2017 Volume 81 Issue 5 Pages 635-636
2017 Volume 81 Issue 5 Pages 635-636
Activation of the renin-angiotensin-aldosterone system (RAAS) and natriuretic peptides (NPs) plays a central role in the pathophysiology of heart failure (HF), namely cardiovascular remodeling and disease progression. These neurohumoral systems initially exert a counter-regulatory action to maintain peripheral perfusion through vasoconstriction and fluid retention. However, as HF progresses, the sustained activation of the RAAS, coupled with a diminished response of NPs, leads to an increase in angiotensin II and aldosterone levels, which facilitates sodium reabsorption and provokes hypertrophy and fibrosis in the heart and vasculature. Consequently, this promotes a vicious cycle in the cardiovascular system (i.e., cardiorenal syndrome), leading to poor prognosis in patients with HF. Indeed, blockage of the RAAS is now a fundamental therapeutic strategy for HF, and the significant reduction in HF mortality over the past 20 years has been accomplished by RAAS modulation with angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin II receptor blockers (ARBs), and aldosterone-receptor blockers. Nevertheless, HF still remains a major and growing cause of cardiovascular death worldwide, with 5-year mortality approaching 50% after diagnosis. Accordingly, a novel approach to modulating the RAAS and NP systems is warranted and may confer additional survival benefits in this disease population.
Article p 709
Since 1989 when Tei et al developed a new form of thermal therapy, named “Waon therapy”,1 accumulating evidence has shown its favorable effect on ischemic heart disease, peripheral artery disease, and chronic HF.2–4 Unlike the conventional sauna, Waon therapy requires a dry sauna kept at 60℃ with a far infrared ray, which increases cardiac output and reduces systemic vascular resistance through thermal peripheral vasodilation.1 Besides the acute hemodynamic effects, Waon therapy is shown to improve endothelial function by enhancing nitric oxide (NO) availability secondary to increased expression of NO synthase (eNOS).3,5 This is clinically relevant because endothelial dysfunction is well documented and closely associated with severe clinical symptoms, as well as lowered exercise capacity, in patients with HF.6,7 In support of this, a multicenter prospective randomized trial (WAON-CHF study) has recently demonstrated that Waon therapy improves the New York Heart Association functional class, 6-min walk distance, and cardiothoracic ratio in patients with advanced HF.8 Impaired NO-mediated vasodilation is potentially attributable to activation of the RAAS and NPs, as well as to oxidative stress and sympathetic nervous system activity. As such, this clinical study further implicates the possible link between Waon therapy and modulation of the RAAS and NP systems in the context of HF (Figure).9,10
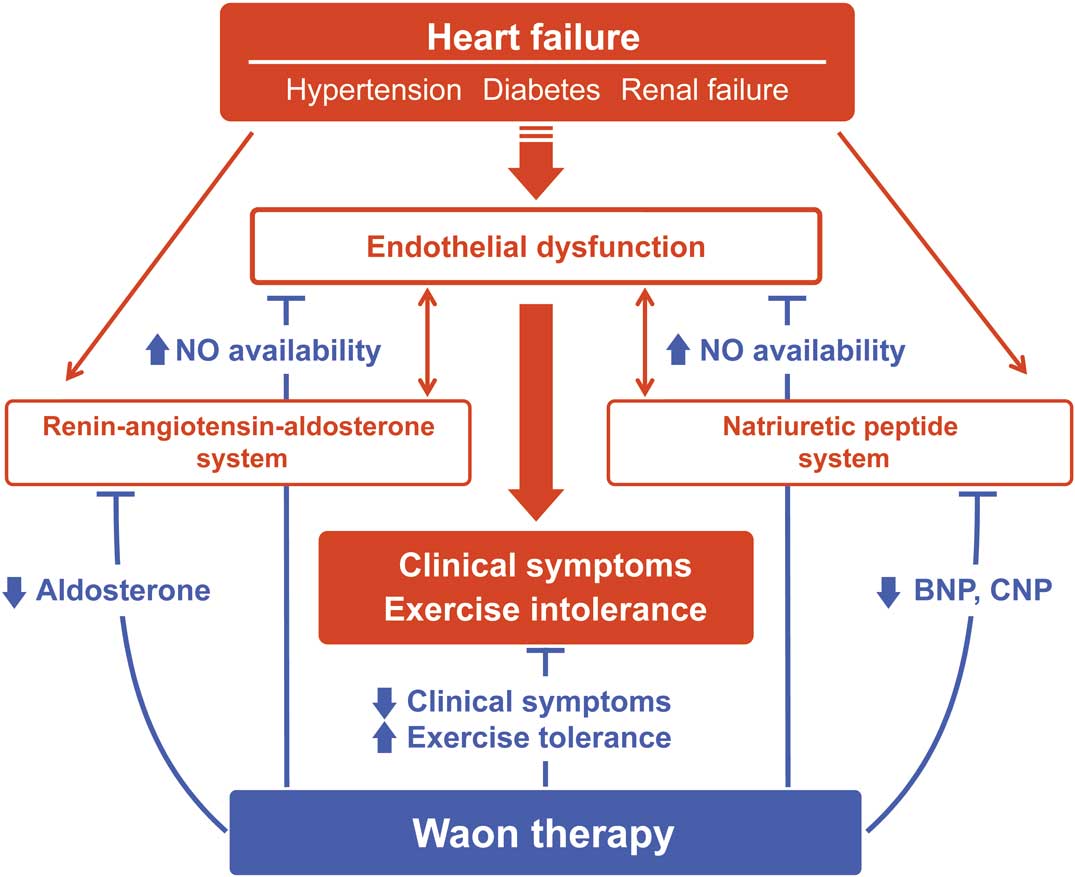
Possible pathways by which Waon therapy improves clinical symptoms and exercise tolerance in heart failure patients with diabetes, hypertension, and renal failure. Waon therapy can restore endothelial dysfunction through inhibition of the RAAS and NP systems, enhancing NO-dependent vasodilation, which results in the improvement of clinical symptoms and exercise tolerance. NO, nitric oxide; NP, natriuretic peptide; RAAS, renin-angiotensin-aldosterone system.
In this issue of the Journal, Ichiki et al11 evaluate whether 2-week Waon therapy reduces neurohumoral factors, including B-type NP (BNP), C-type NP (CNP), and aldosterone levels in a subanalysis of the WAON-CHF study. Although the changes in NP levels did not reach statistical significance before or after Waon therapy, the subgroup analysis revealed that BNP levels in HF patients treated with ACEI or ARBs were markedly diminished by Waon therapy. BNP level is known as the best indicator of elevated filling pressure, and is decreased by preload reduction caused by vasodilation. Thus, decreased BNP levels under the combination of RAAS inhibitor and Waon therapy may reflect enhancement of the vasodilatory effects of both treatments on HF. Moreover, aldosterone levels were lowered by Waon therapy, especially in HF patients with diabetes mellitus, hypertension, or inotrope use. RAAS activation is closely correlated with the endothelial dysfunction seen in patients with hypertension or diabetes. Specifically, excess aldosterone was shown to activate NAD(P)H oxidase, thereby promoting oxidative stress with decreased NO availability in an experimental model of HF.12 Therefore, Waon therapy-mediated inhibition of aldosterone levels, or possibly attenuated oxidative stress, appears to be more pronounced in HF patients with advanced endothelial dysfunction. CNP is the 3rd member of NP family, which is mainly produced by endothelial cells and precipitates endothelial dysfunction via binding to peptide receptor C.13 It is reported that CNP production is increased in the kidney and independently associated with poor outcomes in acute decompensated HF.14 Notably, Ichiki et al show that CNP levels were significantly inhibited by Waon therapy in HF patients with renal impairment, suggesting that worsening renal function associated with HF (cardiorenal syndrome) could be a potential target for Waon therapy.11 On the basis of these findings, it would appear that Waon therapy ameliorates activation of the RAAS and NP systems, particularly in the advanced clinical stages of HF with optimal RAAS inhibitors or comorbidities such as hypertension, diabetes, or renal failure. Furthermore, it is also anticipated that Waon therapy-induced neurohumoral modulation may reverse cardiac remodeling, leading to improved adverse outcomes in those specific HF subtypes, as shown by a retrospective study that Waon therapy reduced cardiac death and rehospitalization in chronic HF patients.15 Given the pivotal role of Waon therapy in the optimization of systemic neurohumoral factors, the subanalysis study provides a novel insight into the pathophysiological mechanism of Waon therapy for chronic HF.11 However, it should be noted that the follow-up period of the current study was relatively short, and thus the long-term effect of Waon therapy on cardiac function and prognosis of HF remains to be determined. Second, the statistical power of this study to detect significant differences in NP levels after treatment might be low because of the limited number of participating patients. Third, no direct evidence was found to support a plausible association between RAAS/NPs and endothelial dysfunction, as well as oxidative stress, inflammation, and sympathetic nervous activity.
In conclusion, Waon therapy represents a potential incremental approach by mitigating the activation of the RAAS and NP systems associated with HF. The present study will give us a deeper understanding of the mechanisms responsible for Waon therapy effects and allow for improved application of this therapy in HF patients with advanced vascular dysfunction.
We thank Naoya Kakutani for his assistance with this manuscript.
The authors declare no conflicts of interest.