Abstract
Background:
There is discordance regarding the effect of symptom status before aortic valve replacement (AVR) on long-term outcome after AVR in severe aortic stenosis (AS).
Methods and Results:
The CURRENT AS registry is a multicenter retrospective registry enrolling 3,815 consecutive patients with severe AS. Among 1,196 patients managed with the initial AVR strategy, long-term clinical outcomes were compared between the symptomatic patients (n=905), and asymptomatic patients (n=291). Median follow-up interval was 1337 days with a 91% follow-up rate at 2 years. AVR was performed in 886 patients (98%) in the symptomatic group and in 287 patients (99%) in the asymptomatic group. Symptomatic patients were older and more often had comorbidities than asymptomatic patients with similar echocardiographic AS severity. The cumulative 5-year incidences of all-cause death and heart failure (HF) hospitalization were significantly higher in symptomatic patients than in asymptomatic patients (25.6% vs. 15.4%, P=0.001, and 14.2% vs. 3.8%, P<0.001, respectively). On landmark analysis at 30 days after AVR, the differences in mortality and HF hospitalization between the 2 groups were mainly observed beyond 30 days.
Conclusions:
When managed with the initial AVR strategy, the long-term outcomes of symptomatic severe AS were worse than those of asymptomatic severe AS. Early AVR strategy might be recommended in some selected asymptomatic severe AS patients with reasonable operative risk.
In the current guidelines, aortic valve replacement (AVR) is recommended as a class I indication for symptomatic severe aortic stenosis (AS), while the strategy of watchful waiting for AVR until symptoms emerge is generally recommended in asymptomatic severe AS, except in the case of left ventricular (LV) dysfunction, very severe AS, suitability for other cardiac surgery or abnormal exercise test.1,2
We recently reported, however, the propensity-score matching analysis from the large-scale multicenter Contemporary outcomes after sURgery and medical tREatmeNT in patients with severe Aortic Stenosis (CURRENT AS) registry, demonstrating that the conservative strategy as compared with the initial AVR strategy in asymptomatic severe AS was associated with a much higher risk for all-cause death and heart failure (HF) hospitalization.3–8
One of the reasons for the poorer outcomes of the conservative strategy was that a significant proportion of patients did not undergo AVR despite emergence of symptoms during follow-up. Another possible reason could be that the outcomes of AVR after emergence of symptoms during follow-up might be worse than that of AVR at the asymptomatic stage. There is a lack, however, of previous large-scale studies on the influence of symptom status before AVR on the long-term outcome of AVR.9,10
The aim of this study was therefore to compare the long-term clinical outcomes of severe AS treated with the initial AVR strategy according to the presence or absence of symptoms at baseline in the CURRENT AS registry.
Methods
Subjects
The CURRENT AS registry is a retrospective, multicenter registry enrolling consecutive patients with severe AS at 27 centers in Japan between January 2003 and December 2011. We searched the hospital database of transthoracic echocardiography, and enrolled consecutive patients who met the definition of severe AS (peak aortic jet velocity [Vmax] >4.0 m/s, mean aortic pressure gradient [PG] >40 mmHg, or aortic valve area [AVA] <1.0 cm2) for the first time during the study period.11
We excluded patients with a history of surgical aortic valve repair/replacement or percutaneous aortic balloon valvuloplasty. The study design, echocardiographic evaluation, data management practices, and patient enrollment have been previously described in detail.3
The institutional review boards at all 27 participating centers approved the protocol. Written informed consent from each patient was waived in this retrospective study, because we used clinical information obtained in routine clinical practice, and no patients refused to participate in the study when contacted for follow-up.
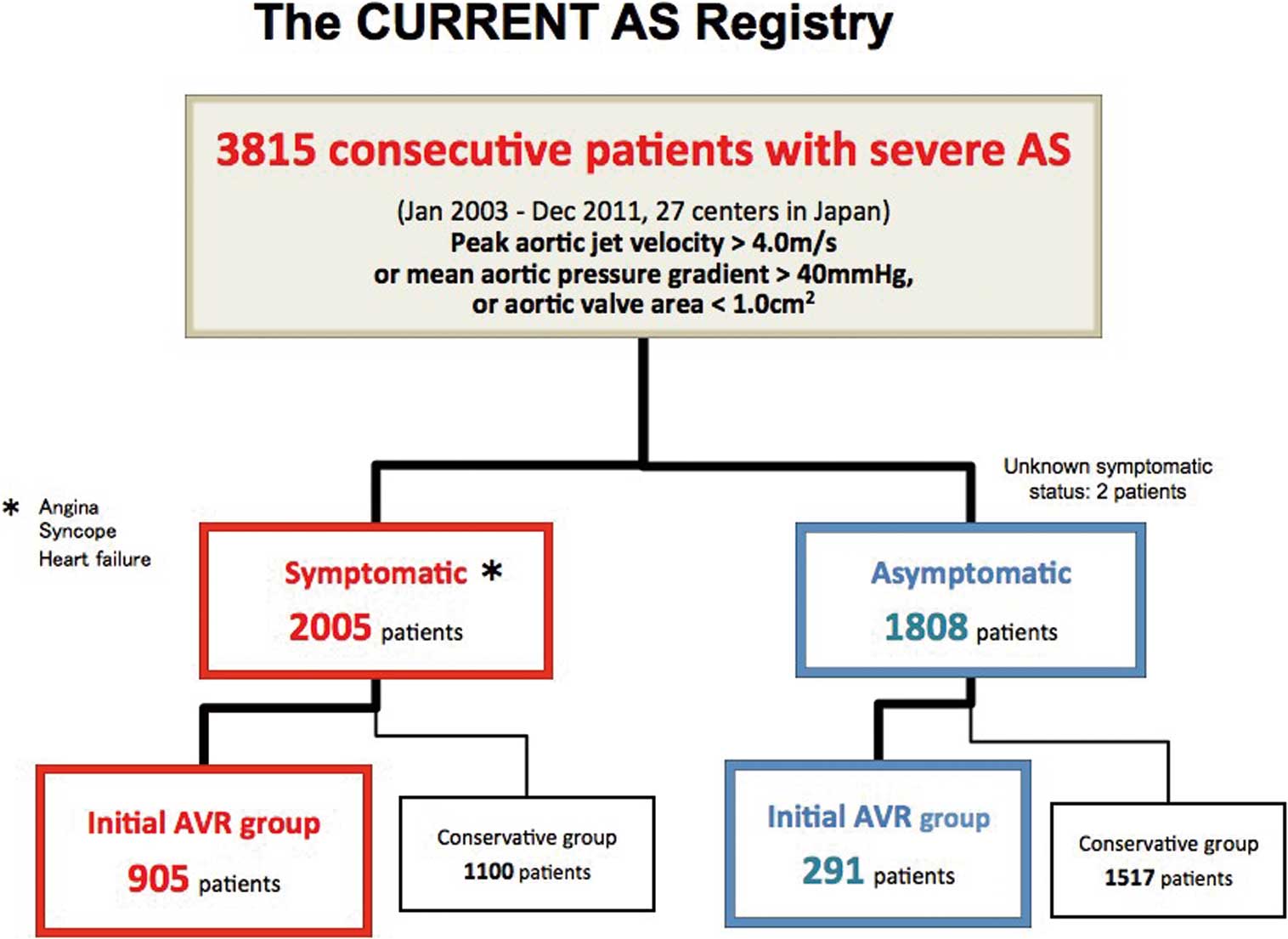
Among 3,815 patients enrolled in this registry, 1,197 patients were managed with the initial AVR strategy, excluding 2,618 patients who were managed with the conservative strategy. Excluding 1 patient whose symptom status was not available, 905 patients (76%) had symptoms thought to be related to AS (symptomatic AVR group), while 291 patients (24%) were asymptomatic at the time of index echocardiography (asymptomatic AVR group;
Figure 1).
AVR Procedures
AVR included both surgical AVR and transcatheter aortic valve implantation (TAVI). Among the 27 participating centers, on-site surgical facilities were available at 20 centers, and TAVI was available at 2 centers. During this period, TAVI was not yet approved in Japan and was conducted only in the context of the pivotal clinical trial. Surgical AVR was performed in the standard fashion under general anesthesia with cardiopulmonary bypass. TAVI was performed using the SAPIEN XT balloon expandable valve (Edwards Lifesciences; Irvine, CA, USA) either through the transfemoral or transapical approach under general anesthesia without cardiopulmonary bypass.
Definitions and Outcome Measures
Collection of baseline clinical information was conducted via hospital chart or database review. Symptoms related to AS were classified into angina, syncope, chronic exertional dyspnea, or acute HF requiring hospitalization. Follow-up data were mainly collected via review of hospital charts; otherwise, data were collected via contact with patients, relatives, and/or referring physicians via mail with questions regarding vital status, symptoms, and subsequent hospitalizations.
For the current analysis, the primary outcome measures were all-cause death and HF hospitalization. Secondary outcome measures included cardiovascular death, aortic valve-related death, and non-cardiovascular death. Cause of death was classified according to the Valve Academic Research Consortium (VARC) definitions, and adjudicated by a clinical event committee.12,13
Aortic valve-related death included aortic valve procedure-related death, sudden death, and death due to HF possibly related to aortic valve. Sudden death was defined as unexplained death in previously stable patients. HF hospitalization was defined as hospitalization due to worsening HF requiring i.v. drug therapy.
Statistical Analysis
In the present study, we compared the baseline clinical, echocardiographic, and procedural characteristics as well as the long-term clinical outcomes between the 2 groups of patients with or without symptoms treated with the initial AVR strategy. Symptom status was assessed at the time of index echocardiography. The main analysis was made according to the intention-to-treat (ITT) principle regardless of the actual performance of AVR. Follow-up was commenced on the day of index echocardiography.
With regard to sensitivity analysis, we compared the long-term clinical outcomes between the asymptomatic and symptomatic AVR groups after excluding those patients who had concomitant surgical procedures other than AVR, or those patients who initially presented with acute HF (Figures S1,S2). We also conducted as-treated analysis in patients who underwent AVR in each group, in which the follow-up was commenced on the day of AVR. Landmark analysis at 30 days after AVR was also performed to distinguish the effects of symptoms on the short-term and long-term outcomes after AVR.
Categorical variables are presented as numbers and percentages, and compared using the chi-squared test or the Fisher exact test. Continuous variables are expressed as mean±SD or median (IQR). Based on their distributions, continuous variables were compared using the Student’s t-test or Wilcoxon rank sum test. We used the Kaplan-Meier method to estimate the cumulative incidence of events and assessed the differences on log-rank test. To adjust for the differences in baseline and procedural characteristics, we used multivariable Cox proportional hazard models incorporating 19 clinically relevant risk-adjusting variables: age; sex; body mass index; hypertension; current smoking; diabetes on insulin; coronary artery disease (CAD); prior myocardial infarction (MI); prior symptomatic stroke; aorta/peripheral artery disease; serum creatinine; hemodialysis; anemia; liver cirrhosis; malignancy currently under treatment; chronic lung disease; AS severity; concomitant coronary artery bypass grafting (CABG); and concomitant valve surgery (Tables 1,2). The centers was incorporated in the stratification variables. Consistent with our previous report, the continuous variables other than age were dichotomized using the median or clinically meaningful reference values. Proportional hazard assumptions for the risk-adjusting variables including categorized age in quartiles were assessed using plots of log (time) vs. log[−log (survival)] stratified by the variable, and were verified to be acceptable. We did not include those factors related to evolution of symptoms such as LV function, pulmonary hypertension, and atrial fibrillation as the risk-adjusting variables. The risks of the symptomatic AVR group relative to the asymptomatic AVR group for the clinical outcome measures are expressed as hazard ratios (HR) and 95% CI.
Table 1.
Baseline Subject Characteristics
| Variables |
Asymptomatic AVR group
(n=291) |
Symptomatic AVR group
(n=905) |
P value |
| Clinical characteristics |
| Age (years)* |
71.6±8.7 |
73.9±8.9 |
0.0002 |
| Age ≥80 years |
49 (17) |
249 (28) |
0.0002 |
| Male* |
126 (43) |
381 (42) |
0.72 |
| BMI (kg/m2) |
22.1±3.3 |
22.4±3.6 |
0.28 |
| BMI <22* |
146 (50) |
476 (53) |
0.47 |
| BSA (m2) |
1.51±0.17 |
1.49±0.18 |
0.21 |
| Hypertension* |
188 (65) |
618 (68) |
0.24 |
| Dyslipidemia |
116 (40) |
360 (40) |
0.98 |
| On statin therapy |
72 (25) |
267 (30) |
0.12 |
| Current smoking* |
22 (8) |
61 (7) |
0.63 |
| History of smoking |
74 (25) |
240 (27) |
0.71 |
| Diabetes mellitus |
59 (20) |
217 (24) |
0.19 |
| On insulin therapy* |
11 (4) |
47 (5) |
0.33 |
| Diagnosis of CAD at time of AVR* |
64 (22) |
337 (37) |
<0.0001 |
| Prior MI* |
5 (2) |
46 (5) |
0.014 |
| Prior PCI |
21 (7) |
82 (9) |
0.33 |
| Prior CABG |
7 (2) |
25 (3) |
0.74 |
| Prior open heart surgery |
13 (4) |
35 (4) |
0.65 |
| Prior symptomatic stroke* |
25 (9) |
82 (9) |
0.81 |
| Atrial fibrillation and flutter |
39 (13) |
168 (19) |
0.04 |
Aortic/peripheral vascular disease (treated or planned
to be treated after AVR)* |
23 (8) |
47 (5) |
0.09 |
| Serum creatinine (mg/dL)* |
0.80 (0.62–1.0) |
0.84 (0.70–1.16) |
0.64 |
| Creatinine >2 mg/dL |
34 (12) |
118 (13) |
0.55 |
| Hemodialysis* |
32 (11) |
103 (11) |
0.86 |
| Anemia*,‡ |
130 (45) |
499 (55) |
0.002 |
| Liver cirrhosis (Child-Pugh B or C)* |
1 (0.3) |
5 (0.6) |
1 |
| Malignancy |
34 (12) |
97 (11) |
0.65 |
| Malignancy currently under treatment* |
7 (2) |
17 (2) |
0.58 |
| Chest wall irradiation |
1 (0.3) |
6 (1) |
1 |
| Immunosuppressive therapy |
4 (1) |
27 (3) |
0.13 |
| CLD (moderate-severe)* |
2 (0.7) |
17 (2) |
0.19 |
| Logistic EuroSCORE |
5.5 (3.7–8.3) |
7.6 (4.8–12.5) |
<0.0001 |
| EuroSCORE II |
1.5 (1.1–2.3) |
2.5 (1.5–4.1) |
<0.0001 |
| STS score (PROM) |
2.0 (1.4–3.3) |
3.1 (1.9–5.2) |
<0.0001 |
| Etiology of aortic stenosis |
| Degenerative |
220 (76) |
753 (83) |
0.004 |
| Congenital (unicuspid, bicuspid, or quadricuspid) |
53 (18) |
101 (11) |
| Rheumatic |
9 (3) |
44 (5) |
| Infective endocarditis |
3 (1) |
3 (0.3) |
| Others (%) |
6 (2) |
4 (0.4) |
| Echocardiographic variables |
| Vmax (m/s) |
4.8±0.8 |
4.7±0.8 |
0.15 |
| Vmax ≥4 m/s* |
245 (84) |
748 (83) |
0.54 |
| Vmax ≥5 m/s |
114 (39) |
314 (35) |
0.17 |
| Peak aortic PG (mmHg) |
93±32 |
90±31 |
0.14 |
| Mean aortic PG (mmHg) |
54±20 |
53±19 |
0.48 |
| AVA (equation of continuity) (cm2) |
0.67±0.16 |
0.64±0.18 |
0.04 |
| AVA index (cm2/m2) |
0.45±0.11 |
0.43±0.12 |
0.13 |
| Eligibility for severe AS |
| Vmax >4 m/s |
240 (82) |
730 (81) |
0.51 |
| Mean aortic PG >40 mmHg |
174/220 (79) |
510/706 (72) |
0.04 |
| Vmax >4 m/s or mean aortic PG >40 mmHg |
243 (84) |
733 (81) |
0.34 |
| AVA <1.0 cm2 alone with LVEF <50% |
5 (2) |
59 (7) |
0.0015 |
| AVA <1.0 cm2 alone with LVEF ≥50% |
43 (15) |
113 (12) |
0.31 |
| LVEDD (mm) |
45±6 |
48±7 |
<0.0001 |
| LVESD (mm) |
28±6 |
32±9 |
<0.0001 |
| LVEF (%) |
67±10 |
61±15 |
<0.0001 |
| LVEF <40% |
4 (1) |
99 (11) |
<0.0001 |
| LVEF <50% |
19 (7) |
186 (21) |
<0.0001 |
| IVST in diastole (mm) |
12±2 |
12±2 |
0.99 |
| PWT in diastole (mm) |
12±2 |
12±2 |
0.85 |
| Any combined valvular disease (moderate or severe) |
81 (28) |
397 (44) |
<0.001 |
| Moderate or severe AR |
55 (19) |
238 (26) |
0.01 |
| Moderate or severe MS |
7 (2) |
44 (4) |
0.07 |
| Moderate or severe MR |
26 (9) |
201 (22) |
<0.0001 |
| Moderate or severe TR |
22 (8) |
125 (14) |
0.0047 |
| TR PG ≥40 mmHg |
21 (7) |
159 (18) |
<0.0001 |
| Symptom type |
| Angina |
|
291 (32) |
|
| Syncope |
|
110 (12) |
|
| Chronic exertional dyspnea |
|
659 (73) |
|
| Acute heart failure |
|
270 (30) |
|
Data given as n (%), mean±SD, or median (IQR). *Potential independent variables selected for the Cox proportional hazard models. ‡Anemia was defined according to the World Health Organization criteria (hemoglobin <12.0 g/dL in women and <13.0 g/dL in men). AR, aortic regurgitation; AVA, aortic valve area; AVR, aortic valve replacement; BMI, body mass index; BSA, body surface area; CABG, coronary artery bypass grafting; CAD, coronary artery disease; CLD, chronic lung disease; IVST, interventricular septum thickness; LVEDD, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction; LVESD, left ventricular end-systolic diameter; MI, myocardial infarction; MR, mitral regurgitation; MS, mitral stenosis; PCI, percutaneous coronary intervention; PG, pressure gradient; PROM, predicted risk of mortality; PWT, posterior wall thickness; STS, Society of Thoracic Surgeons; TR, tricuspid regurgitation; Vmax , peak aortic jet velocity.
Table 2.
Procedural Surgical AVR Characteristics
| Variables |
Asymptomatic AVR
group |
Symptomatic AVR
group |
P value |
| No. patients evaluated |
286 |
876 |
|
| Combined surgical procedures |
| AVR with revascularization therapy |
44 (15) |
244 (28) |
<0.0001 |
| CABG* |
44 (15) |
244 (28) |
<0.0001 |
| PCI |
0 |
0 |
– |
| AVR with any valve surgery* |
38 (13) |
142 (16) |
0.24 |
| AVR with mitral valve surgery |
30 (11) |
113 (13) |
0.28 |
| Mitral valve replacement |
16 (6) |
58 (7) |
0.54 |
| Mitral valve repair |
14 (5) |
55 (6) |
0.39 |
| AVR with tricuspid valve surgery |
23 (8) |
71 (8) |
0.97 |
| Tricuspid valve replacement |
1 (0.4) |
2 (0.2) |
0.57 |
| Tricuspid valve repair |
22 (8) |
69 (8) |
0.92 |
| AVR with ascending aorta replacement |
43 (15) |
62 (7) |
<0.0001 |
| AVR with annular dilatation |
2 (0.7) |
6 (0.7) |
1 |
| AVR with maze operation |
15 (5) |
53 (6) |
0.61 |
| Bioprosthetic valve |
226 (79) |
672 (77) |
0.57 |
| Mechanical valve |
59 (21) |
197 (22) |
|
| Unknown† |
1 (0.4) |
7 (0.8) |
|
| Valve size |
| Bioprosthetic valve |
226 |
672 |
|
| 19 mm |
64 (28) |
283 (42) |
|
| 21 mm |
92 (41) |
248 (37) |
|
| 23 mm |
45 (20) |
95 (14) |
|
| 25 mm |
18 (8) |
33 (5) |
|
| 27 mm |
2 (0.9) |
3 (0.5) |
|
| 29 mm |
2 (0.9) |
0 (0) |
|
| Unknown |
3 (1) |
10 (1) |
|
| Mechanical valve |
59 |
197 |
|
| 16 mm |
3 (5) |
8 (4) |
|
| 17 mm |
6 (10) |
31 (16) |
|
| 18 mm |
1 (2) |
15 (8) |
|
| 19 mm |
24 (41) |
51 (26) |
|
| 20 mm |
1 (2) |
5 (3) |
|
| 21 mm |
10 (17) |
46 (24) |
|
| 22 mm |
1 (2) |
8 (4) |
|
| 23 mm |
10 (17) |
25 (13) |
|
| 25 mm |
1 (2) |
2 (1) |
|
| 27 mm |
1 (2) |
2 (1) |
|
| 29 mm |
1 (2) |
1 (0.5) |
|
| Unknown |
0 (0) |
3 (2) |
|
Data given as n (%). *Potential independent variables selected for Cox proportional hazard models. In the analysis for the surgical procedural characteristics, we excluded 23 patients who did not undergo AVR, and 11 patients who underwent TAVI. †Regarding the prosthetic valve types, we did not have information in 8 patients who were operated on in hospitals other than the study participating centers. TAVI, transcatheter aortic valve implantation. Other abbreviations as in Table 1.
All statistical analysis was conducted by the physicians (T.T. and S.S.) and the statistician (T.M.) using JMP 12.0.1 or SAS 9.4 (both SAS Institute, Cary, NC, USA). All reported P-values are 2-tailed, and P<0.05 was considered statistically significant.
Results
Baseline Clinical and Echocardiographic Characteristics
There were several important differences in the baseline clinical characteristics between the asymptomatic and symptomatic AVR groups (Table 1). Patients in the symptomatic group were older, and more often had CAD, prior MI, atrial fibrillation/flutter, and anemia. Surgical risk scores such as Logistic EuroSCORE, EuroSCORE II, and Society of Thoracic Surgeons (STS) score were significantly higher in the symptomatic AVR group than in the asymptomatic AVR group. Regarding the echocardiographic characteristics, the symptomatic patients compared with the asymptomatic patients more often had combined valvular disease such as mitral regurgitation, tricuspid regurgitation, and aortic regurgitation, and had more depressed LV function as indicated by the lower LV ejection fraction (LVEF), larger LV dimensions, and higher incidence of suspected pulmonary hypertension. The severity of AS assessed using Vmax was not different between the 2 groups (Table 1).
AVR Procedural Characteristics
In the symptomatic AVR group, surgical AVR and TAVI were performed in 876 patients (96.7%), and in 10 patients (1.1%), respectively, with a median interval of 33 days from index echocardiography. In the asymptomatic AVR group, surgical AVR and TAVI were performed in 286 patients (98%), and in 1 patient (0.3%), respectively, with a median interval of 44 days from index echocardiography (Figure 2A).

Regarding the procedural characteristics of surgical AVR, bioprosthetic valves were more frequently used than mechanical valves in both the asymptomatic and symptomatic AVR groups. Relatively small valves were frequently implanted, particularly in the symptomatic AVR group (Table 2). Concomitant CABG was more often performed in the symptomatic AVR group than in the asymptomatic AVR group, while replacement of ascending aorta was more often performed in the asymptomatic AVR group than in the symptomatic AVR group. The prevalence of combined mitral valve and/or tricuspid valve surgery was not different between the 2 groups (Table 2).
AVR Procedural Outcomes
In-hospital mortality after AVR (surgical AVR or TAVI) was numerically, but not statistically significantly, higher in the symptomatic AVR group than in the asymptomatic AVR group (4.2% vs. 2.1%, P=0.10;
Table 3). The duration of intensive care unit stay was significantly longer, and the rate of acute kidney injury as defined by the VARC 2 criteria was significantly higher in the symptomatic AVR group than in the asymptomatic AVR group (Table 3).
Table 3.
Outcomes of Surgical AVR and TAVI (n=1,173)
| |
Asymptomatic AVR group
(n=287) |
Symptomatic AVR group
(n=886) |
P value |
| In-hospital mortality |
6 (2.1) |
37 (4.2) |
0.10 |
| Duration of ICU stay (days) |
3 (2–4) (n=273) |
4 (3–5) (n=822) |
<0.001 |
| Duration of hospital stay after AVR (days) |
19 (15–27) (n=283) |
21 (15–28) (n=864) |
0.08 |
| Atrial fibrillation after AVR |
60/285 (21) |
198/871 (23) |
0.55 |
| Stroke (ischemic or hemorrhagic) |
10/284 (4) |
22/869 (3) |
0.38 |
| Pacemaker implantation after AVR |
2/285 (1) |
19/872 (2) |
0.10 |
| Re-thoracotomy |
14/285 (5) |
51/871 (6) |
0.55 |
| Mediastinitis |
5/285 (2) |
10/871 (1) |
0.43 |
| AKI* after AVR |
13/283 (5) |
87/868 (10) |
0.005 |
| New-onset CLBBB |
2/285 (1) |
1/869 (0.1) |
0.15 |
| New-onset advanced/complete AV block |
4/284 (1) |
18/868 (2) |
0.48 |
Data given as n (%) or median (IQR). AVR included both surgical AVR and TAVI. In the analysis for the outcomes after AVR, we excluded 23 patients who did not undergo AVR, but included 11 patients who underwent TAVI. *Defined using the VARC 2 criteria. AKI, acute kidney injury; AV, atrioventricular; CLBBB, complete left bundle branch block; VARC, Valve Academic Research Consortium. Other abbreviations as in Tables 1,2.
The median follow-up duration of the present survivors was 3.4 years (IQR, 2.4–4.7 years) with 91% follow-up completed at 2 years. The cumulative 5-year incidence of all-cause death was significantly higher in the symptomatic AVR group than in the asymptomatic AVR group on ITT analysis (25.6% vs. 15.4%, P=0.001;
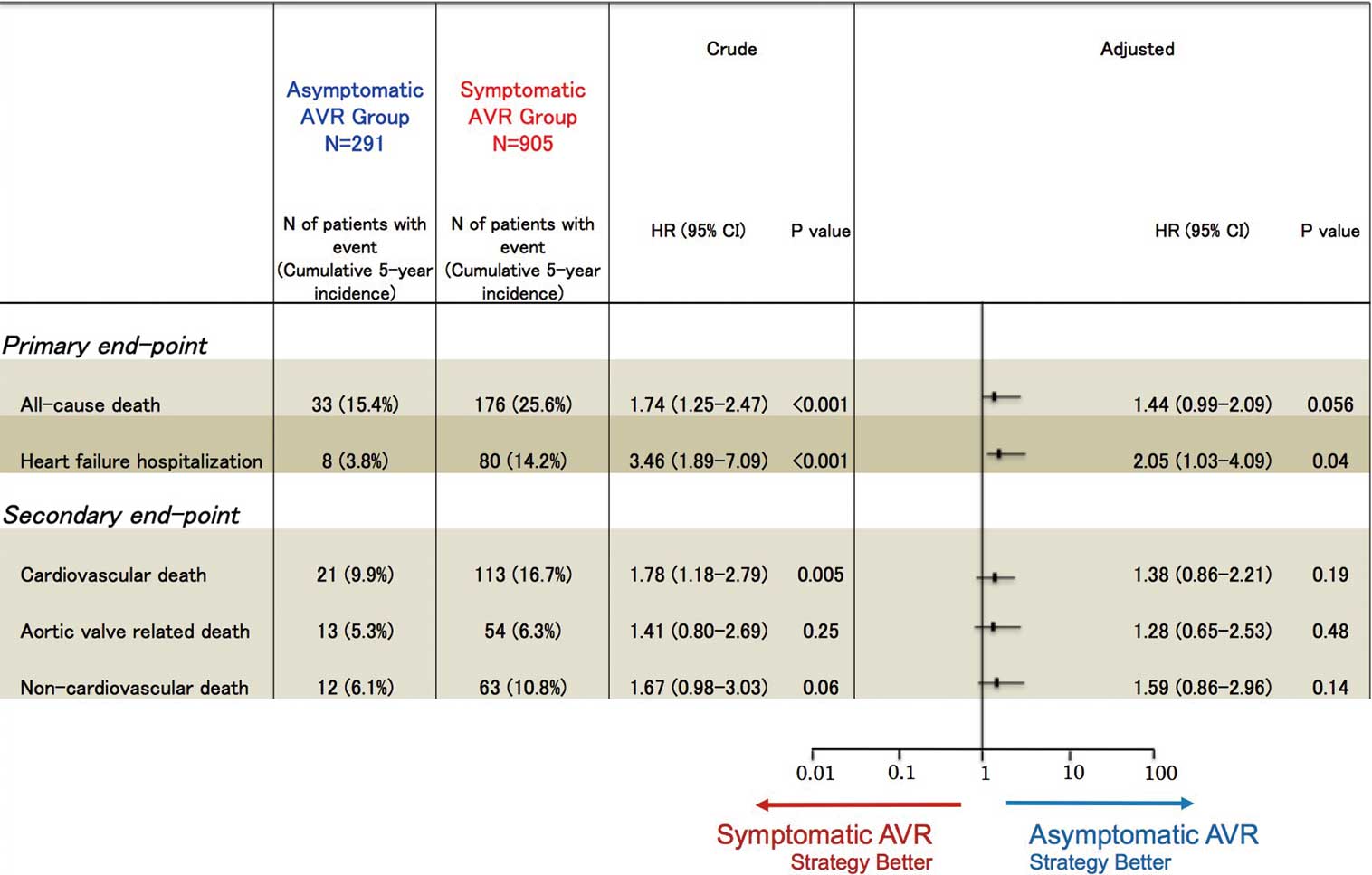
Figure 2B). After adjusting for confounders, the risk for all-cause death trended to be higher in the symptomatic AVR group than in the asymptomatic AVR group (HR, 1.44; 95% CI: 0.99–2.09; P=0.056;
Figure 3;
Table S1). The cumulative 5-year incidence of HF hospitalization was also significantly higher in the symptomatic AVR group than in the asymptomatic AVR group (14.2% vs. 3.8%, P<0.0001;
Figure 2C). After adjusting for confounders, the excess risk of the symptomatic AVR group relative to the asymptomatic AVR group for HF hospitalization remained significant (HR, 2.05; 95% CI: 1.03–4.09; P=0.04;
Figure 3;
Table S1). On sensitivity analysis, all-cause death and HF hospitalization were also compared between the symptomatic and asymptomatic AVR groups without combination surgery or without acute HF admission. On sensitivity analysis, among the patients without combination surgery or acute HF, statistically significant trends favoring asymptomatic AVR relative to symptomatic AVR were seen for both all-cause death and HF hospitalization, in agreement with the results for the whole group (Figures S1,S2).
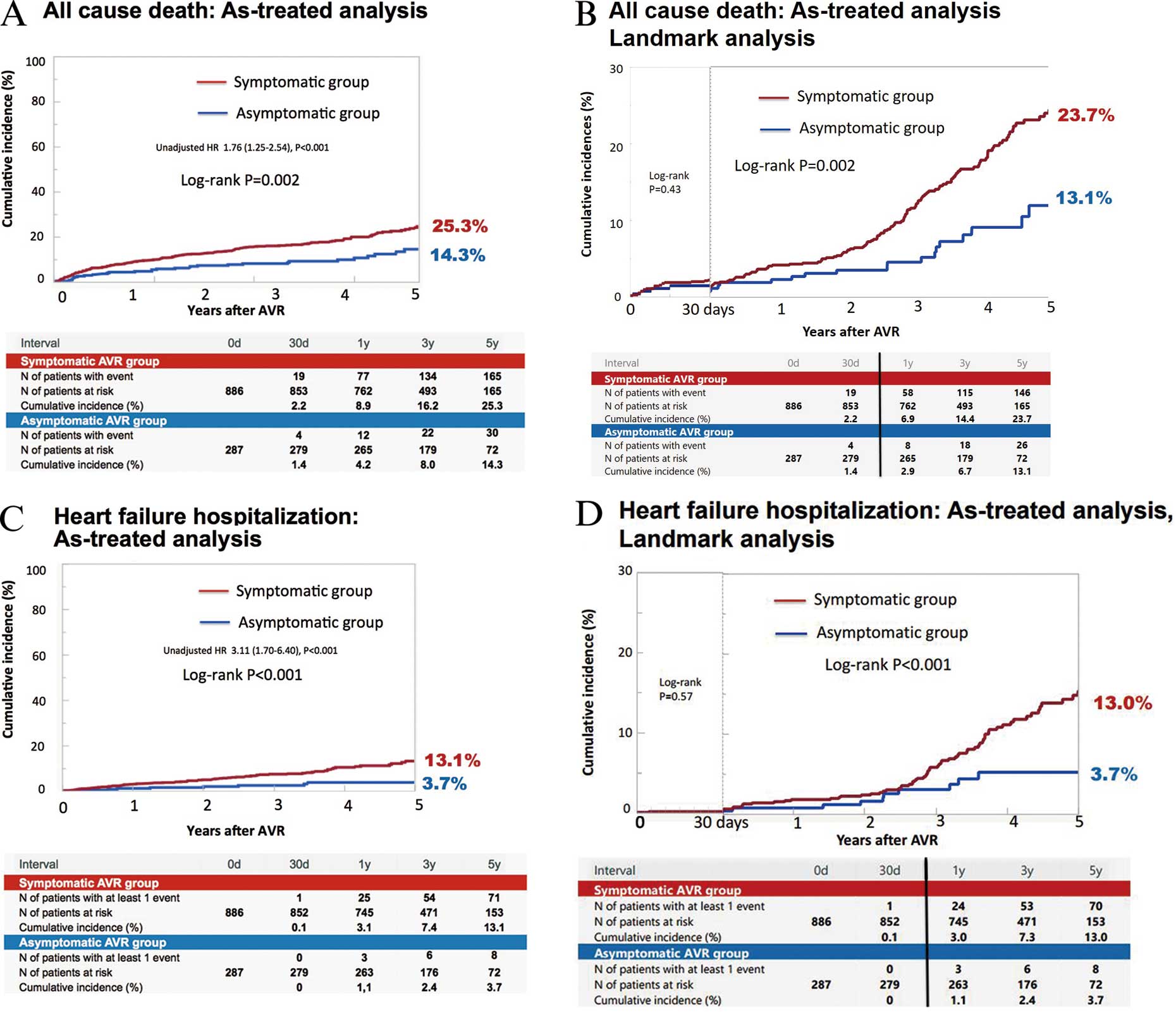
In the asymptomatic group, 9 patients (3.1%) became symptomatic before AVR surgery. The cumulative 5-year incidences of all-cause death and HF hospitalization were significantly higher in the symptomatic AVR group than in the asymptomatic AVR group on as-treated analysis (25.3% vs. 14.3%, P=0.002, and 13.1% vs. 3.7%, P<0.001, respectively;
Figure 4). After adjusting for confounders, the excess risk of the symptomatic AVR group relative to the asymptomatic AVR group remained significant for all-cause death (HR, 1.53; 95% CI: 1.04–2.26, P=0.03), while it was not statistically significant, although borderline, for HF hospitalization (HR, 1.91; 95% CI: 0.95–3.85; P=0.07). On landmark analysis at 30 days, the differences in mortality and HF hospitalization between the 2 groups were mainly observed beyond 30 days, and the Kaplan-Meier curves continued to diverge through 5-year follow-up (Figure 4).
Discussion
The main findings of the present study are as follows: (1) initial AVR strategy in asymptomatic patients with severe AS was associated with better survival and less HF hospitalization compared with the symptomatic patients; and (2) the benefits of AVR in asymptomatic patients compared with symptomatic patients became pronounced at long-term follow-up.
In a few previous small single-center studies, there was discordance regarding the effect of symptom status before AVR on long-term outcomes after AVR.9,10
Among 622 consecutive patients with asymptomatic severe AS seen at the Mayo Clinic, 207 patients underwent AVR after symptom development during follow-up, and 145 patients underwent AVR while they remained asymptomatic.14
The effect of symptom status before AVR on long-term outcome after AVR was evaluated in 265 patients (symptomatic, n=166 patients; asymptomatic, n=97) who were operated on at the Mayo Clinic. The cumulative 10-year survival rate was not different between the 2 groups (symptomatic, 64% vs. asymptomatic, 64%, P=0.92), although 30-day mortality was numerically higher in the symptomatic group than in the asymptomatic group (2% vs. 1%, P=0.43). There seemed, however, to be a bias related to the selection of patients who could be transferred to the Mayo Clinic. In the whole group, the 10-year survival rate tended to be lower in the symptomatic AVR patients than in the asymptomatic AVR patients (62% vs. 70%). More recently, among 812 patients with severe AS aged ≥65 years who underwent AVR at the Cliniques Universitaires St-Luc, the operative mortality was higher and long-term survival was lower in 452 patients with New York Heart Association (NYHA) class 3–4 than in 360 patients with NYHA class 1–2 (10% and 6%, P=0.036, and 56% and 72%, P=0.002, respectively).10
The present study has clearly demonstrated that the initial AVR strategy in asymptomatic patients as compared with that in symptomatic patients was associated with numerically lower hospital mortality and significantly lower long-term mortality and HF hospitalization.
It seems very likely that the morbid preoperative conditions, particularly those associated with acute HF, could have an adverse effect on short-term outcomes after AVR. Furthermore, there are clear pathophysiologic mechanisms underlying the worse long-term clinical outcomes of AVR after symptom development. In severe AS, the increases of afterload and ventricular wall stress stimulate LV hypertrophy, which contributes to the development of symptoms.15
Histopathology of the hypertrophied myocardium has demonstrated apoptosis, and the rate of apoptosis might increase in accordance with increasing afterload.16
In the areas of myocyte apoptosis, the fibroblasts infiltrate into the myocardium and secrete extracellular matrix proteins to establish scar formation after myocyte injury and death.17
A mid-wall pattern of fibrosis on cardiac magnetic resonance imaging using late gadolinium enhancement was observed in up to 38% of patients with moderate or severe AS and has been reported to be associated with increased mortality.16–18
Both surgical and transcatheter AVR are the most effective interventions for eliminating the pressure overload in patients with AS, but even AVR may fail to completely reverse the pathologic changes seen in the myocardium of severely symptomatic AS patients.19
Even mild reduction of LVEF was reported to be a potent predictor of adverse long-term outcomes after AVR.20
The watchful waiting strategy involves waiting for the development of mild symptoms. In the present study, the worse clinical outcomes after AVR in symptomatic patients as compared with asymptomatic patients were seen consistently, even after excluding those patients with acute HF. Therefore, even the presence of relatively mild symptoms was associated with the less favorable outcomes after AVR. Furthermore, watchful waiting for the development of mild symptoms could often not be safely achieved in real clinical practice, because sudden death commonly occurs without preceding symptoms and initial presentation with acute HF is not uncommon during follow-up of asymptomatic severe AS.21
In the previous large study from Mayo clinic, sudden death was observed in 11 (4.1%) of the 270 patients who did not undergo AVR, and all the sudden deaths were not preceded by any AS-related symptoms.14
Also, the in-hospital mortality rate after AVR in asymptomatic patients was only 2.1% in the present study, which was much lower than the in-hospital mortality rates after AVR reported previously.22
Therefore, the present study could provide additional support for the early AVR strategy in asymptomatic severe AS.
We have some study limitations. First, this study was retrospective, and we were unable to exclude the possibility of ascertainment bias for symptoms related to AS at baseline. Second, selection bias for AVR toward less morbid patients with expected low operative mortality might be more prevalent in the asymptomatic than in the symptomatic group. Third, the baseline clinical and surgical procedural characteristics were significantly different between the symptomatic and asymptomatic patients. The worse outcome of the symptomatic patients might be mainly related to the worse baseline characteristics of those patients. We conducted adjusted analyses with the Cox proportional hazard models using the clinical, echocardiographic, and procedural factors as the risk-adjusting variables. The confounding factors might be classified into 2 types: (1) those that emerge while waiting for symptoms; and (2) those that increase the operative risk of AVR, which might delay AVR. We believe it is not appropriate to insert all the factors into the multivariable Cox models in the comparison between AVR in symptomatic and asymptomatic patients. Therefore, we did not include LV function, pulmonary hypertension, and atrial fibrillation as the risk-adjusting variables, because these factors are related to evolution of symptoms. Several other prognostically important factors included as the risk-adjusting variables such as age and concomitant CABG might also be related to waiting for symptoms. Therefore, we should be careful in the interpretation of the adjusted results, considering the complicated relationship between the confounding factors and the timing of AVR. We should wait for the completion of ongoing randomized trials comparing early AVR strategy with the watchful waiting strategy in patients with asymptomatic severe AS, to draw definitive conclusions on the optimal timing of AVR.23
Fourth, we did not collect information on electrocardiographic parameters, blood pressure, frailty, and socioeconomic status, which are often taken into consideration in deciding the indications for AVR in real clinical practice. Fifth, we included those patients with low-gradient AS (AVA <1.0 cm2
alone), in whom the diagnosis of severe AS might be sometimes equivocal, although the proportion of patients with low-gradient AS was relatively small. Finally, we did not evaluate the morbidities associated with AVR and obligatory anticoagulant therapy such as reoperation, and bleeding complications.
Conclusions
When managed with the initial AVR strategy, the long-term outcomes of symptomatic patients with severe AS were worse than those of asymptomatic patients. Early AVR strategy might be recommended in some selected asymptomatic severe AS patients with reasonable operative risk, although we should wait for completion of the ongoing randomized trials comparing early AVR strategy with the watchful waiting strategy.
Acknowledgments
The authors are indebted to Miho Hasegawa and Akimitsu Yukitake for assistance with the manuscript and with the registry database.
Disclosures
The authors declare no conflict of interest.
Grants
The Research Institute for Production Development (Kyoto, Japan).
Supplementary Files
Supplementary File 1
Figure S1.
Kaplan-Meier curves in the subgroup of aortic stenosis patients without combination surgery for (A) all-cause death, and (B) heart failure hospitalization.
Figure S2.
Kaplan-Meier curves in the subgroup of aortic stenosis patients without acute heart failure for (A) all-cause death, and (B) heart failure hospitalization.
Table S1.
Risks for all-cause death and HF hospitalization
Please find supplementary file(s);
http://dx.doi.org/10.1253/circj.CJ-16-0998
References
- 1.
Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP 3rd, Guyton RA, et al. 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 129: e521–e643.
- 2.
Vahanian A, Alfieri O, Andreotti F, Antunes MJ, Baron-Esquivias G, Baumgartner H, et al. Guidelines on the management of valvular heart disease (version 2012). Eur Heart J 2012; 33: 2451–2496.
- 3.
Taniguchi T, Morimoto T, Shiomi H, Ando K, Kanamori N, Murata K, et al. Initial surgical versus conservative strategies in patients with asymptomatic severe aortic stenosis. J Am Coll Cardiol 2015; 66: 2827–2838.
- 4.
Banovic M, Nikolic SD, Putnik S. A randomized trial in patients with asymptomatic severe aortic stenosis: A future has begun! J Am Coll Cardiol 2016; 67: 1970–1971.
- 5.
Castillo-Moreno JA, Garcia-Escribano IA. Might outcome of patients with asymptomatic severe AS be improved by an initial surgical strategy? J Am Coll Cardiol 2016; 67: 1971–1972.
- 6.
Dominguez-Rodriguez A, Abreu-Gonzalez P. Is it time for a new paradigm in asymptomatic severe aortic stenosis? J Am Coll Cardiol 2016; 67: 1968–1969.
- 7.
Marquis-Gravel G, Genereux P. Asymptomatic severe aortic stenosis: Oxymoron? J Am Coll Cardiol 2016; 67: 1969–1970.
- 8.
Taniguchi T, Morimoto T, Sakata R, Kimura T. Reply: Is it time for a new paradigm in asymptomatic severe aortic stenosis? J Am Coll Cardiol 2016; 67: 1972–1973.
- 9.
Brown ML, Pellikka PA, Schaff HV, Scott CG, Mullany CJ, Sundt TM, et al. The benefits of early valve replacement in asymptomatic patients with severe aortic stenosis. J Thorac Cardiovasc Surg 2008; 135: 308–315.
- 10.
Pierard S, de Meester C, Seldrum S, Pasquet A, Gerber B, Vancraeynest D, et al. Impact of preoperative symptoms on postoperative survival in severe aortic stenosis: Implications for the timing of surgery. Ann Thorac Surg 2014; 97: 803–809.
- 11.
Baumgartner H, Hung J, Bermejo J, Chambers JB, Evangelista A, Griffin BP, et al. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. J Am Soc Echocardiogr 2009; 22: 1–23; quiz 101–102.
- 12.
Leon MB, Piazza N, Nikolsky E, Blackstone EH, Cutlip DE, Kappetein AP, et al. Standardized endpoint definitions for transcatheter aortic valve implantation clinical trials: A consensus report from the Valve Academic Research Consortium. J Am Coll Cardiol 2011; 57: 253–269.
- 13.
Kappetein AP, Head SJ, Genereux P, Piazza N, van Mieghem NM, Blackstone EH, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: The Valve Academic Research Consortium-2 consensus document. J Am Coll Cardiol 2012; 60: 1438–1454.
- 14.
Pellikka PA, Sarano ME, Nishimura RA, Malouf JF, Bailey KR, Scott CG, et al. Outcome of 622 adults with asymptomatic, hemodynamically significant aortic stenosis during prolonged follow-up. Circulation 2005; 111: 3290–3295.
- 15.
Dweck MR, Boon NA, Newby DE. Calcific aortic stenosis: A disease of the valve and the myocardium. J Am Coll Cardiol 2012; 60: 1854–1863.
- 16.
Hein S, Arnon E, Kostin S, Schonburg M, Elsasser A, Polyakova V, et al. Progression from compensated hypertrophy to failure in the pressure-overloaded human heart: Structural deterioration and compensatory mechanisms. Circulation 2003; 107: 984–991.
- 17.
Dweck MR, Joshi S, Murigu T, Alpendurada F, Jabbour A, Melina G, et al. Midwall fibrosis is an independent predictor of mortality in patients with aortic stenosis. J Am Coll Cardiol 2011; 58: 1271–1279.
- 18.
Martos R, Baugh J, Ledwidge M, O’Loughlin C, Conlon C, Patle A, et al. Diastolic heart failure: Evidence of increased myocardial collagen turnover linked to diastolic dysfunction. Circulation 2007; 115: 888–895.
- 19.
Weidemann F, Herrmann S, Stork S, Niemann M, Frantz S, Lange V, et al. Impact of myocardial fibrosis in patients with symptomatic severe aortic stenosis. Circulation 2009; 120: 577–584.
- 20.
Dahl JS, Eleid MF, Michelena HI, Scott CG, Suri RM, Schaff HV, et al. Effect of left ventricular ejection fraction on postoperative outcome in patients with severe aortic stenosis undergoing aortic valve replacement. Circ Cardiovasc Imaging 2015; 8: e002917.
- 21.
Genereux P, Stone GW, O’Gara PT, Marquis-Gravel G, Redfors B, Giustino G, et al. Natural history, diagnostic approaches, and therapeutic strategies for patients with asymptomatic severe aortic stenosis. J Am Coll Cardiol 2016; 67: 2263–2288.
- 22.
Masuda M, Kuwano H, Okumura M, Amano J, Arai H, Endo S, et al. Thoracic and cardiovascular surgery in Japan during 2012: Annual report by The Japanese Association for Thoracic Surgery. Gen Thorac Cardiovasc Surg 2014; 62: 734–764.
- 23.
Banovic M, Iung B, Bartunek J, Asanin M, Beleslin B, Biocina B, et al. Rationale and design of the Aortic Valve replAcemenT versus conservative treatment in Asymptomatic seveRe aortic stenosis (AVATAR trial): A randomized multicenter controlled event-driven trial. Am Heart J 2016; 174: 147–153.