmonary arterial pressure; PCWP, pulmonary capillary wedge pressure; PP, pulse pressure; PVRI, pulmonary vascular resistance index; RAP, right atrial pressure; SBP, systolic blood pressure; SVI, stroke volume index; SVRI, systemic vascular resistance index. Other abbreviations as in Table 1.
Figure 1
shows the relationships between Hb levels and hemodynamic parameters in all patients and each patient group. SVI, CI and SVRI correlated with Hb levels in all patients (r=−0.18, −0.20, and 0.32, respectively, P<0.05). In the non-HC group, none of SVI, CI, SVRI and eLVSWI correlated significantly with Hb level. In contrast, SVI, CI and SVRI had significant and moderate correlations with Hb level (r=−0.39, −0.34, and 0.42, respectively, P<0.05). Notably, eLVSWI, a surrogate of intrinsic LV pump performance, was similar between non-anemic and anemic patients and there was no correlation between Hb level and eLVSWI in either the non-HC or HC group. These results strongly suggested that a fall in SVRI is directly associated with increases in CI but unrelated to intrinsic LV performance in anemic patients in the HC group. Univariate and multivariate linear regression analyses were performed to identify the determinants of SVRI (Table 3) in the HC group, and Hb level was the strongest and independent determinant of SVRI after adjusting for age, sex, prevalence of diabetes mellitus and hypertension, administration of vasodilators, LVEF, serum total protein and eGFR.

Table 3.
Univariate and Multivariate Linear Regression of Factors That Correlated With Systemic Vascular Resistance Index in HF Patients
| |
Univariate |
Multivariate |
| β |
P value |
β |
P value |
| Age |
−0.19 |
0.07 |
0.15 |
0.27 |
| Male sex |
0.29 |
<0.01 |
0.15 |
0.14 |
| Diabetes mellitus |
−0.07 |
0.52 |
−0.09 |
0.34 |
| Hypertension |
0.04 |
0.68 |
0.16 |
0.14 |
| Vasodilators |
−0.15 |
0.13 |
−0.11 |
0.29 |
| LVEF |
−0.22 |
0.03 |
0.03 |
0.83 |
| Hemoglobin level |
0.42 |
<0.01 |
0.47 |
<0.01 |
| Log BNP |
0.24 |
0.02 |
0.28 |
0.01 |
| Total protein |
−0.03 |
0.76 |
−0.07 |
0.47 |
| eGFR |
0.24 |
0.02 |
0.06 |
0.64 |
Abbreviations as in Table 1.
The in-hospital clinical course of each patient population is shown in
Table 4. Hospital stay was longer in the HC group than in the non-HC group for anemic patients, but was similar between non-anemic and anemic patients in each group. A total of 17 patients (18%) in the HC group required intravenous inotropic support during hospitalization, with higher prevalence in the anemic subgroup than in the non-anemic subgroup; 3 patients in the HC group died from HF.
Table 4.
In-Hospital Clinical Course of HF Patients
| |
All
(n=198) |
Non-HC group (n=101) |
HC group (n=97) |
ANOVA
P value |
Non-anemia
(n=56) |
Anemia
(n=45) |
Non-anemia
(n=44) |
Anemia
(n=53) |
| Hospital stay, days |
13 (5–24) |
9 (5–16) |
7 (6–16) |
19 (8–30) |
22 (16–36)† |
0.02 |
| Intravenous inotropic support, n (%) |
17 (9) |
0 (0) |
0 (0) |
5 (11) |
12 (23)*,† |
<0.01 |
| In-hospital death, n (%) |
3 (2) |
0 (0) |
0 (0) |
1 (2) |
2 (4) |
0.32 |
*P<0.05 vs. non-anemic patients in each HC group; †P<0.05 vs. non-HC group in each anemic/non-anemic patient population. Abbreviations as in Table 1.
Figure 2A
shows scatter plots of the hemodynamic profile according to the Forrester hemodynamic subsets among non-anemic and anemic patients in the HC group. Only 3 (60%) of 5 patients and 5 (42%) of 12 patients who required intravenous inotropic support were characterized as subset IV in the non-anemic and anemic subgroups, respectively. These results indicated that hemodynamic assessment using the Forrester hemodynamic subsets did not appropriately predict the need for intravenous inotropic support, especially for patients with anemia, and therefore the optimal hemodynamic parameters were re-examined to propose a novel hemodynamic subsets using ROC analysis. As a result, mean RAP and eLVSWI best predicted the need for intravenous inotropic support with the highest area under the ROC curves (Table 5). Stepwise multiple logistic regression analysis among the 8 hemodynamic parameters presented in
Table 5
also revealed that only mean RAP and eLVSWI predicted the need for intravenous inotropic support (mean RAP: odds ratio 1.20, 95% confidence interval 1.06–1.36, eLVSWI: odds ratio 0.92, 95% confidence interval 0.85–0.99). Our novel hemodynamic subsets based on mean RAP and eLVSWI showed that 83% and 75% of patients who required intravenous inotropic support among the non-anemic and anemic patients were characterized as subset IV (Figure 2B), which was a better predictive ability than the conventional Forrester hemodynamic subsets (sensitivity: 82.4% vs. 47.1%, specificity: 83.8% vs. 75.0%, positive predictive value: 51.9% vs. 28.6%, negative predictive value: 95.7% vs. 87.0% and accuracy: 83.5% vs. 70.1%, respectively).

Table 5.
Comparison of Area Under the ROC Curves, Their Optimal Cutoff Values, Sensitivity, Specificity, Positive and Negative Predictive Values, and Accuracy Between Different Hemodynamic Parameters in the HC Group of HF Patients
| |
AUC |
Asymptotic
Sig. |
Cutoff |
Sensitivity |
Specificity |
PPV |
NPV |
Accuracy |
| SBP |
0.663 |
0.04 |
128.0 |
88.2 |
38.8 |
23.4 |
93.9 |
47.4 |
| Mean PCWP |
0.721 |
<0.01 |
20.5 |
82.4 |
60.0 |
30.4 |
94.1 |
63.9 |
| Mean PAP |
0.650 |
0.05 |
|
|
|
|
|
|
| Mean RAP |
0.838 |
<0.01 |
11.5 |
100.0 |
65.0 |
37.8 |
100.0 |
71.1 |
| CI |
0.626 |
0.11 |
|
|
|
|
|
|
| eLVSWI |
0.750 |
<0.01 |
27.4 |
70.6 |
62.5 |
28.7 |
90.9 |
63.9 |
| PVRI |
0.584 |
0.28 |
|
|
|
|
|
|
| SVRI |
0.460 |
0.61 |
|
|
|
|
|
|
AUC, area under the curve; PPV, positive predictive value; NPV, negative predictive value; ROC curve, receiver-operating characteristic curve. Other abbreviations as in Tables 1,2.
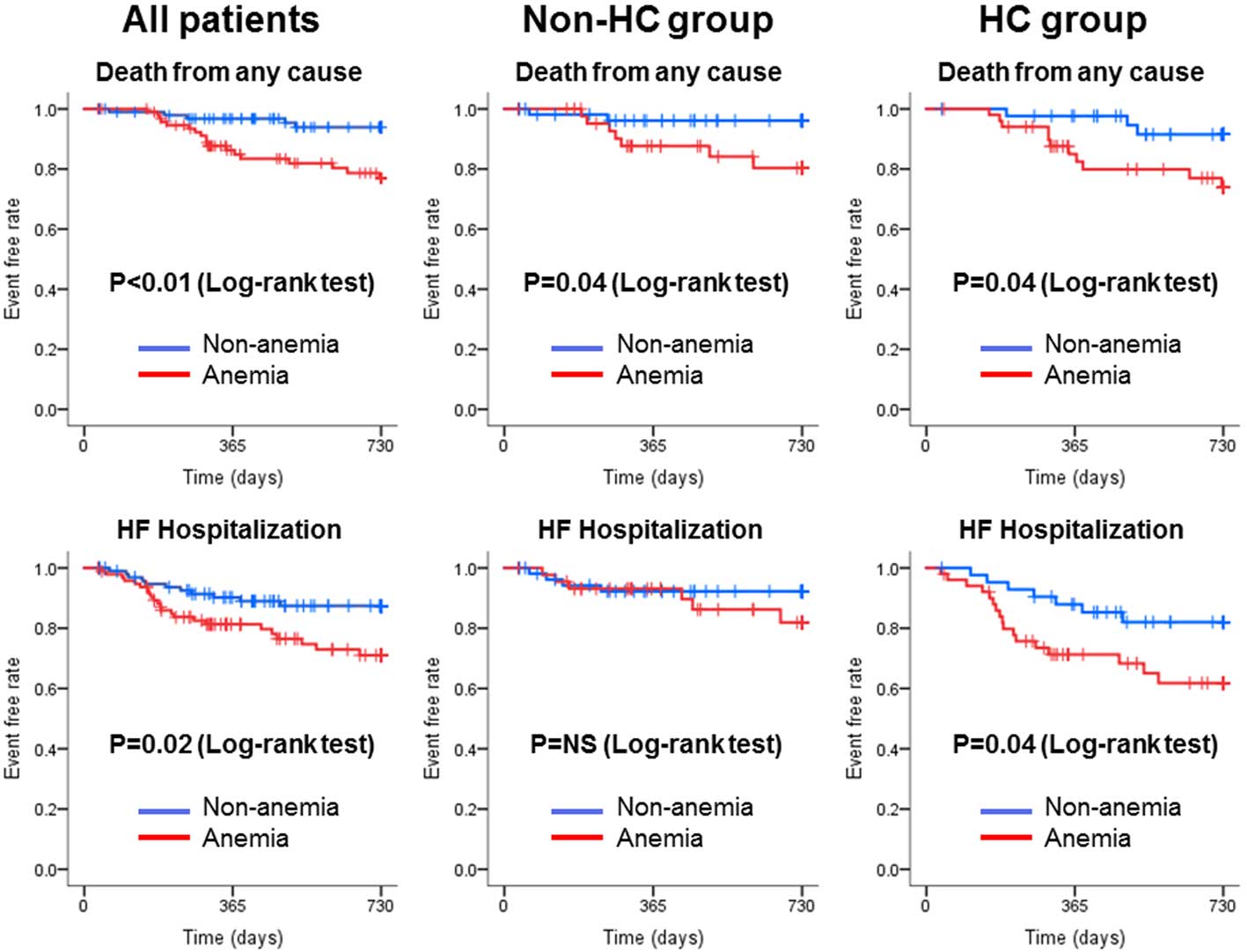
Of the195 patients who were discharged alive from hospital, we were able to obtain follow-up evaluations for 194, with a median of 726 days and range of 34–730 days. There were 23 all-cause deaths and 34 HF hospitalizations; 13 (57%) of the 23 deaths were from cardiovascular causes. The Kaplan-Meier curves in
Figure 3
show that anemic patients had worse outcomes than non-anemic patients among all HF patients (log-rank test, P<0.05).
Figure 3
also shows that the probability of all-cause death was significantly higher among anemic patients than non-anemic patients in both groups (log-rank test, P=0.04 and 0.04, respectively), while that of HF hospitalization was significantly higher for anemic patients than non-anemic patients in the HC group only (log-rank test, P=0.04).
Discussion
The present study is the first to evaluate the effect of anemia on hemodynamic status and in-hospital clinical course of HF patients, and introduces novel hemodynamic subsets for predicting the need for intravenous inotropic support regardless of the presence or absence of anemia. The Hb level was directly associated with SVRI, and subsequently CI, only in HF patients with HC, which could lead to underestimation of in-hospital HF severity despite it being a poor cardiovascular prognosis in the anemic population. Indeed, the Forrester hemodynamic subsets did not appropriately predict the need for intravenous inotropic support, especially among anemic patients. We successfully introduced novel hemodynamic subsets based on mean RAP and eLVSWI to improve the prediction of the need for intravenous inotropic support.
Anemia in HF
Approximately half of HF patients in the present study had anemia. Anemic patients were older and had worse renal function than non-anemic patients, as previously reported.1,15
Interestingly, CCBs were much more frequently prescribed in anemic patients only in the HC group. A large population-based study evaluated the risk of idiopathic aplastic anemia in users of CCBs.16
Another study reported that lower Hb was significantly associated with CCB use in patients with chronic kidney disease.17
These medications can confound the relationship between Hb levels and hemodynamic parameters, especially SVRI. However, our multivariate analysis revealed that medication did not independently contribute to low SVRI in the HC group.
Relationship Between Hemodynamic Parameters and Hemoglobin Levels
A clinical study in 1945 demonstrated that very severe anemia with a Hb <7.0 g/dL induces abrupt increases in cardiac output in patients with non-cardiac disease and caused subsequent high-output HF.18
However, the hemodynamic effects of anemia in HF patients with primary myocardial disease have never been evaluated. The present study revealed that the Hb level was linearly correlated with SVRI, and that the severity of anemia was independently associated with low SVRI only in patients with HC in the range of Hb 6.9–18.7 g/dL. It has been recognized that a low Hb level reduces SVR as a result of decreases in blood viscosity and enhanced nitric oxide-mediated vasodilation,6,7,19
and also induces abnormally low oxygen delivery to organ tissue in general. According to the Fick principle, tissue oxygen extraction increases to compensate for low oxygen delivery secondary to low Hb levels by displacing the Hb-oxygen dissociation curve to the right to maintain oxygen delivery in healthy individuals.19
Notably, the Hb level had no influence on cardiovascular parameters, including SVRI, in patients without HC in the present study. These individuals may have a substantial capacity for maintaining oxygen delivery to peripheral tissue by increased tissue oxygen extraction that needs no further compensatory cardiovascular response including reduction in SVR and increase in cardiac output when anemia is not very severe. In contrast, among patients with HC, disability of compensative increases in 2,3-diphosphoglycerate (2,3-DPG) levels in red cells, a crucial allosteric effector of the affinity of Hb for oxygen,20
and/or tissue damage may evoke further compensatory reduction in SVR and increased cardiac output. This arterial vasodilation causes hypotension and subsequent renal and visceral hypoperfusion, which can lead to elevated sympathetic activity, RAS activity and finally to volume retention.4
However, the causal relationship between anemia and HC remained unclear in the present study and the precise mechanisms underlying variation in cardiovascular response to anemia warrant further investigation. Interestingly, eLVSWI, a surrogate of intrinsic LV pump performance,21
was similar in non-anemic and anemic patients, indicating that anemia may not directly regulate intrinsic cardiac contractility.
Clinical Outcomes
Patients with HC had a higher requirement for intravenous inotropic support and longer hospital stay than those without HC, as predicted. Notably, anemic patients much more frequently received intravenous inotropic support, despite their lower SVRI and higher CI than non-anemic patients, when they had HC. Our results are consistent with recent clinical research that demonstrated low BP and anemia on hospital admission predicted early necessity of ventricular assist device implantation in advanced HF patients.22
In addition, Kaplan-Meier curves showed that re-hospitalization for HF was significantly more frequent for anemic patients than for non-anemic patients in the HC group only. The compensatory reduction in SVR and subsequent high-output hemodynamic status may exert favorable effects, but not sufficiently to overcome unfavorable effects related to anemia. In addition, these hemodynamic responses may lead to underestimation of the hemodynamic severity of HF and mislead clinicians into inappropriate diagnostic and therapeutic approaches.
Novel Hemodynamic Subsets in Predicting the Need for Intravenous Inotropic Support
The Forrester hemodynamic subsets, originally applied to patients with acute HF secondary to acute myocardial infarction,23
did not appropriately predict the need for intravenous inotropic support, especially in anemic HF patients with various etiologies of cardiac disease. The present study revealed that eLVSWI and mean RAP had the highest predictive value, and our novel hemodynamic subsets based on these 2 variables best predicted the need for intravenous inotropic support. Mean PCWP and CI are not independent indices of circulation, and CI is directly influenced by changes in systemic arterial circulation. In contrast, eLVSWI and mean RAP are likely to be independent indices, and none of them differed between the non-anemic and anemic populations.
Study Limitations
Potential limitations of the present study include the small sample population in a single center and the retrospective nature of data collection. Secondly, patients who had already been treated with intravenous inotropic agents and/or vasodilators, respirators, intra-aortic balloon pumping, extracorporeal membrane oxygenation or hemodialysis prior to RHC were excluded, which may have introduced selection bias, and the effect of anemia on cardiovascular hemodynamics and the usefulness of the new hemodynamic subsets in much more severely acute decompensated HF remains unclear. Thirdly, we did not evaluate the etiologies of anemia. Although the causes of anemia in HF are various and complex, they should be assessed for a better understanding of the physiological interactions between anemia and cardiovascular hemodynamics. Fourthly, although left- and right-sided HC was defined as mean PCWP ≥15 mmHg and mean RAP ≥10 mmHg in the present study, the optimal cutoff values of these parameters that determine HC may vary according to a study’s design including selection of the target population and the treatment strategies.11–14
Fifthly, the therapeutic decisions regarding the use of inotropic agents depended on the clinical judgment of the physicians assessing their patients, which may not always maximize patient outcomes and thus may affect the accuracy of novel hemodynamic subsets for predicting the requirement of inotropic support. Finally, we did not evaluate whether treatment of anemia improved cardiovascular hemodynamics and patient outcomes. Recent clinical trials failed to show a beneficial effect of darbepoetin alfa on improvement of clinical outcomes in patients with systolic HF and mild-to-moderate anemia.24
Those authors concluded that the Hb level is simply a marker of poor prognosis rather than a therapeutic target. Treatment of anemia may cause SVR elevation and subsequent reduction of cardiac output that may at least partially offset the improvement of oxygen delivery to the peripheral tissue in patients with HC. The association between cardiac hemodynamics and optimal Hb target warrants further investigation in the treatment of anemia in HF.
Conclusions
Anemia has a direct effect on cardiovascular hemodynamics and clinical outcomes in patients with HF and HC. To avoid underestimation of the hemodynamic severity of HF and to appropriately identify patients who require intravenous inotropic support, novel hemodynamic subsets based on the combination of mean RAP and eLVSWI rather than the conventional Forrester hemodynamic subsets can be applied in daily clinical practice.
Conflict of Interest Statement
K.D. received lecture fees of equal to or more than 500,000 yen from Otsuka Pharma Inc. in 2016. N.Y. received lecture fees of more than 500,000 yen from Bayer Yakuhin, Ltd. and Bristol-Myers Squibb in 2016. M.I. received lecture fees of more than 500,000 yen from Bayer Yakuhin, Ltd., Daiichi Sankyo Co. Ltd. and Mitsubishi Tanabe Pharma Corporation in 2016. The Department of Cardiology and Nephrology, Mie University Graduate School of Medicine is supported in part by unrestricted research grants of equal to or more than 1,000,000 yen from Daiichi Sankyo Co. Ltd., Shionogi & Co., Ltd., Sumitomo Dainippon Pharma Co., Ltd., MSD K.K., Astellas Pharma Inc., Takeda Pharmaceutical Company Limited and Pfizer Japan Inc. in 2016.
References
- 1.
Hamaguchi S, Tsuchihashi-Makaya M, Kinugawa S, Yokota T, Takeshita A, Yokoshiki H, et al. Anemia is an independent predictor of long-term adverse outcomes in patients hospitalized with heart failure in Japan: A report from the Japanese Cardiac Registry of Heart Failure in Cardiology (JCARE-CARD). Circ J 2009; 73: 1901–1908.
- 2.
Go AS, Yang J, Ackerson LM, Lepper K, Robbins S, Massie BM, et al. Hemoglobin level, chronic kidney disease, and the risks of death and hospitalization in adults with chronic heart failure: The Anemia in Chronic Heart Failure: Outcomes and Resource Utilization (ANCHOR) Study. Circulation 2006; 113: 2713–2723.
- 3.
Yamauchi T, Sakata Y, Takada T, Nochioka K, Miura M, Tadaki S, et al; CHART-2 investigators. Prognostic impact of anemia in patients with chronic heart failure-with special reference to clinical background: Report from the CHART-2 Study. Circ J 2015; 79: 1984–1993.
- 4.
Anand IS. Anemia and chronic heart failure implications and treatment options. J Am Coll Cardiol 2008; 52: 501–511.
- 5.
Anand IS, Chandrashekhar Y, Ferrari R, Poole-Wilson PA, Harris PC. Pathogenesis of oedema in chronic severe anaemia: Studies of body water and sodium, renal function, haemodynamic variables, and plasma hormones. Br Heart J 1993; 70: 357–362.
- 6.
Ni Z, Morcos S, Vaziri ND. Up-regulation of renal and vascular nitric oxide synthase in iron-deficiency anemia. Kidney Int 1997; 52: 195–201.
- 7.
Anand IS, Chandrashekhar Y, Wander GS, Chawla LS. Endothelium-derived relaxing factor is important in mediating the high output state in chronic severe anemia. J Am Coll Cardiol 1995; 25: 1402–1407.
- 8.
WHO. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Vitamin and Mineral Nutrition Information System (WHO/NMH/NHD/MNM/11.1). Geneva: World Health Organization, 2011. http://www.who.int/vmnis/indicators/haemoglobin.pdf (accessed March 20, 2017).
- 9.
Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 2009; 53: 982–992.
- 10.
Fincke R, Hochman JS, Lowe AM, Menon V, Slater JN, Webb JG, et al; SHOCK Investigators. Cardiac power is the strongest hemodynamic correlate of mortality in cardiogenic shock: A report from the SHOCK trial. J Am Coll Cardiol 2004; 44: 340–348.
- 11.
Binanay C, Califf RM, Hasselblad V, O’Connor CM, Shah MR, Sopko G, et al. Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness: The ESCAPE trial. JAMA 2005; 294: 1625–1633.
- 12.
Ma TS, Paniagua D, Denktas AE, Jneid H, Kar B, Chan W, et al. Usefulness of the sum of pulmonary capillary wedge pressure and right atrial pressure as a congestion index that prognosticates heart failure survival (from the Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness Trial). J Am Coll Cardiol 2016; 118: 854–859.
- 13.
Drazner MH, Hamilton MA, Fonarow G, Creaser J, Flavell C, Stevenson LW. Relationship between right and left-sided filling pressures in 1000 patients with advanced heart failure. J Heart Lung Transplant 1999; 18: 1126–1132.
- 14.
Iida N, Seo Y, Sai S, Machino-Ohtsuka T, Yamamoto M, Ishizu T, et al. Clinical implications of intrarenal hemodynamic evaluation by Doppler ultrasonography in heart failure. JACC Heart Fail 2016; 4: 674–682.
- 15.
Kajimoto K, Sato N, Takano T. Association between anemia, clinical features and outcome in patients hospitalized for acute heart failure syndromes. Eur Heart J Acute Cardiovasc Care 2015; 4: 568–576.
- 16.
Myers MW, Vasilakis C, Kaufman MR, Jick H. Antihypertensive drugs and the risk of idiopathic aplastic anaemia. Br J Clin Pharmacol 2000; 49: 604–608.
- 17.
Cikrikcioglu MA, Karatoprak C, Cakirca M, Kiskac M, Zorlu M, Cetin G, et al. Association of calcium channel blocker use with lower hemoglobin levels in chronic kidney disease. Eur Rev Med Pharmacol Sci 2013; 17: 2530–2537.
- 18.
Brannon ES, Merrill AJ, Warren JV, Stead EA. The cardiac output in patients with chronic anemia as measured by the technique of right atrial catheterization. J Clin Invest 1945; 24: 332–336.
- 19.
Metivier F, Marchais SJ, Guerin AP, Pannier B, London GM. Pathophysiology of anaemia: Focus on the heart and blood vessels. Nephrol Dial Transplant 2000; 15(Suppl 3): 14–18.
- 20.
Sharma S, Brugnara C, Betensky RA, Waikar SS. Reductions in red blood cell 2,3-diphosphoglycerate concentration during continuous renal replacment therapy. Clin J Am Soc Nephrol 2015; 10: 74–79.
- 21.
Grossman W. Evaluation of systolic and diastolic function of the ventricles and myocardium. In: Baim DS, editor. Grossman’s cardiac catheterization, angiography, and intervention. 7th edn. Philadelphia, PA: Lippincott, Williams & Wilkins,
2006; 315–324.
- 22.
Fujino T, Kinugawa K, Hatano M, Imamura T, Muraoka H, Minatsuki S, et al. Low blood pressure, low serum cholesterol and anemia predict early necessity of ventricular assist device implantation in patients with advanced heart failure at the time of referral from non-ventricular assist device institutes. Circ J 2014; 78: 2882–2889.
- 23.
Forrester JS, Diamond GA, Swan HJ. Correlative classification of clinical and hemodynamic function after acute myocardial infarction. Am J Cardiol 1977; 39: 137–145.
- 24.
Swedberg K, Young JB, Anand IS, Cheng S, Desai AS, Diaz R, et al. Treatment of anemia with darbepoetin alfa in systolic heart failure. N Engl J Med 2013; 368: 1210–1219.