Abstract
Background:
Acute kidney injury (AKI) is associated with poor outcome after acute myocardial infarction (AMI), but whether hemodynamic status at presentation influences this prognostic significance is unknown.
Methods and Results:
A total of 2,798 AMI patients admitted within 48 h after symptom onset and who underwent urgent coronary angiography were enrolled in the present study. AKI was defined as an increase in serum creatinine ≥0.3 mg/dL or ≥50% within 48 h during hospitalization. Patients were classified into 3 groups according to Killip class on admission: Killip 1, n=2,164; Killip 2–3, n=366; and Killip 4, n=268. AKI occurred more frequently with increasing Killip class (Killip 1, 2–3, and 4: 6.3%, 15.3%, and 31.3%, respectively; P<0.001). AKI was associated with increased in-hospital mortality, regardless of Killip class (non-AKI and AKI patients: 1.1% vs. 6.6% in Killip 1; 5.2% vs. 35.7% in Killip 2–3, and 28.8% vs. 45.2% in Killip 4, P<0.01 for all). On multivariate analysis, the adjusted OR of AKI for in-hospital mortality in Killip 1, Killip 2–3, and Killip 4 were 3.79 (95% CI: 1.54–9.33, P=0.004), 5.35 (95% CI: 2.67–10.7, P<0.001), and 1.48 (95% CI: 0.94–2.35, P=0.093), respectively.
Conclusions:
In AMI patients undergoing urgent coronary angiography, AKI was significantly associated with increased in-hospital mortality in Killip 1 as well as Killip 2–3 at presentation, but not in Killip 4.
During the past decades, mortality after acute myocardial infarction (AMI) has dramatically decreased owing to the development and widespread use of primary percutaneous coronary intervention (PCI), including stent implantation.1,2
Further decreases in mortality, however, have not been achieved.3
The identification of high-risk patients likely to benefit from more aggressive strategies is needed.
A growing number of studies have shown that acute kidney injury (AKI) is strongly related to increased morbidity and mortality after AMI.4–8
Although possible mechanisms for worsening of renal function during hospitalization for AMI are multifactorial and complex,9,10
hemodynamic instability such as cardiogenic shock has been shown to be strongly related to the occurrence of AKI.6,11
AKI might reflect renal impairment secondary to hypoperfusion associated with hemodynamic instability. The poor outcomes of AKI patients may be explained at least in part by the confounding effects of hemodynamic instability. Whether AKI can be used as a prognostic factor to identify high-risk AMI patients who present with cardiogenic shock is unknown. On the other hand, in the current interventional era, mortality in AMI patients who present with Killip class 1 without hemodynamic instability is very low, as compared with those who present with Killip class 2 or higher.12–14
Whether AKI negatively affects clinical outcome even in AMI patients with Killip class 1 has yet to be investigated. The aim of this study was therefore to elucidate the relationship between AKI and hemodynamic status, as estimated by Killip class at presentation, in a recent large prospective multicenter study of AMI patients, and to assess the effects of this relationship on in-hospital outcome.
Methods
The present patients were selected from the Japanese registry of acute Myocardial INfarction diagnosed by Universal dEfiniTion (J-MINUET), a prospective observational multicenter study. A total of 3,283 consecutive patients admitted within 48 h after onset of AMI to 28 Japanese medical institutions were enrolled between July 2012 and March 2014. The design, inclusion criteria, and results of the J-MINUET study have been previously reported.15
Briefly, AMI was diagnosed using the new universal definition, in which cardiac troponin is the preferred biomarker of myocardial injury.16
ST-segment elevation AMI was diagnosed in the presence of new ST elevation at the J point in at least 2 contiguous leads ≥0.2 mV in men or ≥0.15 mV in women in leads V2–3, or ≥0.1 mV in another 2 contiguous leads.17,18
This study was conducted in accordance with the Declaration of Helsinki. The protocol was approved by the ethics committee of each participating institution.
In the J-MINUET study, urgent coronary angiography was performed in 3,053 patients within 48 h after hospital admission. Among these patients, 255 were excluded from the study for one or more of the following reasons: hemodialysis (n=199), missing data related to Killip class on admission (n=18), or missing or unavailable serum creatinine (SCr; n=48) during hospitalization. Therefore, the final study group consisted of 2,798 patients.
Estimated glomerular filtration rate (eGFR) was calculated from creatinine concentration using the modified glomerular filtration rate estimating equation for Japanese patients with chronic kidney disease (CKD): eGFR (mL/min/1.73 m2)=194×SCr−1.094×age−0.287
(×0.739 if female).19
CKD was defined as eGFR on admission <60 mL/min/1.73 m2.20
AKI was defined as an increase in SCr by ≥0.3 mg/dL or ≥50% within 48 h during hospitalization.21
Anemia was defined using the World Health Organization diagnostic criteria (hemoglobin <13.0 g/dL in men, <12.0 g/dL in women).22
Clinical events were collected at the time of discharge. The primary endpoint was in-hospital mortality. The secondary endpoint was major adverse cardiac events (MACE), defined as a composite of all-cause death, cardiac failure, ventricular tachycardia or ventricular fibrillation (or both), and bleeding during hospitalization. Cardiac failure was defined as congestive heart failure or cardiogenic shock (or both) that required treatment.
Statistical Analysis
Continuous variables are presented as median (IQR). Differences between continuous variables were analyzed using Mann-Whitney U-test. Categorical data are expressed as percentages and were compared using the chi-squared test or Fisher’s exact test. Differences were considered statistically significant at P<0.05. Univariate and multivariate analyses were performed to identify predictors of in-hospital mortality and MACE. Stepwise selection for multivariable logistic regression with P≤0.1 for backward elimination was used to select the best predictive model. Odds ratios (OR) and 95% CI were calculated. Missing values were imputed with the use of a multivariate normal model and the chained equations approach.23
The multiple imputation method has been described previously.24
Statistical analysis was performed with SPSS version 19.0 (SPSS, Chicago, IL, USA) and STATA, version 12 (StataCorp LP, College Station, TX, USA).
Results
Patient Characteristics
AKI occurred in 276 patients (9.9%). Clinical patient characteristics according to presence of AKI are listed in
Table 1. Patients with AKI were older, more likely to have a previous history of hypertension and diabetes mellitus, presented with a lower systolic blood pressure and a more rapid heart rate, and more frequently had anemia and CKD than those without AKI.
Figure 1
shows the incidence of AKI development according to the Killip class on admission. The proportion of patients with AKI increased in parallel to an increase in Killip class. Patients with AKI more frequently had multivessel disease, less frequently underwent primary PCI, and more frequently underwent intra-aortic balloon pumping. Infarct size as estimated using maximum creatine kinase was larger in patients with AKI.
Table 2
lists in-hospital outcome according to presence of AKI. The rates of both in-hospital mortality and MACE were higher in the AKI patients.
Table 1.
Baseline Characteristics
| |
Non-AKI
(n=2,522) |
AKI
(n=276) |
P value |
| Age (years) |
68 (59–77) |
72 (64–81) |
<0.001 |
| Male |
1,922 (76.2) |
215 (77.9) |
0.531 |
| Hypertension |
1,639 (65.4) |
197 (71.6) |
0.039 |
| Diabetes |
687 (27.6) |
114 (42.5) |
<0.001 |
| Previous MI |
272 (10.8) |
36 (13.1) |
0.250 |
| STEMI |
1,808 (71.7) |
200 (72.5) |
0.786 |
| SBP (mmHg) |
138 (118–160) |
134 (107–158) |
0.005 |
| HR (beats/min) |
76 (64–89) |
86 (70–104) |
<0.001 |
| Killip class |
|
|
<0.001 |
| Class 1 |
2,028 (80.4) |
136 (49.3) |
|
| Class 2–3 |
310 (12.3) |
56 (20.3) |
|
| Class 4 |
184 (7.3) |
84 (30.4) |
|
| Anemia |
608 (24.1) |
138 (50.0) |
<0.001 |
| SCr (mg/dL) |
0.82 (0.68–1.02) |
1.40 (1.01–2.22) |
<0.001 |
| CKD |
895 (35.5) |
222 (80.4) |
<0.001 |
| Multivessel disease |
1,043 (41.5) |
171 (62.0) |
<0.001 |
| Primary PCI |
2,323 (92.1) |
242 (87.7) |
0.011 |
| IABP |
382 (15.2) |
120 (44.1) |
<0.001 |
| Max CK (IU/L) |
1,487 (552–3,162) |
2,384 (1,011–5,176) |
<0.001 |
Data given as median (IQR) or n (%). AKI, acute kidney injury; CK, creatinine kinase; CKD, chronic kidney disease; HR, heart rate; IABP, intra-aortic balloon pumping; MI, myocardial infarction; PCI, percutaneous coronary intervention; SBP, systolic blood pressure; SCr, serum creatine; STEMI, ST-segment elevation myocardial infarction.
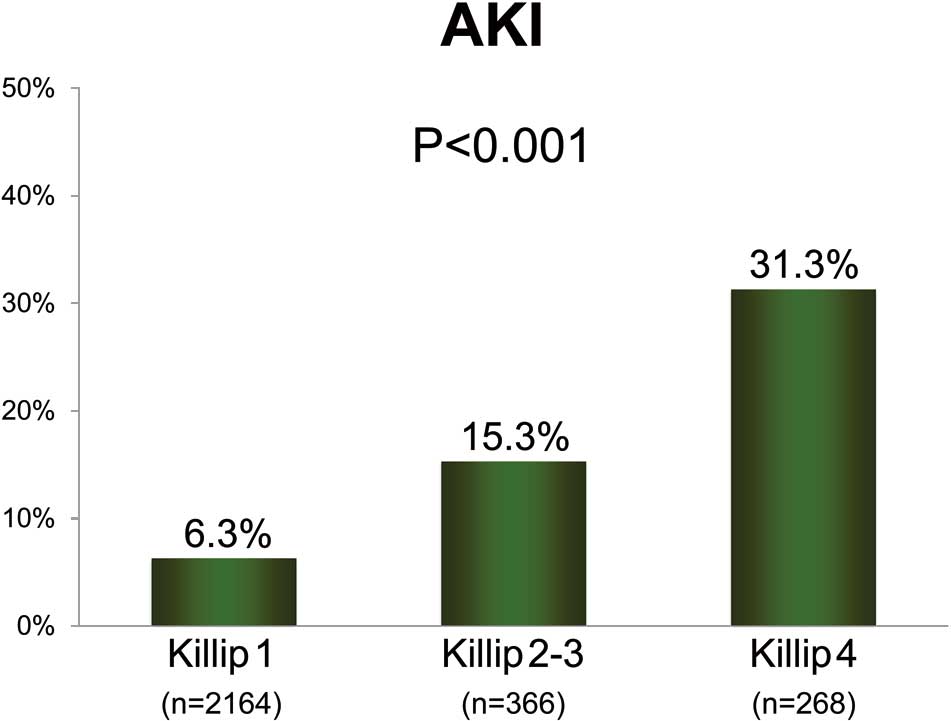
Table 2.
In-Hospital Outcomes
| |
Non-AKI
(n=2,522) |
AKI
(n=276) |
P value |
| Mortality |
91 (3.6) |
67 (24.3) |
<0.001 |
| MACE |
355 (14.1) |
137 (49.6) |
<0.001 |
| Cardiac failure |
273 (11.0) |
118 (43.5) |
<0.001 |
| VT/VF |
77 (3.1) |
29 (10.7) |
<0.001 |
| Bleeding |
45 (1.8) |
23 (8.5) |
<0.001 |
Data given as n (%). AKI, acute kidney injury; MACE, major adverse cardiac events; VF, ventricular fibrillation; VT, ventricular tachycardia.
Tables 3,4
list the univariate and multivariate predictors of in-hospital mortality and MACE. Killip class 2 or higher and AKI were significant independent predictors of both in-hospital mortality and MACE (P-values of the interaction between Killip class and AKI: P<0.001 and P<0.001, respectively).
Table 3.
Independent Predictors of In-Hospital Mortality
| |
Univariate |
Multivariate |
| OR (95% CI) |
P value |
OR (95% CI) |
P value |
| Age (per 1 year) |
1.04 (1.03–1.06) |
<0.001 |
1.04 (1.02–1.05) |
<0.001 |
| Male |
1.39 (0.98–1.96) |
0.065 |
|
|
| Hypertension |
0.78 (0.55–1.10) |
0.149 |
|
|
| Diabetes |
1.20 (0.84–1.72) |
0.307 |
|
|
| Previous MI |
1.17 (0.70–1.97) |
0.552 |
|
|
| STEMI |
1.90 (1.27–2.85) |
0.002 |
|
|
| SBP (per 1 mmHg) |
0.97 (0.96–0.98) |
<0.001 |
|
|
| HR (per 1 beat/min) |
1.02 (1.01–1.03) |
<0.001 |
|
|
| Killip class |
| Class 1 |
Ref. |
|
Ref. |
|
| Class 2–3 |
7.89 (4.75–13.1) |
<0.001 |
5.00 (2.97–8.42) |
<0.001 |
| Class 4 |
46.4 (29.8–72.3) |
<0.001 |
19.0 (11.5–31.4) |
<0.001 |
| Anemia |
1.77 (1.27–2.47) |
0.001 |
|
|
| CKD |
7.75 (5.03–11.9) |
<0.001 |
|
|
| Multivessel disease |
2.10 (1.50–2.94) |
<0.001 |
|
|
| Primary PCI |
2.30 (1.01–5.21) |
0.046 |
|
|
| IABP |
5.53 (3.98–7.68) |
<0.001 |
1.40 (0.98–2.00) |
0.065 |
| Log Max CK |
2.46 (2.05–2.95) |
<0.001 |
1.55 (1.29–1.86) |
<0.001 |
| AKI |
7.76 (5.58–10.8) |
<0.001 |
2.36 (1.66–3.37) |
<0.001 |
Abbreviations as in Table 1.
Table 4.
Independent Predictors of In-Hospital MACE
| |
Univariate |
Multivariate |
| OR (95% CI) |
P value |
OR (95% CI) |
P value |
| Age (per 1 year) |
1.02 (1.01–1.02) |
<0.001 |
|
|
| Male |
0.97 (0.78–1.19) |
0.740 |
|
|
| Hypertension |
1.03 (0.85–1.26) |
0.761 |
|
|
| Diabetes |
1.21 (0.99–1.47) |
0.060 |
|
|
| Previous MI |
1.35 (1.03–1.78) |
0.030 |
|
|
| STEMI |
1.87 (1.50–2.34) |
<0.001 |
1.10 (0.87–1.40) |
0.427 |
| SBP (per 1 mmHg) |
0.98 (0.98–0.98) |
<0.001 |
|
|
| HR (per 1 beat/min) |
1.02 (1.01–1.02) |
<0.001 |
|
|
| Killip class |
| Class 1 |
Ref. |
|
Ref. |
|
| Class 2–3 |
4.44 (3.50–5.62) |
<0.001 |
2.96 (2.31–3.81) |
<0.001 |
| Class 4 |
16.6 (13.4–20.5) |
<0.001 |
6.99 (5.37–9.11) |
<0.001 |
| Anemia |
1.52 (1.26–1.83) |
<0.001 |
|
|
| CKD |
3.56 (2.93–4.32) |
<0.001 |
1.76 (1.41–2.19) |
<0.001 |
| Multivessel disease |
1.92 (1.59–2.31) |
<0.001 |
|
|
| Primary PCI |
1.00 (0.73–1.37) |
0.992 |
|
|
| IABP |
4.63 (3.86–5.56) |
<0.001 |
1.68 (1.37–2.05) |
<0.001 |
| Log Max CK |
1.80 (1.65–1.98) |
<0.001 |
1.27 (1.16–1.40) |
<0.001 |
| AKI |
4.02 (3.28–4.92) |
<0.001 |
1.43 (1.15–1.78) |
0.001 |
Abbreviations as in Tables 1,2.
Figure 2A
shows the in-hospital mortality in the presence or absence of AKI stratified by Killip class on admission. In patients with Killip class 1 and Killip class 2–3, in-hospital mortality in the presence of AKI was 6-fold and 7-fold higher than in the absence of AKI, respectively, while there was a smaller relative increase in in-hospital mortality in AKI patients for Killip class 4.
Figure 2B
shows the rates of MACE in the presence or absence of AKI stratified by Killip class on admission. In patients with Killip class 1 or Killip class 2–3, the rates of MACE were significantly higher in the AKI patients than in those without AKI. In patients with Killip class 4, however, the rate of MACE in the AKI patients tended to be higher than in patients without AKI, but the difference did not reach statistical significance.
Table 5
lists the multivariate adjusted OR of AKI for in-hospital mortality and MACE according to Killip class on admission. AKI was a significant predictor of both in-hospital mortality and MACE in Killip class 1 as well as in Killip class 2–3, but not in Killip class 4.

Table 5.
Multivariate Adjusted OR of AKI for In-Hospital Outcome According to Killip Class on Admission
| |
OR (95% CI) |
P value |
| Mortality |
| Killip class 1 |
3.79 (1.54–9.33) |
0.004 |
| Killip class 2–3 |
5.35 (2.67–10.7) |
<0.001 |
| Killip class 4 |
1.48 (0.94–2.35) |
0.093 |
| MACE |
| Killip class 1 |
1.93 (1.25–3.00) |
0.003 |
| Killip class 2–3 |
1.64 (1.08–2.49) |
0.021 |
| Killip class 4 |
1.10 (0.80–1.50) |
0.569 |
Abbreviations as in Table 2.
Discussion
In this substudy of the J-MINUET study, evaluating 2,798 AMI patients who underwent urgent coronary angiography, the prevalence of AKI was 9.9%, similar to that in previous studies of AMI patients.6,25
The present analysis supports the association of AKI with poor short-term cardiovascular outcome, including mortality, as reported previously.6–8,25
In the present study, the prevalence of AKI increased in a stepwise fashion with increasing Killip class on admission, and there was a close link between the development of AKI and poor in-hospital outcome regardless of Killip class. Of note, on multivariate analysis AKI maintained its negative prognostic impact on in-hospital clinical outcomes in patients who presented with Killip class 1, as well as Killip class 2–3, but not in those with Killip class 4. To the best of our knowledge, this is the first report to show the differential impact of AKI on in-hospital clinical outcome after AMI by Killip class at presentation in the current troponin era. The present findings highlight the importance of the interaction between AKI and Killip class on admission in early risk stratification of AMI patients.
In the current era of primary PCI for AMI, AMI patients who are in Killip class 1 at presentation are generally recognized to have excellent outcomes.12
In the present study, primary PCI was performed in 2,565 patients (91.7%), and the in-hospital mortality in Killip class 1 was very low (1.4%). AKI had a significant adverse impact on in-hospital outcome, including mortality, even in low-risk patients with Killip class 1. This suggests that confounding by hemodynamic instability, which has been shown to be strongly related to the development of AKI after AMI,6,11,26
does not completely explain the poor outcomes of AMI patients in whom AKI develops. The interpretation of higher rates of in-hospital mortality and MACE after the development of AKI, however, is challenging because of pre-existing clinical variables related to both AKI development and adverse outcomes. Consistent with previous studies,4–6,8
patients with AKI were older and had higher rates of hypertension, diabetes mellitus, anemia, CKD, and more severe coronary artery disease, as indicated by the higher prevalence of multivessel disease. These high-risk profiles more likely explain the poor outcomes in patients with AKI. The contribution of AKI to poor outcome, however, continued to be significant even after adjustment for baseline characteristics. In addition to contrast-induced nephropathy,4,5
links between renal dysfunction and oxidative stress, inflammation, altered cytokine levels, platelet activation, and hypercoagulable or thromboresistant state have been confirmed, and these factors are more likely to increase the risk of adverse events after AMI.9,10,27
Regardless of causality, the present findings highlight the importance of AKI as a predictor of adverse outcomes in patients with Killip class 1 on presentation, as well as those with Killip class 2–3. Thus, close monitoring of SCr during hospitalization is important for identifying high-risk patients, and the targeting of patients with AKI for more careful and aggressive treatment may potentially improve short-term survival after AMI in patients with Killip class 1–3 on presentation.
Another important finding was that AKI was not a significant independent predictor of in-hospital adverse outcome, including mortality, in patients with Killip class 4 at presentation. Mortality in AMI patients with cardiogenic shock remains very high, even in the current interventional era for AMI.28
In the present study, in-hospital mortality in the Killip class 4 group reached 34.0%, and AKI commonly developed in this subgroup of patients (prevalence, 31.3%) and was closely linked to adverse outcomes; nonetheless, the significant adverse effects of AKI on in-hospital outcome were not evident after multivariate adjustment. Although the reason why AKI did not predict adverse outcome in the Killip class 4 group remains unclear, several factors may account for the results. First, baseline SCr may already reflect impairment associated with acute hemodynamic instability in this particular clinical setting, and we may have thus potentially underestimated the true incidence of AKI development. Second, because the number of patients who presented with Killip 4 was relatively small, the subgroup analysis might have been underpowered; J-MINUET, however, was a prospective multicenter study in which a relatively large number of consecutive patients were enrolled. Therefore, the present findings can be generalized to AMI patients in a real-world clinical setting. Further prospective studies in a larger number of patients, however, are needed to verify the present results.
The present study had several important limitations. First, multivariate analysis was performed, but there were many differences in baseline clinical characteristics between the patients with and without AKI. We therefore we cannot rule out the possibility of unassessed confounding factors. Second, AKI was defined solely on the basis of an increase in SCr within 48 h during hospitalization. Because SCr often changes during the acute phase in AMI patients,7
we might not have estimated the true incidence of AKI development. The present definition of AKI is widely used and was strongly associated with clinical outcome in the present study, as well as in previous studies,6,21,25
but it might be, at least in part, arbitrary. Third, we did not have data on the course of AKI (whether AKI resolved or persisted) and the need for hemodialysis or renal replacement therapy during or after the index hospitalization, which have significant prognostic implications.8
Fourth, the sample size for stratified analysis in Killip class 4 might have been too small to show an association between AKI and in-hospital mortality, although there was a marginally significant association on multivariate analysis. Finally, the volume of contrast agent used during coronary angiography and PCI was not available in this study. Higher contrast volume has been reported to be associated with higher rates of contrast-induced AKI and in-hospital death in AMI patients undergoing primary PCI.29
Minimizing contrast volume may help to prevent AKI and improve clinical outcome in AMI patients. Because of the limited ability to study this issue, further studies are required to determine the risk factors for AKI, and whether the prevention of AKI can help to improve clinical outcome after AMI.
Conclusions
In AMI patients, the development of AKI during hospitalization had a significant adverse impact on in-hospital clinical outcome in patients in Killip class 1, as well in Killip class 2–3 on admission, but not in Killip class 4.
Acknowledgments
This study was supported by the Intramural Research Fund, grant number 23-4-5, for Cardiovascular Diseases of the National Cerebral and Cardiovascular Center. The authors thank all the enrolled patients, participating cardiologists, medical and other staff who have contributed to this study. The J-MINUET investigators are listed in
Appendix.
Disclosures / Conflict of Interests
None.
Grants
This study was supported by the Intramural Research Fund, grant number 23-4-5, for Cardiovascular Diseases of the National Cerebral and Cardiovascular Center.
Appendix. J-MINUET Investigators
Masaharu Ishihara, Hyogo College of Medicine (principal investigator); Nobuaki Kokubu, Sapporo Medical University; Tadaya Sato, Akita Medical Center; Teruo Inoue, Dokkyo Medical University; Shigeru Oshima, Gunma Prefectural Cardiovascular Center; Hiroshi Funayama, Saitama Medical Center Jichi Medical University; Ken Kozuma, Hiroyuki Kyono, Teikyo University; Wataru Shimizu, Nippon Medical School; Satoru Suwa, Juntendo University Shizuoka Hospital; Kengo Tanabe, Mitsui Memorial Hospital; Tetsuya Tobaru, Sakakibara Heart Institute; Kazuo Kimura, Yokohama City University Medical Center; Junya Ako, Kitasato University; Mafumi Owa, Suwa Red Cross Hospital; Yasuhiro Morita, Ogaki Municipal Hospital; Yukio Ozaki, Fujita Health University; Satoshi Yasuda, Teruo Noguchi, Masashi Fujino, Yoshihiro Miyamoto, Kunihiro Nishimura, National Cerebral and Cardiovascular Center; Junichi Kotani, Osaka University Graduate School of Medicine; Takashi Morita, Osaka General Medical Center; Atsunori Okamura, Sakurabashi Watanabe Hospital; Yoshihiko Saito, Nara Medical University; Masaaki Uematsu, Kansai Rosai Hospital; Hiroyuki Okura, Kawasaki Medical School; Atsushi Hirohata, Sakakibara Heart Institute of Okayama; Yasuharu Nakama, Hiroshima City Hospital; Keijiro Saku, Fukuoka University School of Medicine; Seiji Hokimoto, Kumamoto University Graduate School of Medical Sciences; Koichi Nakao, Saiseikai Kumamoto Hospital Cardiovascular Center; Kazuteru Fujimoto, National Hospital Organization Kumamoto Medical Center; Yoshisato Shibata, Miyazaki Medical Association Hospital; Kazuhito Hirata, Okinawa Prefectural Chubu Hospital.
References
- 1.
Rogers WJ, Frederick PD, Stoehr E, Canto JG, Ornato JP, Gibson CM, et al. Trends in presenting characteristics and hospital mortality among patients with ST elevation and non-ST elevation myocardial infarction in the National Registry of Myocardial Infarction from 1990 to 2006. Am Heart J 2008; 156: 1026–1034.
- 2.
Jernberg T, Johanson P, Held C, Svennblad B, Lindback J, Wallentin L. Association between adoption of evidence-based treatment and survival for patients with ST-elevation myocardial infarction. JAMA 2011; 305: 1677–1684.
- 3.
Roe MT, Messenger JC, Weintraub WS, Cannon CP, Fonarow GC, Dai D, et al. Treatments, trends, and outcomes of acute myocardial infarction and percutaneous coronary intervention. J Am Coll Cardiol 2010; 56: 254–263.
- 4.
Giacoppo D, Madhavan MV, Baber U, Warren J, Bansilal S, Witzenbichler B, et al. Impact of contrast-induced acute kidney injury after percutaneous coronary intervention on short- and long-term outcomes: Pooled analysis from the HORIZONS-AMI and ACUITY trials. Circ Cardiovasc Interv 2015; 8: e002475.
- 5.
Narula A, Mehran R, Weisz G, Dangas GD, Yu J, Genereux P, et al. Contrast-induced acute kidney injury after primary percutaneous coronary intervention: Results from the HORIZONS-AMI substudy. Eur Heart J 2014; 35: 1533–1540.
- 6.
Fox CS, Muntner P, Chen AY, Alexander KP, Roe MT, Wiviott SD. Short-term outcomes of acute myocardial infarction in patients with acute kidney injury: A report from the national cardiovascular data registry. Circulation 2012; 125: 497–504.
- 7.
Marenzi G, Lauri G, Assanelli E, Campodonico J, De Metrio M, Marana I, et al. Contrast-induced nephropathy in patients undergoing primary angioplasty for acute myocardial infarction. J Am Coll Cardiol 2004; 44: 1780–1785.
- 8.
Wi J, Ko YG, Kim JS, Kim BK, Choi D, Ha JW, et al. Impact of contrast-induced acute kidney injury with transient or persistent renal dysfunction on long-term outcomes of patients with acute myocardial infarction undergoing percutaneous coronary intervention. Heart 2011; 97: 1753–1757.
- 9.
Ronco C, Haapio M, House AA, Anavekar N, Bellomo R. Cardiorenal syndrome. J Am Coll Cardiol 2008; 52: 1527–1539.
- 10.
Marenzi G, Cosentino N, Bartorelli AL. Acute kidney injury in patients with acute coronary syndromes. Heart 2015; 101: 1778–1785.
- 11.
Queiroz RE, de Oliveira LS, de Albuquerque CA, Santana Cde A, Brasil PM, Carneiro LL, et al. Acute kidney injury risk in patients with ST-segment elevation myocardial infarction at presentation to the ED. Am J Emerg Med 2012; 30: 1921–1927.
- 12.
Desta L, Jernberg T, Lofman I, Hofman-Bang C, Hagerman I, Spaak J, et al. Incidence, temporal trends, and prognostic impact of heart failure complicating acute myocardial infarction. The SWEDEHEART Registry (Swedish Web-System for Enhancement and Development of Evidence-Based Care in Heart Disease Evaluated According to Recommended Therapies): A study of 199,851 patients admitted with index acute myocardial infarctions, 1996 to 2008. JACC Heart Fail 2015; 3: 234–242.
- 13.
de Carvalho LP, Gao F, Chen Q, Sim LL, Koh TH, Foo D, et al. Long-term prognosis and risk heterogeneity of heart failure complicating acute myocardial infarction. Am J Cardiol 2015; 115: 872–878.
- 14.
Steg PG, Dabbous OH, Feldman LJ, Cohen-Solal A, Aumont MC, Lopez-Sendon J, et al. Determinants and prognostic impact of heart failure complicating acute coronary syndromes: Observations from the Global Registry of Acute Coronary Events (GRACE). Circulation 2004; 109: 494–499.
- 15.
Ishihara M, Fujino M, Ogawa H, Yasuda S, Noguchi T, Nakao K, et al. Clinical presentation, management and outcome of Japanese patients with acute myocardial infarction in the troponin era: Japanese Registry of Acute Myocardial Infarction Diagnosed by Universal Definition (J-MINUET). Circ J 2015; 79: 1255–1262.
- 16.
Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, et al. Third universal definition of myocardial infarction. J Am Coll Cardiol 2012; 60: 1581–1598.
- 17.
Alpert JS, Thygesen K, Antman E, Bassand JP. Myocardial infarction redefined: A consensus document of the Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. J Am Coll Cardiol 2000; 36: 959–969.
- 18.
O’Gara PT, Kushner FG, Ascheim DD, Casey DE Jr, Chung MK, de Lemos JA, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; 61: e78–e140.
- 19.
Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 2009; 53: 982–992.
- 20.
K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis 2002; 39: S1–S266.
- 21.
Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, et al. Acute Kidney Injury Network: Report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007; 11: R31.
- 22.
Nutritional anaemias. Report of a WHO scientific group. World Health Organ Tech Rep Ser 1968; 405: 5–37.
- 23.
Little RJA, Rubin DB. Statistical analysis with missing data, 2nd edn. New Jersey: Wiley-InterScience, 2002.
- 24.
Rubin DB. Inference and missing data. Biometrika 1976; 63: 581–592.
- 25.
Shacham Y, Leshem-Rubinow E, Steinvil A, Assa EB, Keren G, Roth A, et al. Renal impairment according to acute kidney injury network criteria among ST elevation myocardial infarction patients undergoing primary percutaneous intervention: A retrospective observational study. Clin Res Cardiol 2014; 103: 525–532.
- 26.
Vavalle JP, van Diepen S, Clare RM, Hochman JS, Weaver WD, Mehta RH, et al. Renal failure in patients with ST-segment elevation acute myocardial infarction treated with primary percutaneous coronary intervention: Predictors, clinical and angiographic features, and outcomes. Am Heart J 2016; 173: 57–66.
- 27.
Rodrigues FB, Bruetto RG, Torres US, Otaviano AP, Zanetta DM, Burdmann EA. Effect of kidney disease on acute coronary syndrome. Clin J Am Soc Nephrol 2010; 5: 1530–1536.
- 28.
Wayangankar SA, Bangalore S, McCoy LA, Jneid H, Latif F, Karrowni W, et al. Temporal trends and outcomes of patients undergoing percutaneous coronary interventions for cardiogenic shock in the setting of acute myocardial infarction: A report from the CathPCI Registry. JACC Cardiovasc Interv 2016; 9: 341–351.
- 29.
Marenzi G, Assanelli E, Campodonico J, Lauri G, Marana I, De Metrio M, et al. Contrast volume during primary percutaneous coronary intervention and subsequent contrast-induced nephropathy and mortality. Ann Intern Med 2009; 150: 170–177.



