2017 Volume 81 Issue 3 Pages 290-297
2017 Volume 81 Issue 3 Pages 290-297
It is almost a quarter of century that a pioneering work of 2 researchers named Brugada brought the entire scientific community to understanding the molecular, clinical, and electrophysiological aspects of a distinctive syndrome. It affects mainly young adults with syncope and/or sudden cardiac death caused by polymorphic ventricular tachycardia or ventricular fibrillation in the absence of any sign of cardiac degeneration or alteration. Although the involvement of the epicardial layer of the right ventricular outflow tract, and the requirement of pharmacologic challenge for unveiling concealed forms, have been fully characterized, many areas of uncertainties remain to be elucidated, such as the unpredictable usefulness of programmed ventricular stimulation, the role of radiofrequency catheter ablation for reducing ST-segment elevation, and the value of risk stratification in patients diagnosed with upper displacement of right precordial leads. How much Brugada syndrome is an intense field of research is witnessed by 4 different consensus committees that took place in a relatively short period of time considering the recent discovery of this intricate arrhythmogenic disease. The main focus of this review is to describe the milestones in Brugada syndrome from its first phenotypic and genotypic appraisals to recent achievements in electrical therapies proposed for the management of this fascinating rhythm disturbance that, despite new diagnostic and therapeutic learnings, still predisposes to sudden cardiac death.
The Brugada syndrome (BrS) is characterized on ECG by right bundle branch block (RBBB) and an unusual form of ST-T wave elevation (unrelated to ischemia, electrolyte abnormalities or structural heart disease) in the anterior right precordial leads (RPL).1 As circumstantiated by local traditions and cultural myths that attributed some fantastic names (Lai-tai, Bangungut, Pokkuri) to a syndrome that could induce sudden unexpected nocturnal death or simply sudden unexplained death, BrS is more diffused in Japan (0.15–0.27%) and Philippines (0.18%),2 compared with Western countries (Europe: 0–0.017%; North America: 0.005–0.1%),3 although an intermittent ECG pattern often conceals the true prevalence. Despite risk distribution not being uniform among patients, and most not experiencing adverse cardiac events during their lives, the “Brugada sign” usually qualifies patients with syncope and a family history of sudden cardiac death (SCD) for an implantable cardioverter-defibrillator (ICD).
The aims of this review are to summarize the recent appraisals in BrS and to provide updates in the diagnostic and therapeutic paths of a disease that bears a non-negligible risk of SCD.
Provided the ECG abnormalities of the right ventricle (RV) were all associated to SCD,4 Joseph and Pedro Brugada5 reported the full phenotypic characterization of the RBBB in the prognosis of a syndrome in which altered autonomic tone and antiarrhythmic drugs could modulate the extent of ST-segment elevation (Figure 1).6

Milestones in Brugada syndrome (BrS). The appraisals from discovery of the disease to current proposed criteria for diagnosis are reported as a timeline. APHRS, Asia Pacific Heart Rhythm Society; EHRA, European Heart Rhythm Association; HRS, Heart Rhythm Society; ICD, implantable cardioverter-defibrillator; PES, programmed electrical stimulation; RFA, radiofrequency ablation; RV, right ventricle; SCD, sudden cardiac death; VF, ventricular fibrillation.
In 1997, a series initially consisting of 47 patients,7 and subsequently extended to 63, demonstrated the usefulness of the ICD in preventing SCD in patients with RBBB and ST-segment elevation in leads V1–3.1 A few years later, Priori et al analyzed 52 BrS families with 44 asymptomatic family members (FM) displaying positive genetic screening but negative ECGs, hence documenting the effect of family history in the prognosis of the disease.8 Following these studies, a first consensus report was elaborated.9 Subsequently, a second consensus was drafted,10 which introduced the concept of changing eating habits, because cardiac arrest (CA) caused by polymorphic ventricular tachycardia (pVT) or ventricular fibrillation (VF) can occur after a large meal, especially with foods rich in carbohydrates and glutinous rice, which are believed to shift potassium from free circulation into cells.11
In the majority of genetically diagnosed cases, BrS displays an autosomal dominant mode of transmission with low penetrance8,12 (i.e., the abnormal gene is inherited by 50% of the offspring, and both males and females equally inherit the defective gene, but not all will develop the disease). The identification of a loss-of-function mutation affecting the SCN5A (sodium voltage-gated channel α subunit 5) gene on chromosome 3p21–2313 was rapidly followed by reports of other variants (Figure 2)14,15 that can either alter protein synthesis16 or processing/trafficking,17 or affect channel gating, kinetics, permeability, and ion selectivity.18,19 In more detail, missense mutations (in BrS these occur in two-thirds of patients) are point mutations in which a single nucleotide change results in a codon that codes for a different amino acid; in smaller amounts, nonsense mutations (protein truncated at the mutation site), and splice-site, frameshift, insertion, and deletion mutations (protein completely altered after the mutation site) have been discovered as well.14 In the past decades, with accumulated findings suggesting that BrS has a heterogeneous genetic basis, a more complex inheritance has been proved. Nowadays, <40% of BrS cases are familial, whereas other cases are sporadic.12,19 Furthermore, unlike most previously reported sodium channelopathies, overlap syndromes displaying recessive inheritance characteristics and not following simple Mendelian rules, can be found.20

Location of the SCN5A mutations. The amino acid variants (circles, n=279) are shown in different colors according to the type of mutation. Red arrows point towards those pathogenic variants encountered in ≥6 unrelated individuals. Intronic variants (n=17) are not displayed.
In spite of the low yield of genetic screening (<30%, Table), with 65–70% of BrS patients remaining genetically unresolved, mutations in additional genes,15 including different subunits of the L-type cardiac Ca2+ channel,21 remain to be further investigated.
| Genetics | |
|---|---|
| Main genes displaying pathogenic variants | % of BrS carriers |
| SCN5A | 20–25% cases |
| CACNA1C | 5% cases |
| CACNA2D1, KCND3, CACNB2b, GPD1L, HCN4, KCNE3, KCNE5, KCNJ8, RANGRF, TRPM4, SCN1B, SCN2B, SCN3B, SLMAP |
<1% cases, each |
BrS, Brugada syndrome; CACNA1C, calcium voltage-gated channel subunit α1C; CACNA2D1, calcium voltage-gated channel auxiliary subunit α2δ1; CACNB2b, calcium voltage-gated channel auxiliary subunit β2; GPD1L, glycerol-3-phosphate dehydrogenase 1-like; HCN4, hyperpolarization-activated, cyclic nucleotide-gated K+4; KCND3, potassium voltage-gated channel subfamily D member 3; KCNE3, potassium voltage-gated channel subfamily E regulatory subunit 3; KCNE5, potassium voltage-gated channel subfamily E regulatory subunit 5; KCNJ8, potassium voltage-gated channel subfamily J member 8; RANGRF, RAN guanine nucleotide release factor; SCN1B, sodium voltage-gated channel β subunit 1; SCN2B, sodium voltage-gated channel β subunit 2; SCN3B, sodium voltage-gated channel β subunit 3; SCN5A, sodium voltage-gated channel α subunit 5; SLMAP, sarcolemma associated protein; TRPM4, transient receptor potential cation channel subfamily M member 4.
Although repolarization heterogeneity within the epicardium of the right ventricular outflow tract (RVOT) has been linked to phase 2 reentry VT,22 other authors have delineated conduction disturbances (PR-segment prolongation, different degrees of incomplete RBBB) and late potentials (see later) on the surface ECG, associated with H-V interval prolongation in electrophysiologic studies, as substrates for depolarization disorders.23 Postmortem analyses of unexplained sudden death victims in different series were all concordant in describing peculiar tissue abnormalities at the RVOT,24 characterized by reduced connexin-43 expression, interstitial fibrosis,25 and activation slowing that conditions an absent transmural repolarization gradient, and abnormal conduction restitution.26
To date, both theories are believed to be involved in the etiopathogenesis of BrS as demonstrated by an elegant study of ECG imaging on 25 BrS and 6 RBBB patients; unlike BrS, RBBB showed delayed activation in the entire RV, without ST- segment elevation, fractionation, or repolarization abnormalities.27
Fever may induce the appearance of a type 1 BrS ECG pattern and may trigger episodes of pVT/VF in affected patients,28 because of accentuation of the inactivation of the Na+ channel.17 When an increase in body temperature >38℃ occurs, current guidelines recommend close ECG monitoring in combination with antipyretics.3
Hypertestosteronemia appears to be a modulating factor that associates BrS with the male sex (8–10-fold more prevalent than in women);29 the incidence of fatal arrhythmias seems related to ion currents underlying the epicardial action potential notch.2
Diagnostic ECG (type 1, Figure 3A,B) displays a coved ST-segment elevation of at least 2 mm of amplitude recorded in the RPL, either spontaneously or evoked by a 10-min infusion of sodium channel blocking agents (ajmaline 1 mg/kg, flecainide 2 mg/kg, procainamide 10 mg/kg, or pilsicainide 1 mg/kg).1–3,7–10 The saddleback ST-segment elevation ≥2 mm (type 2 BrS ECG, Figure 3A) or <1 mm (either coved or saddleback, type 3 BrS ECG, Figure 3A) is suspicious for BrS and requires further investigation.2,3,30–32 The first consensus in 2002 recommended diagnosis when a type 1 ECG pattern was detected in 2 among V1–3 leads with a gradually descending ST segment;7–9 3 years later, the second consensus focused on the relative weight of a few clinical aspects such as (1) documented VF; (2) self-terminating pVT; (3) family history of SCD (<45 years); (4) coved-type ECGs in FMs; (5) inducibility at programmed electrical stimulation (PES); (6) syncope; and (7) nocturnal agonal respiration.10 Following the report on BrS diagnosed in just 1 RPL,33,34 the Heart Rhythm Society (HRS), the European Heart Rhythm Association (EHRA), and the Asia Pacific Heart Rhythm Society (APHRS), released new guidelines according to spontaneous or drug-induced coved ST-segment elevation ≥2 mm in ≥1 lead (either V1 or V2) located in the 4th, 3rd, or 2nd intercostal space (ICS), and type 2 or type 3 ECG that fully converts into a type 1 ECG morphology.2 In this regard, it has been recently demonstrated that the upper displacement of the RPL on either rest ECG or Holter-ECG (Figure 3B) is able to unveil approximately 21% of new BrS cases35 because it reflects the normal projection of the heart (including the RVOT) onto the anterior chest surface.36 Such appraisals prevent the pro-arrhythmic side effects of class IC drugs,30–32,37,38 but also raise concerns about risk stratification (see later) of such individuals, because the diagnostic pattern is found spontaneously. Finally, a recent consensus of the HRS/EHRA/APHRS and the Latin American Society of Cardiac Pacing and Electrophysiology (SOLAECE) proposed the Shanghai Score System3 that calculates points derived by: ECG findings (spontaneous type 1 ECG at nominal or high leads=3.5; fever-induced type 1 ECG at nominal or high leads=3; type 2 or 3 ECG that converts to type 1 after drug infusion=2); clinical history (unexplained CA or documented VF/pVT=3; nocturnal agonal respirations=2; suspected arrhythmic syncope=2; syncope of unclear mechanism/unclear etiology=1; atrial fibrillation or flutter in patients <30 years without alternative etiology=0.5); family history (1st- or 2nd-degree relative with definite BrS=2; suspicious SCD during fever/nocturnal/under medication to be avoided in BrS=1; unexplained SCD <45 years with negative autopsy=0.5); probable pathogenic mutation in BrS susceptibility gene=0.5. According to this score, diagnosis is probable/definite when the cumulative sum is ≥3.5 points, and possible when 2–3 points are obtained; in cases with <2 points diagnosis is not feasible.
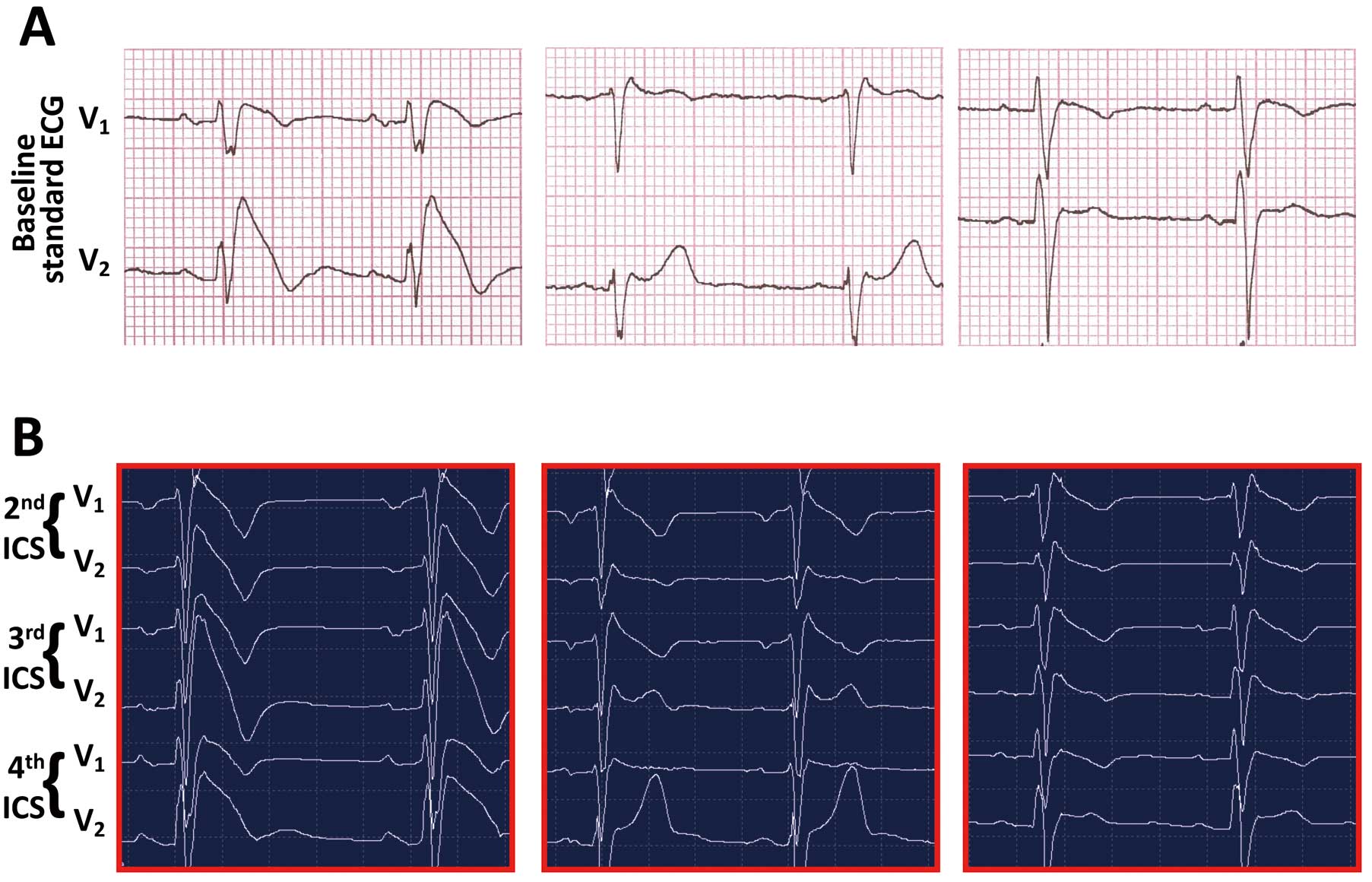
The 3 Brugada syndrome patterns on baseline ECG and on Holter-ECG monitoring. (A) Coved (Left) and saddleback (Center and Right) ST-segment elevations. Paper speed: 25 mm/s; amplitude: large square=1 millivolt. (B) Holter-ECG recordings with thoracic leads in the 2nd, 3rd and 4th intercostal spaces for the 3 patients in (A).
Asymptomatic patients are usually discovered during (1) routine check-ups (pre-operation, sports, insurance), (2) family screening; (3) antiarrhythmic treatment for palpitations or (4) fever.
The complete syndrome is characterized by episodes of rapid pVT in patients with an ECG pattern of RBBB and coved ST-segment elevation in the RPL. When the episodes terminate spontaneously, the patient develops syncopal attacks. When the episodes are sustained, full blown CA and eventually SCD occur. Such a distinction has been questioned recently, because syncope with prodrome, especially blurred vision, suggests a benign etiology of syncope itself in BrS patients;39 in contrast, many patients with suspected autonomic dysfunction who have undergone a tilt-table test, which was positive, and who have been treated accordingly have subsequently died suddenly.40
Family History of Sudden DeathAlthough a family history of BrS was initially considered to play an important role in the diagnostic process,8 different ethnic groups, population selection in each study, inclusion criteria of ECG with different ICS, and the number/timing of the ECG recordings did not confirm such an association.2,3
Sex DifferenceAlthough the original descriptions noted that men present with a greater risk clinical profile than women and have a worse prognosis,41 a recent report provided the first event rate in the female sex (0.7%/year), and focused on SCD or previous sinus node dysfunction as a high-risk factor in future arrhythmic events in women as well.42
Risk and Prognosis in Life StagesAfter the first case of CA in a Polish boy during a febrile illness,5 research groups confirmed the higher prevalence in adults,1–11,28–42 not excluding involvement in children when particularly aggressive phenotypes were manifested.43 In fact, rapid VT and conduction abnormalities,44 as well as sinus node dysfunction and atrial tachyarrhythmias45 in the absence of structural or metabolic abnormalities, have been observed in infancy.46
On the other hand, the elderly have been recently assessed as an epoch of the life with low BrS occurrence,47 although management with ICDs is associated with more inappropriate shocks and lead failure in old patients,48 who perhaps might benefit more from pacemaker therapy.49
Programmed Electrical StimulationFor identification of individuals at major risk who would benefit from ICD therapy, pVT/VF inducibility at PES, became widely accepted.1,9,10,50 Brugada et al1,5,7,33,34 showed that PES is highly sensitive in predicting high risk, whereas the cohorts analyzed by Priori et al,51 Eckardt et al,52 and the FINGER study53 did not provide evidence that PES is a good indicator. Accordingly, PES in BrS has received a Class IIb recommendation.2 The last pooled analysis suggested that induced pVT/VF during PES up to double extrastimuli is associated with a higher risk of CA,54 although non-inducibility is not synonymous with low risk. To consolidate the usefulness of PES, multicenter prospective studies that include large numbers of patients are required.55
Noninvasive Instrumental FeaturesNowadays there is an increasing interest in other noninvasive assessments, such as late potentials analysis and T-wave amplitude variability. These parameters reflect disorders in ventricular depolarization and repolarization, respectively.56
In agreement with these findings, a multicenter prospective registry identified fragmentation of the QRS (defined as ≥2 spikes within the QRS complex in leads V1 –V3) as a predictor of arrhythmic events in BrS patients without a history of pVT/VF.57
However, so far none of the above has proven effective in risk prediction. Considerable evidence supports the concept of the spontaneous type 1 pattern on either ECG or 12-lead Holter-ECG,51 recorded in whichever ICS,58 being associated with worse prognosis.50,51,59 In agreement with such findings, in a cohort of 300 suspected BrS patients, 64 cases that were diagnosed according to the new guidelines displayed 0.11% annual incidence of CA over a mean observation time of 41 years.35 Along the same line, others showed that in highly symptomatic (55% events) patients, type 1 ECG in the 4th and 3rd ICS did not influence prognosis, although the occurrence of diagnosis in only High-ICS and with drug test were very low (19.3 and 11.2%, respectively);58 therefore, both of these clinical observations reinforce the concept that a spontaneous BrS ECG pattern retains its prognostic value even when present in high precordial leads.35,58
On account of the delineated clinical features, a new category of risk, namely intermediate-low, can be hypothesized and included in the pyramid of risk (Figure 4).

Risk stratification according to clinical and instrumental features. High-, intermediate-, and low-risk panels refer to patients diagnosed with Brugada syndrome in the 4th intercostal (standard) space (ICS).
Overall, the risk of lethal or near-lethal arrhythmic episodes among previously asymptomatic patients with BrS varies according to the series: 8% event rate at 33±39 months of follow-up reported by Brugada et al;33 6% event rate at 34±44 months by Priori et al;57 1% event rate after 40±50 months and 30±21 months of follow-up, respectively by Eckardt et al60 and Giustetto et al61 and finally, Probst et al53 reported a 1.5% event rate at 31 months of follow-up.
In the absence of effective pharmacologic therapies that prevent from SCD, the ICD is the only therapeutic option for BrS patients (Figure 5A). It is important to remark that ICDs are not free from several disadvantages, especially in young patients who will undergo plural device replacements for battery exhaustion.62 To this regard, at 10-years post-implantation, the rates of inappropriate shocks and/or device malfunction caused by lead failure are 37% and 29%, respectively.63 Endocardial lead dysfunction requires extraction and replacement, with further complications. Recently, the possibility of an entirely subcutaneous device (S-ICD) that avoids the side effects associated with transvenous electro-catheters has added options to the available armamentarium.64 Such devices have recently overcome the initial concerns of a potential risk of undersensing tachyarrhythmias (Figure 5B). To date, the role of the anti-tachypacing modality offered by traditional transvenous ICD in preventing degeneration of monomorphic VT is not considered essential in this category of patients, because low amplitude wave VF and polymorphic VT are the most common arrhythmias found in BrS patients; intriguingly, a recent multicenter retrospective study observed that monomorphic VTs account for only 4.2% of BrS patients implanted with an ICD and that there is a strong likelihood of arrhythmia disappearance after endocardial and/or epicardial ablation;65 this technical aspect corroborates the therapeutic role of S-ICD, and its association with fewer complications over a lifetime qualifies the subcutaneous defibrillator as the present and future indication for this complex arrhythmogenic disease.

Device-based arrhythmia treatment in Brugada syndrome. (A) Polymorphic ventricular tachycardia treated by transvenous ICD. (B) Subcutaneous ICD defibrillation test. ICD, implantable cardioverter-defibrillator; VF, ventricular fibrillation.
The early attempts at catheter ablation to treat BrS patients were limited to a few reported cases of patients with electrical storms. The former approach was designed to target the initiating PVCs that trigger VF with radiofrequency ablation (RFA) at the endocardial site of the RVOT.66 However, this approach did not prove successful in all cases, because BrS patients rarely have PVCs frequently enough to allow mapping and to provide a precise target for ablation.
The first epicardial RFA of the RVOT was followed by pVT/VF inducibility in 22% cases, while BrS pattern disappeared after procedure in 3 above 9 patients at follow-up.67
A recent report evaluated 14 BrS patients with ICD for targeting fragmented and delayed potentials and low voltage areas in the basal condition and after flecainide test. In a short time, data reported 100% pattern disappearance under basal conditions and after drug challenge, no pVT/VF inducibility, and no more episodes.68 Although larger studies with longer follow-up are required, these results provide new insights into the electrophysiological mechanisms of BrS; however, areas of uncertainty regarding the clinical outcome after embellishment of the ventricular repolarization have been already observed in the history of cardiac electrophysiology, when digoxin administration, which shortens the QT segment,69 was not associated with better outcome in long QT (LQT) syndrome patients.70 Moreover, whether the elimination of the phenotypic manifestations of the BrS will result in less arrhythmic events during follow-up remains to be established.
Finally, it should be taken into account that in patients in whom BrS is associated with the early repolarization pattern/syndrome, selective ablation of the anterior RV epicardium (including the RVOT) is not ameliorative.3
Because translation to humans of the experimental results in homozygous Scn5a−/− mice with conduction block and reentrant VT71 has been disappointing, mainly because of the lack of testosterone-mediated Ito currents or concordance between the RVOT and ECG in non-human settings, selective pharmacologic strategies have been developed.
Isoproterenol is effective in reducing the arrhythmic burden and electrical storm during energy delivery by the ICD;72,73 its pharmacodynamics seem related to increased Ca2+ currents through L-type Ca2+ channels. Dimethyl lithospermate B slows inactivation of INa, thus increasing INa during the early phases of AP and suppressing arrhythmogenesis.2 Bepridil suppressed VT/VF in several studies of patients with BrS, mainly through Ito inhibition and INa increment (peak and late currents) via upregulation of the sodium channels, while prolonging the QT interval at slow rates.74 Hydroquinidine, a class IA antiarrhythmic drug, inhibits Ito to a greater extent in the epicardium than in the endocardium, and has proven efficacy and safety in a long-term follow-up study.75 Cilostazol, a phosphodiesterase III inhibitor, normalizes the ST-segment, most likely by augmenting ICa as well as by reducing Ito secondary to an increase in cAMP and heart rate.76 Finally, combined use of a Chinese herb extract that inhibits Ito and hydroquinidine has been hypothesized.77
Cellular reprogramming through the technology of human-induced pluripotent stem cells (hIPSCs) has been described for different primary electrical diseases, including LQT1, LQT2, LQT3, LQT8/Timothy syndrome and catecholaminergic pVT, as well as for BrS.78,79 If optimized, a hypothetical hiPSC-based approach could be successful in cases of frequent ICD shocks attributable to VT in spite of aggressive antiarrhythmic treatment, thus realizing “patient-tailored therapy”.
BrS is a primary electrical disorder, characterized by typical ECG signs, and it predisposes to death secondary to VT in the absence of cardiac dysfunction. Recommendations released by scientific societies have been updated several times, mainly for ascertaining diagnosis. Therefore, patients should be evaluated in a dedicated clinic with appropriately trained staff. In the meantime, the increased number of subjects with new diagnosis will require a modified clinical approach, such as hospitalization during fever, as well as extending the already wide list of drugs to be avoided (www.brugadadrugs.org) in all patients affected by BrS.
This work was partially supported by a grant of the Italian Ministry of Education, University and Research (MIUR):PON03PE_00009_4 OPTIMA Cardiopaths.