2017 Volume 81 Issue 11 Pages 1596-1602
2017 Volume 81 Issue 11 Pages 1596-1602
Background: Durable pulmonary vein isolation (PVI) is critical in reducing recurrence after radiofrequency catheter ablation for atrial fibrillation (AF). The VISITAG Module, an automatic annotation system that takes account of catheter stability and contact force (CF), might be useful in accomplishing this.
Methods and Results: In 49 patients undergoing VISITAG-guided AF ablation (group A), we set the following automatic annotation criteria: catheter stability range of motion ≤1.5 mm, duration ≥5 s, CF ≥5 g, time ≥25% and tag diameter at 6 mm. We used ablation >20 s and force-time integral >150 gs at each site, then moved to the next site where a new tag appeared that overlapped with the former tag. Results and outcome were retrospectively compared for 42 consecutive patients undergoing CF-guided AF ablation without this algorithm (group B). Successful PVI at completion of the initial anatomical line was more frequent in group A than B (66.3% vs. 36.9%, P=0.0006) while spontaneous PV reconnection was less frequent (14.2% vs. 30.9%, P=0.0014) and procedure time was shorter (138±35 min vs. 180±44 min, P<0.001). One-year success rate off anti-arrhythmic drugs was higher in group A (91.8% vs. 69.1%, log rank P=0.0058).
Conclusions: An automated annotation algorithm with an optimal setting reduced acute resumption of left atrium-PV conduction, shortened procedure time, and improved AF ablation outcome.
Catheter ablation (CA) is an effective therapy of atrial fibrillation (AF). The cornerstone of CA for AF is pulmonary vein isolation (PVI).1,2 Despite initially successful PVI, however, recurrence of AF after CA occurs frequently and is often related to the recovery of conduction between the pulmonary vein (PV) and left atrium (LA).3–5 Contact force (CF) monitoring of the ablation catheter might help achieve more durable lesions and substantially improve procedural outcomes.6 A recent randomized, multicenter study, however, showed that CF data availability was associated with reduced acute PV reconnections but not improved 1-year success rates, procedure and fluoroscopy times, or complication rates.7 The failure of CF monitoring to improve clinical outcome of AF ablation might be due to a lack of information on the catheter stability as well as the precise location of ablated lesions, and subsequent heterogeneous ablation intensity. A new ablation annotation system (CARTO3 System, VISITAG Module) uses an algorithm for the automated annotation of RF ablation applications based on objective, predefined parameters. Because it provides crucial information on catheter stability and ablation intensity at each site, it may be helpful in accomplishing durable PVI.
The aim of this study was therefore to evaluate the utility of automated ablation lesion annotation by comparing procedure parameters and outcomes between automated annotation-guided and non-guided procedures.
The study was conducted under a retrospective, single-center observational design. We included 91 AF patients who underwent initial PVI by an experienced operator between June 2013 and January 2015. We performed 51 initial AF ablations from June 2014 to January 2015. We excluded from the study 2 patients who underwent empirical liner ablation in addition to PVI. The remaining 49 patients underwent AF ablation using automated annotation algorithm-guided PVI (group A; n=49). The comparison group consisted of 42 consecutive patients who underwent CF-guided PVI without automated annotation algorithm from June 2013 to December 2013 (group B; n=42). We did not include patients treated from January 2014 to May 2014 because PVI during this period was performed using different methods and annotation parameters. In total, the treatment groups consisted of 79 men and 12 women (mean age, 60.4±11.3 years). Fifty-eight patients had paroxysmal AF (PaF) and the other 33 patients had non-paroxysmal AF (non-PaF). Adequate oral anticoagulation was maintained for at least 1 month before the procedure. Warfarin was interrupted 1 day before the procedure and was restarted in the evening of the day of the procedure. Dabigatran, rivaroxaban and apixaban were omitted only on the morning of the procedure. The absence of thrombus in the LA was confirmed on transesophageal echocardiography the day before PVI. Anti-arrhythmic drugs (AAD) were stopped for at least 5 half-lives before the procedure. Transesophageal echocardiography was performed in all patients after admission. All patients gave informed consent for the ablation procedures and the use of their clinical data in a retrospective study. The study protocol was approved by the institutional ethics committee.
LA Multi-Detector Computed TomographyAll patients underwent multi-detector computed tomography (MDCT) with a 160-detector row, dynamic volume scanner for 3-D reconstruction of the LA and PV within the week prior to the ablation procedure using a 64-slice MDCT scanner (Brilliance CT 64, Phillips Medical Systems, Cleveland, OH, USA). A bolus of non-ionic iodinated contrast (Iopamirone 370; Bayer Yakuhin, Osaka, Japan) was injected through an antecubital vein at a flow rate of 0.67 mL/min/kg for 15 s, followed by a saline bolus flush. The scan was initiated according to the bolus-tracking method (6 s after the threshold of 120 Hounsfield units in the descending aorta). Cardiac images from the carina to the apex of the heart were acquired during a single breath-hold at the end-tidal position. 3-D CT was reconstructed at 50% of the R-R interval and recorded as DICOM data.
Cardiac CatheterizationA 6-Fr decapolar catheter was placed in the coronary sinus via the median antebrachial vein, while a 7-Fr decapolar catheter was placed in the superior vena cava and right atrium and a 10-Fr SoundStar ultrasound catheter (Biosense Webster, Diamond Bar, CA, USA) was inserted into right atrium via the femoral vein. Anatomic mapping of the LA using the CartoSound module of a CARTO3 system (Biosense Webster) was performed. Two long sheaths (Daig SL0; St Jude Medical, St Paul, MN, USA) were introduced into the LA using a single trans-septal puncture technique via the femoral vein. An initial i.v. bolus of heparin (150 IU/kg) was followed by continuous infusion to maintain an activated clotting time >300 s. PV angiography was performed by injecting a contrast medium through the trans-septal long sheaths into the LA. Conscious sedation was achieved with a combination of bolus thiamylal sodium and continuous dexmedetomidine hydrochloride. Patient respiration was controlled using bi-level positive airway pressure in all cases.
Integration of CARTO With CTThe ICE (intracardiac echocardiography) images were displayed through the CartoSound module using an echocardiography system (Vivid i, GE). ICE LA plane images were obtained at the end-tidal position and at the end of the T wave. Five to eight contours were sampled between the ostia of the right PV and the left PV, all of which were registered as the LA ICE image. These 2 images were then integrated with the installed surface registration system.
Automated Annotation Algorithm SettingWe set the parameters of the automated ablation annotation system (Carto 3 System, VISITAG Module, Biosense Webster) as follows: (1) catheter stability range of motion ≤1.5 mm; (2) catheter stability duration >5s, and (3) CF ≥5 g, time ≥25%. Tag size was 6 mm in diameter. We intended to ablate thickly so that each tag overlapped the other. We set the minimum force-time integral (FTI) as 150 gs, and attempted to meet or exceed this level at each ablation site.
AF AblationProcedures were performed by integrating the 3-D image using an open-irrigated ThermoCoolSmartTouch catheter (Biosense Webster) and a circular mapping catheter (Lasso, Biosense Webster; adjustable circumference, 15–25 mm; interelectrode pacing, 1–2 mm) in all patients. We performed ipsilateral circumferential PVI in sinus rhythm except in subjects with persistent AF refractory to electrical cardioversion. The present CF was measured based on electromagnetic location technology. RF energy was delivered using a dragging method at 30–35 W except at the site near the esophagus, where we delivered 25–30 W RF energy using a Stockert 70 (Biosense Webster) RF generator. The upper temperature limit was set to 43℃ and saline irrigation flow was 17–30 mL/min (Cool Flow Pump; Biosense Webster). In (group A) 49 patients undergoing AF ablation from June 2014 to January 2015 who received circumferential PVI, RF ablation was guided by the ablation annotation system (Carto 3 System, VISITAG Module, Biosense Webster) using an algorithm for the automated annotation of RF ablation based on objective, predefined parameters as noted. The new tag appeared automatically when all these conditions were satisfied. We set the FTI parameter at 100–150 gs. As FTI increased from 100 gs to 150 gs, the color of the tag gradually changed from white to red. We ablated for at least 20 s at each site, and continued ablation until the tag turned red. Near the esophagus, we aborted RF delivery before the temperature of the esophagus reached 40℃, as monitored with a SensiTherm (St. Jude Medical). Tag size was 6 mm in diameter, and we intended to move the catheter to the next position such that the new tag overlapped the preceding tag (Figure 1A). We continued to ablate each site for >20 s and until the tag turned red even when the local PV potential had disappeared. The 42 control patients (group B) undergoing AF ablation from June 2013 to December 2013 received PVI with CF monitoring but without the use of an automated algorithm (Figure 1B). The ablation strategy and energy settings were similar to that used for group A. We attempted to maintain CF between 10 g and 20 g and moved the RF catheter to the next position every 20–25 s. We continued to ablate each site for >20 s even when the local PV potential had disappeared. Successful PVI was defined as the loss of PV potentials (entrance block) and failure to capture LA during pacing from all bipoles of the circular multielectrode mapping catheter when positioned at the PV ostium (output, 10 mA; pulse width, 2 ms; exit block). Persistent isolation (entrance and exit block) for each PV was confirmed after a waiting period ≥20 min. Dormant conduction was evaluated for each PV separately with i.v. adenosine (triphosphate) at a dose of 0.4 mg/kg body weight under back-up pacing from the right ventricle in 74 patients after persistent block was documented. If ATP triggered dormant conduction, additional ablation was delivered at areas in which the earliest activation was transiently observed on the circular mapping catheter. I.v. ATP was repeated until dormant conduction was no longer present. We also attempted to ablate non-PV premature atrial contractions, including the superior vena cava, if they triggered AF or appeared frequently on continuous i.v. injection of isoproterenol. When atrial flutter or atrial tachycardia was induced by atrial rapid pacing, we eliminated it by also performing linear ablation of the cavotricuspid isthmus, LA roof, and mitral valve isthmus and focal atrium tachycardia ablation. We did not perform empiric linear ablation or defragmentation.

Initial anatomical PVI line in (A) group A and (B) group B. (A) Annotation of ablation lesions during pulmonary vein isolation (PVI) was performed using an automated algorithm with predefined criteria for a catheter stability range of motion ≤1.5 mm and catheter stability duration >5 s (right anterior oblique [RAO], anteroposterior [AP] and left anterior oblique [LAO] view). White automated annotation tag, force-time integral (FTI) <100 gs; pink automated annotation tag, 100 gs≤FTI<150 gs; red automated annotation tag, FTI ≥150 gs. (B) In control group B, PVI was performed using contact force-guided ablation with manual annotation (RAO, AP and LAO view).
All patients were hospitalized under continuous rhythm monitoring for 3 days after the ablation procedure. We directed patients to check their pulse rate and rhythm 3 times per day and to visit the outpatient clinic if they experienced a relapse of AF. All patients were scheduled for visits to the outpatient clinic at 1, 2, 3, 6, 9, and 12 months after ablation. Electrocardiogram was obtained at each visit. Holter electrocardiogram was performed at 6 months after the procedure. Additional monitoring was performed on the basis of symptoms. A 3-month blanking period after ablation was used. AAD were discontinued at 3 months after the procedure. Freedom from atrial tachyarrhythmia was defined as the absence of AF, atrial flutter, or atrial tachycardia lasting >30 s off AAD.
Statistical AnalysisDescriptive statistics are reported as mean±SD or median (IQR) for continuous variables and as absolute frequencies and percentages for categorical variables. Continuous variables were compared using parametric and non-parametric testing based on data distribution. Categorical variables were compared using the chi-squared or Fisher exact test. Event-free survival was reported as the crude event rate and estimated using the Kaplan-Meier survival function. Pairwise comparisons of survival rates were made using log-rank test. Multivariable Cox proportional hazards analysis was used to adjust for other established risk factors of recurrence. All statistical analysis was performed using MedCalc (ver. 12.7.5.0; MedCalc Software, Acacialaan, Ostend, Belgium) and JMP 12.2.0. (SAS Institute, Cary, NC, USA).
Patient characteristics at baseline are listed in Table 1. Hypertension was more frequent in group A than group B, and CHADS2 (congestive heart failure, hypertension, advanced age, diabetes mellitus, stroke) score was lower in group B. There were no significant differences, however, between the groups in age, prevalence of PaF, left ventricular ejection fraction, or LA diameter. All PV were successfully isolated in all patients. There were no major procedural complications in either group (Table 2).
| Variable | Group A (n=49) |
Group B (control) (n=42) |
P-value |
|---|---|---|---|
| Age (years) | 60.8±11.1 | 59.8±11.5 | 0.67 |
| Male | 43 (87) | 36 (85) | 0.77 |
| Height (cm) | 169.3±8.6 | 167.1±8.9 | 0.23 |
| Weight (kg) | 71.9±17.3 | 67.9±11.2 | 0.20 |
| Type of AF | |||
| Paroxysmal | 28 (57) | 30 (71) | 0.15 |
| Persistent | 15 (31) | 7 (17) | |
| Long-lasting | 6 (12) | 5 (12) | |
| CHADS2 score | |||
| 0, 1 | 36 (73) | 35 (83) | 0.014 |
| 2 | 7 (14) | 4 (10) | |
| ≥3 | 6 (12) | 3 (7) | |
| Hypertension | 36 (73) | 18 (43) | 0.0029 |
| Diabetes mellitus | 8 (16) | 4 (10) | 0.33 |
| History of HF | 3 (6) | 3 (7) | 0.84 |
| Prior stroke or TIA | 3 (6) | 1 (2) | 0.37 |
| LAD (mm) | 39.6±5.8 | 38.0±5.6 | 0.18 |
| LVEF (%) | 65.5±7.4 | 64.5±7.7 | 0.52 |
Data given as mean±SD or n (%). AF, atrial fibrillation; HF, heart failure; LAD, left atrial dimension; LVEF, left ventricular ejection fraction; TIA, transient ischemic attack.
| Variable | Group A (n=49) |
Group B (control) (n=42) |
P-value |
|---|---|---|---|
| CTI | 18 (35) | 9 (21) | 0.14 |
| Procedure time (min) | 138±35 | 180±44 | <0.0001 |
| Mean CF (g) | 12.6±2.3 | 10.2±2.9 | <0.0001 |
| RF energy application time (min) | 30.5±9.0 | 35.7±7.3 | 0.0037 |
| Total fluoroscopy time (min) | 32.0±14.5 | 45.8±18.0 | 0.0001 |
| Total fluoroscopy dose (mGy) | 65 (36–116) | 90 (61–154) | 0.019 |
| No. annotated ablation tags | 76.6±17.9 | 86.3±19.7 | 0.015 |
| Complications | 0 (0) | 1 (2)† | NS |
Data given as mean±SD, median (IQR) or n (%). †Pericardial effusion not requiring drainage. CF, contact force; CFAE, complex fractionated atrial electrogram; CTI, cavotricuspid isthmus line; RF, radiofrequency.
Successful PVI at the completion of the initial anatomical line was achieved in the majority of ipsilateral pairs of PV (66%; 65/98) in group A, but in only 31 of 84 (37%) in group B patients, and was more frequently achieved in group A than in group B (P=0.0006; Figure 2A).
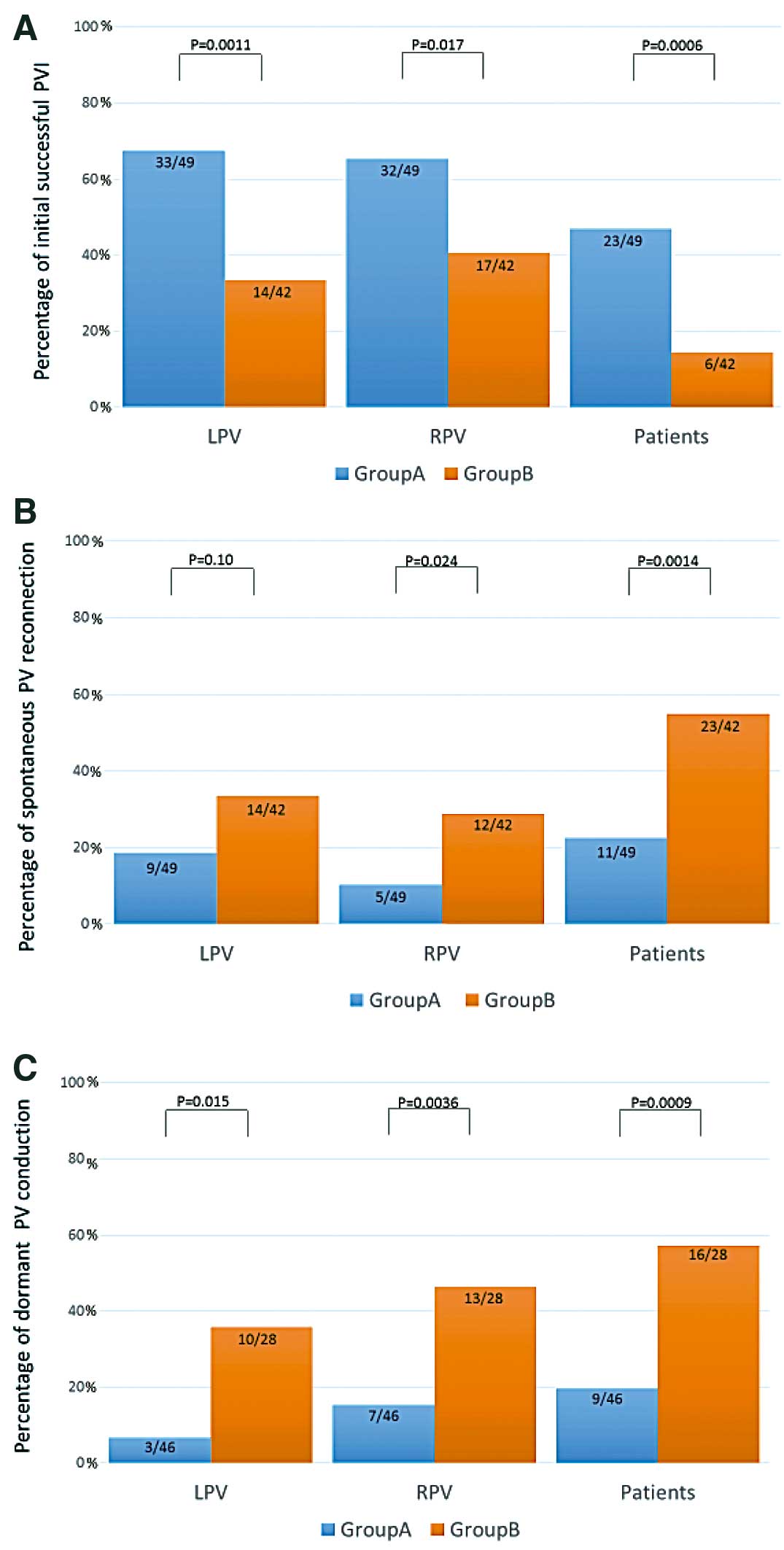
Percentage of (A) initial successful pulmonary vein isolation (PVI); (B) spontaneous pulmonary vein (PV) reconnection; and (C) dormant PV conduction in group A vs. group B (control). In group A, the annotation of ablation lesions during PVI was performed using an automated algorithm, while in group B, the annotation of ablation was performed manually by the operator. LPV, left PV; RPV, right PV.
Spontaneous PV reconnection after waiting >20 min and PV dormant conduction evoked by ATP were observed in only 11 of 49 (22%) and in 9 of 46 (19%) in group A, but in as many as 23 of 42 (55%) and in 16 of 28 (57%) in group B, respectively. These were significantly less frequent in group A than in group B (P=0.0014 and P=0.0009, respectively; Figure 2B,C). Average CF was higher in group A (P<0.0001); and average procedure time, RF energy application time, and fluoroscopy time were significantly shorter in group A (Table 2).
Clinical Recurrence of ArrhythmiaA total of 90 out of 91 patients completed 1-year follow up. The 1-year single procedure success rates on or off AADs were 45/49 (91.8%) in group A and 32/42 in group B (76.2%). The 1-year single procedure success rates and off AADs were 45/49 (91.8%) in group A and 29/42 (69.1%) in group B. On Kaplan-Meier analysis, freedom from recurrent atrial tachyarrhythmia was significantly better in group A (log-rank P=0.0058; Figure 3).

Single procedure success rate (A) on/off anti-arrhythmic drugs and (B) off anti-arrhythmic drugs vs. use of automated annotation algorithm-guided pulmonary vein isolation: Kaplan-Meier analysis of freedom from recurrent atrial tachyarrhythmia at 1 year.
To analyze the predictors of AF recurrence, both univariate and multivariate analysis were performed (Table 3). On univariate Cox proportional analysis, ablation without the VisiTag module was significantly associated with AF recurrence (HR, 4.13; 95% CI: 1.46–14.7; P=0.0065; Table 3A). The association between ablation without VisiTag and AF recurrence was significant even after adjustment on multivariate Cox proportional analysis for non-PaF, LA dimension, age, and female sex. On multivariate analysis, without the VisiTag module was the only independent predictor of AF recurrence (Table 3B).
| A | Univariate P-value |
HR | 95% CI |
|---|---|---|---|
| Ablation without VisiTag module | 0.0065 | 4.13 | 1.46–14.7 |
| Female | 0.18 | 2.25 | 0.63–6.39 |
| Age (years) | 0.08 | 1.04 | 0.99–1.09 |
| Non-PaF | 0.81 | 1.12 | 0.40–2.91 |
| Hypertension | 0.98 | 1.006 | 0.38–2.77 |
| Diabetes | 0.3 | 0.39 | 0.02–1.94 |
| Stroke | 0.73 | 1.45 | 0.08–7.11 |
| CHADS2 score | 0.69 | 1.09 | 0.66–1.70 |
| LAD | 0.75 | 0.98 | 0.90–1.07 |
| LVEF | 0.56 | 1.01 | 0.95–1.09 |
| B | Multivariate P-value |
HR | 95% CI |
| Model 1 | |||
| Ablation without VisiTag module | 0.0067 | 4.11 | 1.45–14.6 |
| Female | 0.19 | 2.23 | 0.62–6.33 |
| Model 2 | |||
| Ablation without VisiTag module | 0.0039 | 4.50 | 1.58–16.0 |
| Age (years) | 0.051 | 1.04 | 0.99–1.09 |
| Model 3 | |||
| Ablation without VisiTag module | 0.0058 | 4.24 | 1.49–15.1 |
| Non-PaF | 0.61 | 1.28 | 0.46–3.36 |
| Model 4 | |||
| Ablation without VisiTag module | 0.0068 | 4.17 | 1.45–14.9 |
| LAD | 0.89 | 1.005 | 0.92–1.09 |
CA, catheter ablation; non-PaF, non-paroxysmal AF; PaF, paroxysmal AF. Other abbrviations as in Table 1.
The spontaneous PV reconnection rate was significantly lower in the automated annotation ablation group A than in the control group B. The procedure time was significantly shorter and the 1-year single procedure success rate was significantly better in group A compared with the control group, B. There were no major complications during or after PVI in either group. Significantly, the automated RF ablation algorithm was demonstrated to facilitate the PVI procedure without an increase in adverse complications.
Importance of Durable Lesion FormationAF recurs after ablation in approximately 10–30% of patients with PaF and in even more patients with persistent AF.1 This recurrence, however, is associated with the resumption of conduction between the previously isolated PV and LA.3–5 Reversible tissue injury stems from incomplete lesion formation, resulting in temporary electric uncoupling without cell death. Cappato et al identified conduction recurrence across RF lesions for PV isolation in approximately 80% of cases at 4 months after ablation, and this late reconnection might contribute significantly to AF recurrence.8,9 A recent study proved the superiority of complete PVI over incomplete PVI with respect to AF recurrence.10
Methods to detect incomplete reversible lesions in clinical practice remain to be fully clarified. The presence of dormant conduction in response to adenosine or ATP that hyperpolarizes atrial cell membranes and triggers transient conduction, might indicate incomplete cell injury.11 The efficacy of additional ablation at these sites, however, is still controversial.12–15
Usefulness of an Automated Annotation SystemAn ablation catheter with the ability to continuously monitor real-time CF has been developed. CF monitoring is useful in AF ablation in reducing ablation time and additional touch-up ablation and in improving the outcome of initial CA compared with conventional irrigated catheters.16,17 We previously reported that high average CF decreased ablation time but did not improve ablation outcome.18 A recent randomized multi-center controlled trial also indicated that the availability of CF data to the operator during an ablation procedure for PAF was not associated with a better 1-year success rate.7 The failure of CF monitoring to improve clinical outcomes of AF ablation might be due to a lack of information on catheter stability during energy delivery, and heterogeneous RF ablation intensity. If so, real-time information on catheter stability and ablation intensity would possibly improve lesion completeness. The VISITAG module is an automated annotation ablation system that visualizes ablation sites precisely, as well as the intensity of ablation at each ablation point. The tag display influences the operator’s manipulation of the catheter, and when settings are optimal, this system might help to achieve more durable PV isolation.19
Optimal Parameter SettingThe tags displayed by the VISITAG module depend on the setting of predefined parameters, and optimal parameter setting is crucial to properly visualize the site and intensity of ablation. We set the ablation tag diameter at 6 mm, catheter stability minimum time at 5 s, and maximum range at 1.5 mm. This means that a new tag will appear if the catheter remains within 1.5 mm for >5 s during the ablation. When we tested a stability maximum range <1.5 mm, new tags appeared one after another, despite maintenance of adequate catheter stability, due mainly to patient breathing. In contrast, with a stability maximum range >1.5 mm, new tags tended to appear at a position that did not overlap the preceding tag when RF was delivered via a dragging technique. Because we aim to overlap the ablation tags as much as possible in order to identify conduction gap locations using ablation tag information, a stability maximum range >1.5 mm is too large. We therefore set the stability maximum range at 1.5 mm. In the force over time category, we set the time at 25% and minimum force at 5 g, meaning that a new tag will appear only if the catheter contact maintains ≥5 g for >25% of the ablation time. We set the FTI category at 100–150 gs: as that FTI increases from 100 gs to 150 gs during ablation, the color of the tag gradually changes from white to red. We set the minimal FTI at 150 gs at each ablation site.
Efficacy of VISITAG ModuleIn this study, use of the VISITAG module was associated with a lower frequency of conduction gaps after the initial anatomical PVI line, lower rate of spontaneous PV reconnection, and of dormant conduction evoked by ATP infusion. The quality of PVI was clearly better in ablations done using this technology with the present setting than in those without it. This indicates that the VISITAG module is successful in providing the operator with precise data on the location of ablation sites and on the intensity of ablation at each site, with consideration of catheter stability. The ablation procedure was significantly shorter in those cases in which it was used, and from our standpoint also easier than the alternate technique. One-year ablation success rate was better in the VISITAG group, possibly due to improvements in the durability of PVI, as evidenced by the markedly low rate of additional ablation procedures beyond PVI in the present subjects.
Previous StudiesPrevious studies of the efficacy of automated annotation algorithm-guided AF ablation are few in number.20 Anter et al reported that the automated and objective annotation of RF ablation enhances the detection of ineffective energy delivery and reduces the incidence of conduction recovery.19 That study did not include a CF category but did include an impedance decrease category. In the present study, average CF was higher in the VisiTag group because the force over time criteria used in the present study meant that the automated tag did not appear until adequate CF was maintained. The use of CF category and FTI might be more effective in achieving transmural lesion during PVI. The EFFICAS I study reported that reconnection in previously isolated PV was strongly correlated with FTI, and suggested a minimal FTI target of 400 gs for each new lesion.21 Although the present minimum FTI was lower than that in the EFFICAS I study, it is reasonable that minimum FTI was different because the settings of automated annotation and distance between ablation tags were different. In the present study, operators needed to ablate densely to make the ablation tags overlap each other, and the minimum FTI was therefore relatively low.
Study LimitationsThis study had some limitations. First, it was a retrospective observational study, hence there must be some potential bias because a historical control was used for comparison. Second, the recurrence rate of AF may have been underestimated because asymptomatic short-duration AF episodes may have been undetected. Third, the results are based on a relatively small sample of patients. Fourth, this is the report of a single experienced operator. Ensuring the efficacy and safety of automated annotation-based ablation will require confirmation in a prospective study with a larger sample size.
In PVI procedures, an automated ablation lesion annotation system with optimal settings is a safe and effective way to reduce the acute resumption of LA-PV conduction and to improve AF ablation outcomes, possibly due to the better durability of PVI.
K. Inoue has received honoraria from Johnson and Johnson KK, Medtronic, Bayer, Boehringer Ingelheim, and Bristol Myers Squibb. The other authors declare no conflict of interest.