2017 Volume 81 Issue 11 Pages 1557-1563
2017 Volume 81 Issue 11 Pages 1557-1563
Cyclophilin A (CyPA) is secreted from vascular smooth muscle cells, inflammatory cells, activated platelets, and cardiac fibroblasts in response to oxidative stress. Excessive and continuous activation of the RhoA/Rho-kinase system promotes the secretion of CyPA, resulting in the development of multiple cardiovascular diseases. Basigin (Bsg), a transmembrane glycoprotein that activates matrix metalloproteinases, is an extracellular receptor for CyPA that promotes cell proliferation and inflammation. Thus, the CyPA/Bsg system is potentially a novel therapeutic target for cardiovascular diseases. Importantly, plasma CyPA levels are increased in patients with coronary artery disease, abdominal aortic aneurysms, pulmonary hypertension, and heart failure. Moreover, plasma CyPA levels can predict all-cause death in patients with coronary artery disease and pulmonary hypertension. Additionally, plasma soluble Bsg levels are increased and predict all-cause death in patients with heart failure, suggesting that CyPA and Bsg are novel biomarkers for cardiovascular diseases. To discover further novel molecules targeting the CyPA/Bsg system, high-throughput screening of compounds found molecules that ameliorate the development of cardiovascular diseases. In addition to CyPA and Bsg, novel therapeutic targets and their inhibitors for patients with pulmonary arterial hypertension have been recently screened and identified. Ultimately, the final goal is to develop novel biomarkers and medications that will be useful for improving the prognosis and quality of life in patients with cardiovascular diseases.
Endothelial cells (ECs) secrete a variety of vasoactive substances,1–4 such as nitric oxide (NO), prostacyclin, and hydrogen peroxide (H2O2), that protect against vascular dysfunction and organ failure.5,6 These substances affect adjacent vascular smooth muscle cells (VSMCs) to activate downstream signaling elements, including soluble guanylyl cyclase (sGC)/cGMP, adenylate cyclase/cAMP, and cGMP-dependent protein kinase 1-α (PKG1α), effecting vascular relaxation.7 In contrast, the RhoA/Rho-kinase pathway plays an important role in various fundamental cellular functions, including contraction, motility, and proliferation of VSMCs, affecting vascular contraction and remodeling.8,9 Thus, the control and balance between EC-mediated relaxation and Rho-kinase-mediated contraction are important for vascular homeostasis and hemodynamics.10 Cardiovascular diseases are initiated by endothelial dysfunction leading to expression of adhesion molecules for inflammatory cells.11,12 Activated inflammatory cells produce large amounts of reactive oxygen species (ROS), secrete cytokines/chemokines and growth factors, and promote cardiovascular diseases.10 During this process, the interactions among ECs, VSMCs, inflammatory cells, and adventitial tissues play crucial roles in deteriorating vascular function and promoting cardiovascular diseases.13–15 In addition to inflammatory cells, VSMCs also secrete a variety of humoral factors that affect vascular cell components in an autocrine/paracrine manner.16,17 Among these factors, the secretion of cyclophilin A (CyPA) is induced by continuous Rho-kinase activation and excessive ROS production.18–20 During the past 15 years, I have focused on a series of studies to elucidate the precise mechanism by which oxidative stress damages vascular function and promotes cardiovascular diseases.16,21–31 In 2017, I received the Sato memorial award from the Japanese Circulation Society for these achievements and here, I review my 15 years’ work, during which I carried out basic cardiovascular research and clinical application projects. In 2002, I started basic research on the pathogenesis of pulmonary hypertension (PH) as a degree research project, expanded the research area to arteriosclerosis and heart failure (HF) during a stay in the USA, and, since returning to Japan, I have been devoted to translational research accomplished through basic research. I will also introduce recent progress that has been made in our understanding of the pathogenesis of cardiovascular diseases, and discuss current challenges to developing clinical applications and conducting translational research.30,32–48
Endothelial dysfunction is an initial trigger for the development of atherosclerotic diseases.2,3,12 AMPK/eNOS signaling plays a crucial role in vascular homeostasis and blood pressure regulation.36 Thirty years ago, CyPA was identified as the main target of the immunosuppressive drug cyclosporine A.19,20 Cyclophilins are a family of highly conserved and ubiquitous proteins; the most abundant cyclophilin, CyPA, is widely distributed in most tissues.18 Intracellular CyPA has a variety of functions, including intracellular trafficking, signal transduction, and transcriptional regulation.4 It plays a crucial role in the translocation of Nox enzymes such as p47phox, which is known to contribute to VSMC proliferation. Because ROS production by Nox enzymes activates other oxidase systems, CyPA and Nox enzymes amplify ROS formation in a synergistic manner, increasing oxidative stress.20 Intracellular CyPA plays an important role as a chaperone in protein folding and trafficking.18 In addition to its intracellular functions, CyPA can be secreted in response to oxidative stress, and extracellular CyPA stimulates ECs to induce adhesion molecules,24 apoptosis,28 and inflammatory cell migration.17 In a series of studies, it has been found that intracellular and extracellular CyPA promote intimal thickening,24 abdominal aortic aneurysms (AAA),26 atherosclerosis,28 cardiac hypertrophy,29 and PH.30 Based on these results, translational research on patients with coronary artery disease (CAD) examined the prognostic importance of CyPA, followed by a series of clinical studies.8,16,30,31,33,39 Plasma levels of CyPA were significantly increased in patients with angiographically proven CAD.16 Additionally, CyPA levels were elevated in patients with risk factors for atherosclerosis (hypertension, diabetes, smoking, dyslipidemia, and advanced age).16 CyPA was a prognostic marker for cardiovascular interventions such as percutaneous coronary intervention and coronary artery bypass grafting.16 These results suggested that circulating CyPA is a novel biomarker for CAD and plays a crucial role in the development of atherosclerosis in humans.20 Adding plasma CyPA to the known cardiovascular risk factors (age, sex, smoking, hypertension, diabetes, and dyslipidemia) significantly improved the overall performance of the logistic-regression model, as reflected in the c-statistic increase from 0.807 to 0.870. Thus, CyPA added prognostic information far beyond that provided by age, sex, family history of ischemic heart disease, presence of hypertension, diabetes, smoking status, body mass index, eGFR, and plasma lipid levels. When the level of high-sensitivity C-reactive protein (hsCRP) was included in the baseline model, the c-statistic increased from 0.807 to 0.873. Excluding 141 patients with high hsCRP did not significantly change the results. Among patients with hsCRP values >1.0, the same trend was observed, suggesting the potential usefulness of this combination of biomarkers for diagnosing CAD.16 In population subgroups with both low and high hsCRP levels, CyPA levels were significantly higher in patients with severe CAD than in those without CAD.16,39 The increased severity of CAD observed in patients with elevated CyPA may be a consequence of a higher frequency of risk factors for atherosclerosis, all of which promote ROS production and CyPA secretion. All of these risk factors create an oxidative stress environment, contributing to higher plasma CyPA levels in patients with severe CAD. Vascular oxidative stress can be induced by mechanical stretch, pressure, shear stress, environmental factors such as hypoxia, and secreted factors such as angiotensin II (AngII). In addition, extracellular CyPA induces ROS production in VSMCs and recruits inflammatory cells, resulting in the augmentation of vascular ROS and atherosclerosis.28 CyPA is also secreted from activated macrophages, lymphocytes, and platelets, all of which are important sources of extracellular CyPA; platelet-bound CyPA is associated with cardiovascular events. These data strongly support the role of CyPA as a novel biomarker for CAD. Secreted CyPA can be accumulated in atherosclerotic plaques in the coronary arteries. Indeed, strong CyPA expression has been observed in the coronary arteries of patients with myocardial infarction.16 Interestingly, this expression was localized to the region beneath the thin fibrous cap of atherosclerotic plaques. During fatty streak formation, CyPA participates in lipid uptake by affecting scavenger receptors.28 At all stages, CyPA plays a role in inflammation by promoting monocyte adhesion and recruitment, and contributing to the oxidative environment.17 Recently, it was examined whether plasma CyPA levels could predict long-term prognosis of patients with CAD, and the incidence of all-cause death, rehospitalization, and revascularization was higher in patients with higher plasma CyPA levels than in those with lower levels.31 In particular, plasma CyPA levels had a strong prognostic impact for future revascularization.31 Plasma CyPA level is a biomarker that exactly reflects the severity of developing arteriosclerosis.39 Thus, agents that inhibit CyPA secretion may suppress the development of atherosclerosis in future (Figure 1).

Early diagnosis, therapy, and prevention by targeting cyclophilin A (CyPA).
AAA formation is a consequence of chronic aortic wall inflammation associated with progressive degeneration of structural components, particularly the elastic lamina.49 Key mechanisms include VSMC senescence, oxidative stress, local proinflammatory cytokine production, and increased matrix metalloproteinase (MMP) activity, which degrade extracellular matrix. All of these characteristics suggest the potential contribution by CyPA to the development of AAA. As expected, AngII-induced aortic aneurysm formation was completely prevented on a CyPA-knockout background.26 In particular, AngII-induced aortic rupture and sudden death, occurring in 40% of control mice, were prevented by CyPA knockout.26 Thus, CyPA could be a novel therapeutic target for the treatment of aortic aneurysms.20,50 The role of CyPA as an essential protein for the development of AAA was highlighted in the New England Journal of Medicine.49
The myocardial extracellular matrix plays an important role in maintaining the structure of the heart. In contrast, myocardial collagen content correlates with both the stiffness of the heart in patients with HF, and reduced left ventricular ejection fraction, dilatation, and resultant HF. Indeed, it was recently demonstrated that myocardial fibrosis, evaluated from biopsy samples, plays an important role in the progression of HF and predicts prognosis in patients with HF.51 Patients with severe HF show post-capillary PH, a disorder with elevated pulmonary arterial pressure and pulmonary vascular resistance. Importantly, it was found that the extent of post-capillary PH is a prognostic factor in patients with HF.52 Additionally, in patients with HF, Rho-kinase activation plays a crucial role in the process of fibrosis.53,54 Thus, further analyses of CyPA-mediated cardiac function regulation were performed and, importantly, CyPA promoted ROS production, fibrosis, and hypertrophy in mouse hearts in response to AngII.29 Therefore, inhibition of CyPA is a useful therapeutic strategy to attenuate cardiac hypertrophy in patients with high oxidative stress levels caused by smoking, hypertension, hyperlipidemia etc. CyPA is a key mediator of Rho-kinase, which generates a vicious cycle of ROS augmentation affecting ECs, VSMCs, and inflammatory cells.19,20 Rho-kinase enhances myosin light-chain phosphorylation and mediates VSMC contraction.15 In ECs, the RhoA/Rho-kinase pathway negatively regulates NO production.8 In VSMCs, this pathway activates gene expression and secretion of growth factors, promoting cell proliferation and migration. A selective Rho-kinase inhibitor, fasudil, reduces ROS-induced CyPA secretion.26 Among the auto/paracrine factors, CyPA has been identified as a ROS-responsive protein secreted by vascular cell components upon Rho-kinase activation. Indeed, Rho-kinase is substantially involved in the vascular effects of various vasoactive factors, including AngII. Vascular ROS formation can be stimulated by mechanical stretch, pressure, shear stress, hypoxia, and growth factors, all of which activate the RhoA/Rho-kinase system. In summary, the RhoA/Rho-kinase system plays a crucial role in EC dysfunction, VSMC contraction/proliferation, and inflammation. Roles for Rho-kinase in the development of cardiac hypertrophy,38 fibrosis,44 arrhythmogenic right ventricular cardiomyopathy (ARVC),40 and PH34,37 have been demonstrated.
In addition to inhibiting CyPA secretion, it is logical to suppose that agents that prevent CyPA binding to its receptors may have therapeutic potential. Elucidation of the extracellular CyPA receptors will contribute to the development of novel therapies for cardiovascular diseases. By blocking the vicious cycle that augments ROS production through CyPA autocrine/paracrine signaling, we may obtain novel therapeutic tools for controlling cardiovascular diseases. Many vascular cell components secrete CyPA via a process that requires ROS production, Rho-kinase activation, and vesicle formation.8 In inflammatory cells, extracellular CyPA works as a chemoattractant in cooperation with other cytokines and chemokines.24 However, despite mounting evidence that CyPA serves multiple intracellular and extracellular functions, surprisingly little is known regarding its effect on specific receptors.18 Basigin (Bsg, also known as CD147 or EMMPRIN) has been proposed as an extracellular receptor for CyPA in inflammatory cells.18 Bsg is a multifunctional protein that promotes myofibroblast differentiation, cell proliferation, and MMP activation. Additionally, Bsg is an essential receptor for multiple ligands, including malaria parasites, CyPA, and soluble Bsg (sBsg) itself. Recently, it was demonstrated that Bsg is a receptor for VSMCs.30 It is expressed in cardiac fibroblasts, secreted by mechanical stretch, and activates MMPs in cardiac tissues.45 Importantly, Bsg disrupts NO metabolism and causes harmful endothelial activation, including Rho-kinase activation.10 Additionally, serum levels of sBsg are significantly increased in patients with HF;45 higher sBsg levels predicted all-cause death and HF hospitalization. Moreover, serum sBsg levels strongly correlated with serum IL-8, adiponectin, and CyPA levels, indicating the importance of the CyPA/Bsg system in patients with HF.45 Thus, serum sBsg levels could be a promising biomarker, and further study will provide additional knowledge on the usefulness of sBsg in patients with HF. Interactions between extracellular CyPA and Bsg may contribute to the development of HF; thus, discovery of CyPA and Bsg inhibitors may provide an effective therapeutic approach to treating patients with HF.
PAH is a distinct pulmonary vascular disease, categorized as Group I within the PH World Health Organization (WHO) clinical classification system. In addition to genetic considerations, many environmental factors, including hypoxia, infection,55 and smoking, are involved in the development of PAH.56 These factors constitute complex interactions that affect the pulmonary vasculature in a multi-stage manner.52 The characteristics of pulmonary artery smooth muscle cells (PASMCs) of patients with PAH (PAH-PASMCs) differ from those of healthy controls. However, we still have limited information as to the basal mechanisms behind these differences. Pulmonary artery ECs are important for pulmonary vascular homeostasis whereas EC dysfunction accelerates the development of PH.15,43,46 As a degree research project, I demonstrated the protective roles of the endogenous erythropoietin (Epo)/Epo receptor (EpoR) system in endothelial function and homeostasis.21 Additionally, EpoR−/−-rescued mice revealed a crucial role of the endogenous Epo/EpoR system in the ischemic heart22 and ischemia-induced angiogenesis.23 Overall, the intrinsic Epo/EpoR system is important for endothelial function in cardiovascular diseases.33,57 However, clinical trials failed to show the benefit of exogenous Epo administration to patients with cardiovascular diseases. Interestingly, a binding partner of CyPA, cyclosporine A, induced excessive Epo production by Epo-producing cells.18 Additionally, the expression of CyPA is upregulated by hypoxia-inducible factor α (HIF-1α), which is important for Epo production.4 Thus, Epo and CyPA are mechanistically connected, contributing to the regulation of oxygen supply, consumption, and resultant oxidative stress. PAH is associated with endothelial dysfunction, PASMC proliferation, and inflammation58 in which Rho-kinase is again substantially involved.8 Consistently, long-term treatment with fasudil suppressed the development of monocrotaline-induced PH in rats and hypoxia-induced PH in mice.34,37 Inhibition of CyPA secretion by fasudil may have contributed to the therapeutic effects; therefore, the hypothesis that the CyPA/Bsg system promotes the development of PH was tested. Coincidentally, Bsg is an essential receptor for malaria parasites, which disrupt NO metabolism inducing harmful endothelial activation, including Rho-kinase activation.18 As expected, extracellular CyPA and vascular Bsg played a crucial role in hypoxia-induced PH by inducing growth factor secretion, inflammatory cell recruitment, and VSMC proliferation.30 Moreover, extracellular CyPA induced secretion of cytokines/chemokines and growth factors from PAH-PASMCs, and this effect was enhanced by hypoxia. In a clinical study, plasma CyPA levels were increased and predicted poor outcomes in patients with PAH.30
In addition to CyPA and Bsg, several novel pathogenic proteins that are potentially useful biomarkers for PAH have been found.59–61 imaging techniques to evaluate pulmonary arterial remodeling in patients with PAH have been developed,62,63 and, in addition, it has been found that pravastatin and metformin ameliorate hypoxia-induced PH in mice.25,46 Recently, microarray analyses using PAH-PASMCs to identify further common pathogenic proteins showed significant changes in 1,858 genes in PAH-PASMCs compared with control PASMCs. Some novel targets to develop therapeutic agents for patients with PAH were identified (Figure 2).
Screening of novel therapeutic target of pulmonary arterial hypertension (PAH). PASMCs, pulmonary artery smooth muscle cells.
Chronic thromboembolic pulmonary hypertension (CTEPH) is a distinct pulmonary vascular disease, categorized as group IV in the WHO classification of PH.64–67 Before the emergence of balloon pulmonary angioplasty, CTEPH was a serious disorder causing severe right ventricular failure and death.68–71 The main feature of CTEPH is obstruction of pulmonary arteries by organized thrombi;64,68 however, the pathogenesis remains obscure and finding the key CTEPH molecule and elucidating the pathogenesis of this disorder are current research goals. It was recently demonstrated that plasma levels of thrombin-activatable fibrinolysis inhibitor (TAFI) are significantly elevated in patients with CTEPH compared with those with PAH or controls.42 Importantly, the minor allele of CPB2 was found in patients with CTEPH compared with the standard Asian population;42 plasma levels of TAFIa negatively correlated with clot lysis time in these patients.42 Finally, using genetically modified mice, the role of TAFI in the development of CTEPH has been demonstrated.47 Importantly, TAFIa levels were markedly increased in the plasma of patients with CTEPH.47 To evaluate the effects of TAFIa inhibition in PH, in silico screening using the Life Science Knowledge Bank (LSKB) database was performed and it found several TAFIa inhibitors, one of which ameliorated PH in mice.47 Additionally, TAFI inhibitors currently used in clinical settings were screened and peroxisome proliferator-activated receptor-α (PPARα) agonists were found; these significantly reduced liver TAFI synthesis with resultant amelioration of PH in mice and rats.47 Thus, TAFIa is a novel and realistic therapeutic target of CTEPH.
Life phenomena are complex and closely interconnected, and even with small studies the more you keep on, the more you can discover. Collaborative research activities and discussions of common interests with researchers around the world can make the researcher’s life fulfilling and fruitful again. I am grateful for the good luck and guidance that I have enjoyed during my research over the past 15 years. However, it is disappointing to learn that the number of research articles from Japan has been dramatically decreasing during the 21st century.72 The number of publications in the American Heart Association journals (Circulation, Circulation Research, and Arteriosclerosis, Thrombosis, and Vascular Biology) has also decreased.72 Recent data clearly demonstrate the devastating situation of basic research in Japan compared with other top countries in the world.72 In addition to the shocking situation in cardiovascular research, the number of publications from Japan in engineering science also sharply declined during the past decade compared with the average number of publications from other countries.72 To rescue the current critical situation and regenerate medical research in Japan, the Japanese government established the Japan Agency for Medical Research and Development (AMED) in April 2015. AMED aims to achieve the highest levels of research, from basic to clinical, by establishing and maintaining an environment for the growth of medical research and development. Additionally, the Japanese government has started a plan to increase the number of young researchers (under 40 years of age) working in universities by supporting initiatives that offer more tenure positions and allowing more flexible use of grants to hire young researchers. In addition, the Japanese Circulation Society supports cardiovascular research in various ways, including encouraging and aiding cardiovascular research, commending achievements, and giving grants to young researchers for their studies abroad. I hope these efforts may improve the situation in Japan in the future.
I have reviewed 15 years of challenges during the development of novel therapies for cardiovascular diseases by clinical application of basic research (Figure 3). The mechanism of onset of cardiovascular diseases is very complicated, and there are still many points to be clarified. From now on, translational research will become increasingly important. Even in basic research using genetically modified animals, it is important to confirm clinical significance using human-derived pathological specimens to drive development of diagnostic tools and therapies. Thus, the importance of research environments that integrate a wide range of research from basic to clinical is increasing. I would like to continue research that will deliver the findings obtained by basic research to the clinic, and to deeply consider the questions raised in clinical practice through basic research.
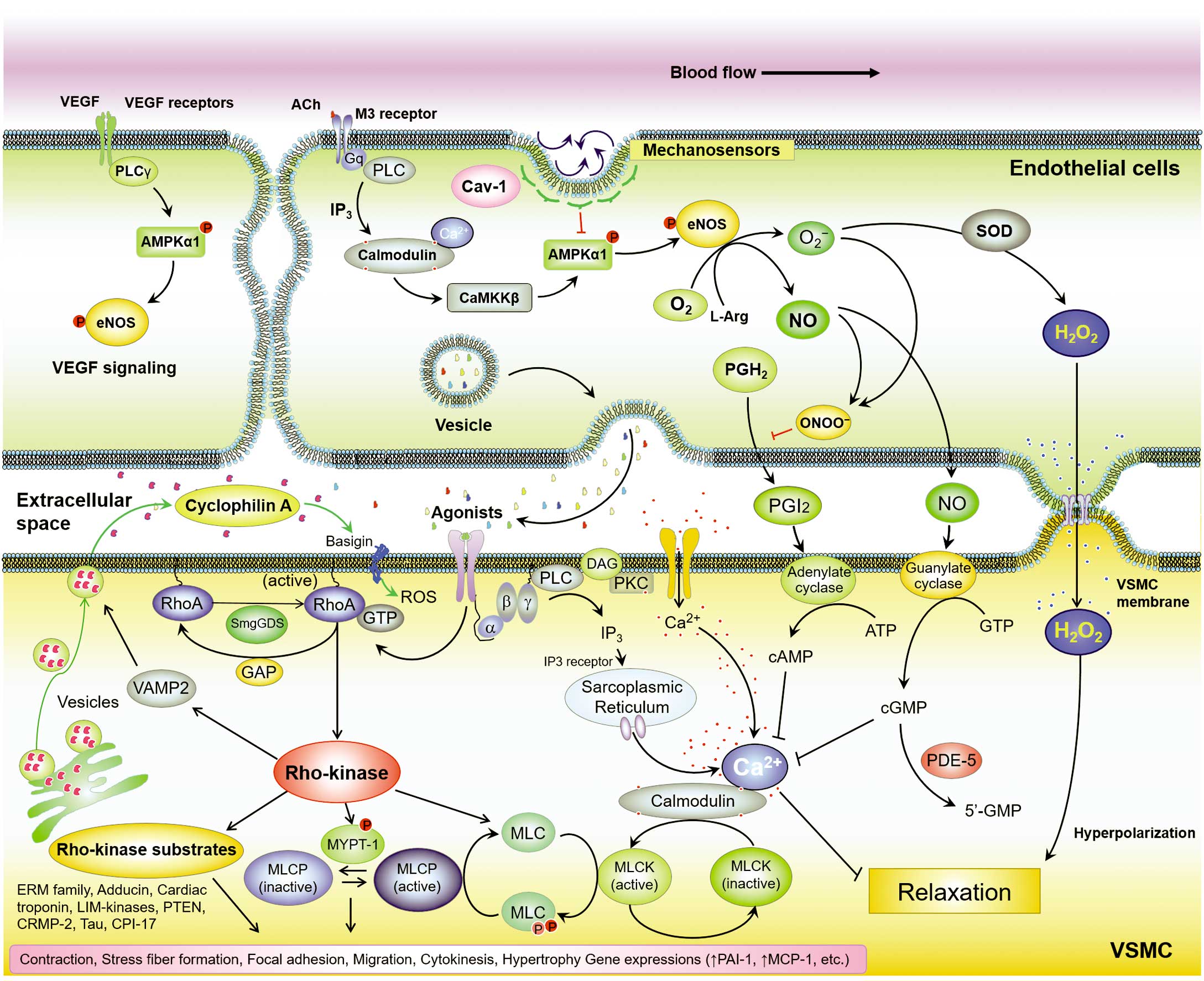
Novel therapeutic targets for cardiovascular diseases. Ach, acetylcholine; eNOS, endothelial nitric oxide synthase; NO, nitric oxide; PKC, protein kinase C; PLC, phospholipase C; ROS, reactive oxygen species; SOD, superoxide dismutase; VEGF, vascular endothelial growth factor; VSMC, vascular smooth muscle cells.
It is my great honor and pleasure to receive the Sato memorial Award. I express my enormous appreciation to my mentors for their strong support and guidance: Professor Hiroaki Shimokawa (Tohoku University Graduate School of Medicine) and Professor Bradford C. Berk (University of Rochester, School of Medicine). I also acknowledge all my colleagues, friends, and collaborators around the world, especially past and present co-workers in the Department of Cardiovascular Medicine in Tohoku university, Drs. Satoshi Yasuda, Yoshihiro Fukumoto, Kenta Ito, Satoshi Miyata, Jun Takahashi, Koichiro Sugimura, Minako Oikawa, Makoto Nakano, Yutaka Miura, Tatsuo Aoki, Shunsuke Tatebe, Kotaro Nochioka, Masanobu Miura, Saori Yamamoto, Toru Shimizu, Shohei Ikeda, Nobuhiro Yaoita, Shun Kudo, Tatsuro Minami, Hideaki Suzuki, Kota Suzuki, Taijyu Satoh, Nobuhiro Kikuchi, Junichi Omura, Yohei Satake, Haruka Sato, Ryo Kurosawa, Masamichi Nogi, Shinichiro Sunamura, Tomohiro Ohtsuki, Katsuya Kozu, Kazuhiko Numano, Zhulanqiqige Do.e, Alia Ellawindy, Md. Elias-Al-Mamun, Mohammad Abdul Hai Siddique. This work was supported in part by Grants-in-aid for Scientific Research (15H02535, 15H04816, and 15K15046), all from the Ministry of Education, Culture, Sports, Science and Technology, Tokyo, Japan, Grants-in-aid for Scientific Research from the Ministry of Health, Labour, and Welfare, Tokyo, Japan (10102895), and Grants-in-aid for Scientific Research from the Japan Agency for Medical Research and Development, Tokyo, Japan (15ak0101035 h0001, 16ek0109176 h0001, and 17ek0109227 h0001).
None.