Abstract
Background:
This study determined whether relaxin or matrix metalloproteinase (MMP)-9 influences angiotensin II (AngII)-induced abdominal aortic aneurysms (AAA).
Methods and Results:
Male C57BL/6 or apolipoprotein E−/−
mice were infused with AngII with or without relaxin. Relaxin did not influence AngII-induced AAA in either mouse strain. Infusion of AngII reduced, but relaxin increased, MMP-9 mRNA in macrophages. We then determined the effects of MMP-9 deficiency on AAA in apolipoprotein E−/−
mice. MMP-9 deficiency led to AAA formation in the absence of AngII, and augmented AngII-induced aortic rupture and AAA incidence.
Conclusions:
MMP-9 deficiency augmented AngII-induced AAA.
Relaxin, a peptide hormone in the insulin family, has spawned interest as a therapeutic for heart failure.1
Relaxin elevates matrix metalloproteinase (MMP)-9 expression in certain cell types.2
MMP-9 is associated with abdominal aortic aneurysms (AAA).3–6
Our initial purpose was to determine whether relaxin augments angiotensin (Ang) II-induced AAA through an MMP-9-mediated mechanism. Relaxin did not augment AngII-induced AAA, although it increased MMP-9 mRNA abundance in the macrophages of AngII-infused mice. We then determined whether MMP-9 genetic deficiency influenced AngII-induced AAA.
Results
No Augmentation of AngII-Induced AAA by Relaxin
To determine whether relaxin increases AngII-induced AAA, we first used male C57BL/6 mice because the incidence of AngII-induced AAA is low in this mouse strain.7
Four groups of mice were infused with: (1) vehicle, (2) AngII, (3) AngII and relaxin 0.3 mg/kg/day, and (4) AngII and relaxin 0.6 mg/kg/day. Detection of plasma relaxin concentrations by ELISA were confirmed in mice infused with either dose of relaxin. Two mice died of aortic rupture (n=2/12, 17%) in mice infused with AngII alone, but no aortic rupture-induced deaths were observed in the other 3 groups. AngII infusion led to AAA formation, but neither dose of relaxin augmented incidence of AngII-induced AAA (Figure 1A).
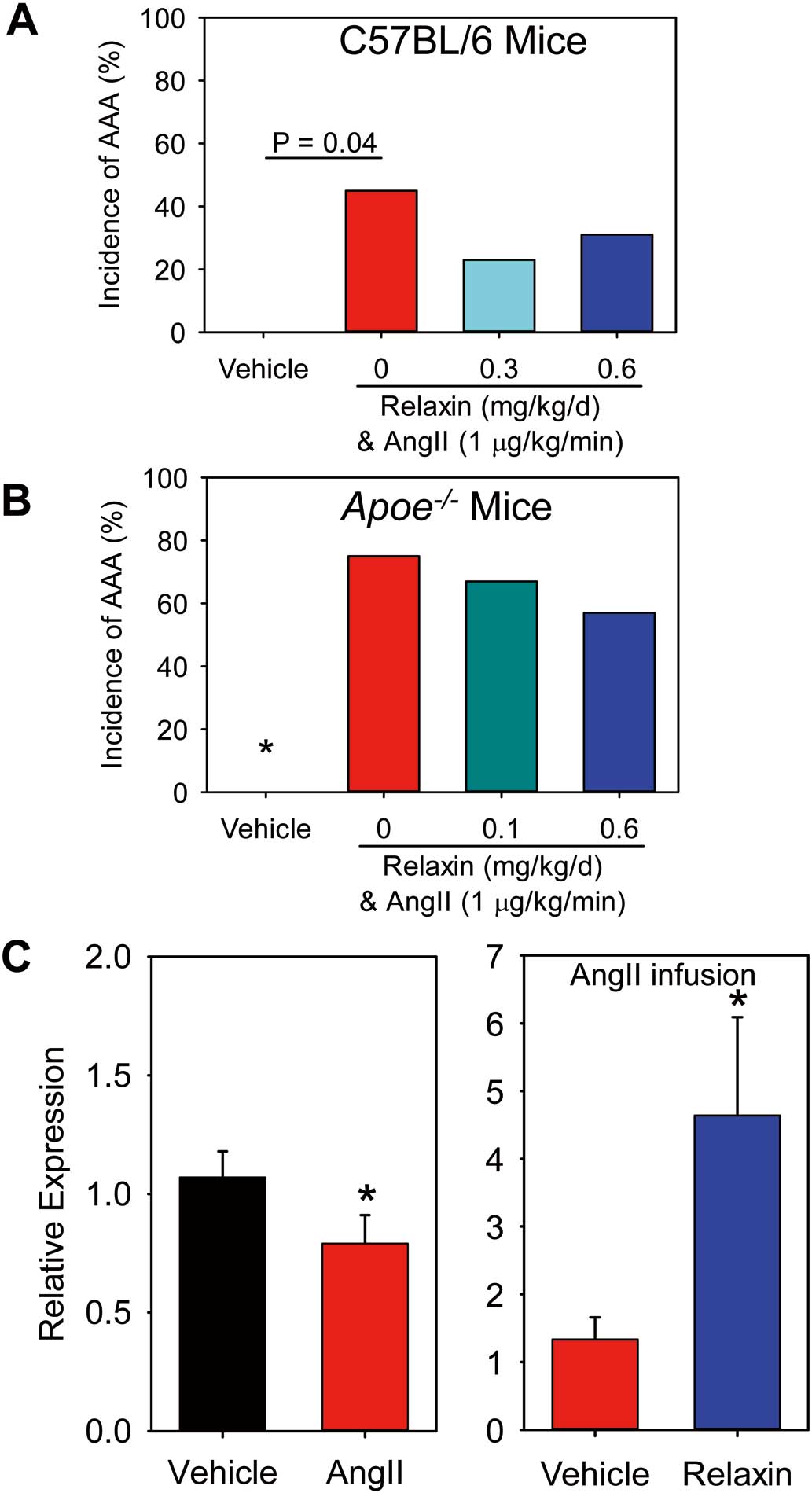
To rule out the possibility that relaxin may reduce AngII-induced AAA, male apolipoprotein
(Apo)e−/−
mice, a common mouse model for studying AngII-induced AAA,7,8
were infused with: (1) vehicle, (2) AngII, (3) AngII and relaxin 0.1 mg/kg/day, and (4) AngII and relaxin 0.6 mg/kg/day. Death from aortic rupture was 0%, 13% (n=2/16), 7% (n=1/14), and 20% (n=3/15) in the vehicle, AngII, AngII and relaxin 0.1 mg/kg/day, and AngII and relaxin 0.6 mg/kg/day groups, respectively. Relaxin did not affect body weight or systolic blood pressure in mice infused with AngII. AngII infusion led to AAA formation, but neither dose of relaxin influenced the incidence of AngII-induced AAA (Figure 1B).
To determine whether relaxin influences MMP expression, we performed two short term studies: (1) saline or AngII was infused for 7 days, and (2) AngII alone or AngII and relaxin were infused for 7 days. AngII infusion led to reductions of MMP-9 mRNA abundance, but co-infusion of relaxin with AngII significantly increased MMP-9 mRNA abundance in peritoneal macrophages (Figure 1C), but not in suprarenal aortas.
MMP-9 Deficiency Augmented AngII-Induced AAA
To determine the effects of MMP-9 on AAA formation,
Mmp9+/+
and
Mmp9−/−
mice in an
Apoe−/−
background were infused with either saline or AngII for 28 days. MMP-9 deficiency had no effect on body weight or AngII-induced high blood pressure. None of the saline-infused mice died. In the AngII-infused groups, 2 of 21 (10%)
Mmp9+/+
mice and 11 of 26 (42%)
Mmp9−/−
mice died of aortic rupture (Figure 2A). Surprisingly, 4 of 14 (29%) saline-infused
Mmp9−/−
mice developed AAA. AngII infusion had a significant effect on the incidence of AAA. Incidence of AAA between the 2
Mmp9
genotypes was also significantly different in both saline-infused and AngII-infused mice (Figure 2B).
Discussion
The initial purpose of this study was to evaluate the effects of relaxin in AngII-induced AAA. Relaxin had no effect on AngII-induced AAA formation in either C57BL/6 or
Apoe−/−
mice. Relaxin and AngII synergistically increase MMP-9 secretion from prostate tumors.9
In our study, relaxin increased the mRNA abundance of MMP-9 in macrophages, but not in the suprarenal aorta of AngII-infused mice. Although many studies have demonstrated beneficial effects of relaxin in heart failure,1
a recent study reported that relaxin had no effect on AngII-induced pathologies.10
Therefore, relaxin may have complex roles in AngII-mediated MMP-9 expression and cardiovascular diseases.
The most interesting finding was that MMP-9 genetic deficiency in hypercholesterolemic mice induced AAA formation in the absence of AngII. Furthermore, MMP-9 deficiency increased the incidence of aortic rupture and AAA in AngII-infused mice. This finding contradicted previously reported studies that MMP-9 deficiency attenuates elastase or calcium chloride-induced AAA.4,5
Apparent differences between the reported studies and ours are the different manipulations in different mouse strains. In the reported studies, elastase4
or calcium chloride5
was applied directly to infrarenal aortas of normocholesterolemic mice. In our study, hypercholesterolemic mice were infused subcutaneously with either saline or AngII. Hypercholesterolemia augments AngII-induced AAA.7
However, this does not explain why MMP-9 deficiency in the saline-infused mice also induced AAA, because saline infusion itself does not lead to AAA in mice.
Differences in the genetic background of mouse strains may contribute to differences in experimental outcomes.11
The
Mmp9−/−
mice used in previously reported studies4,5
and in our study were originally developed in a mixed background,12
which was further complicated by different breeding approaches in different institutes.
Mmp9−/−
mice used in elastase- or calcium chloride-induced AAA models were described as 129/SvEv,4,5
whereas in our study
Mmp9−/−
mice were on a mixed background of 129/SvEv and C57BL/6. However, it is unclear whether and how the genetic differences of these mice contributed to the conflicting findings.
Despite being born grossly normal,
Mmp9−/−
mice have abnormal development of growth plates in their long bones, including delayed vascularization and ossification.12
MMP-9 depletion impairs collagen organization and angiogenesis, as well as smooth muscle cell migration and the capacity to contract collagen, indicating that its absence may have detrimental effects on repairing abnormal vascular constructs.13
Consistent with our findings, Aikawa’s group has reported that genetically engineered resistance for MMP collagenases augments AngII-induced AAA, although accumulation of collagen in the aortic adventitia is enhanced.14
Because new collagen biosynthesis is impaired in human AAA,15
it is possible that MMP-9 deficiency impairs the integrity of vascular constructs. AngII infusion into
Mmp9−/−
mice may accelerate the process of damaging vascular integrity, thereby augmenting AAA and increasing the aortic rupture risk. Our study and those reported by Aikawa’s group14
highlight the need for additional work to solidify the role of MMP-9 under different conditions and stages of AAA, and address the translation of different animal models to the human disease.
Acknowledgments
We thank Mr. Mark Bostrom for his technical assistance with the MMP-9 genetic mouse studies and Dr. Richard Charnigo in the Department of Statistics, University of Kentucky for helping with the statistical analyses.
Sources of Funding
Relaxin study was supported by an award from Merck & Co., Inc.
Mmp9−/−
mouse study was supported by the National Institutes of Health under award numbers R01 HL107319 and HL133723. The content of this report is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosures
D.G.J. is a Merck employee; M.D., V.D.C., and D.E.G. were Merck employees during the execution of the experiments.
Supplementary Files
Supplementary File 1
Supplementary Methods
Please find supplementary file(s);
http://dx.doi.org/10.1253/circj.CJ-17-0229
References
- 1.
Du XJ, Hewitson TD, Nguyen MN, Samuel CS. Therapeutic effects of serelaxin in acute heart failure. Circ J 2014; 78: 542–552.
- 2.
Ahmad N, Wang W, Nair R, Kapila S. Relaxin induces matrix-metalloproteinases-9 and -13 via RXFP1: Induction of MMP-9 involves the PI3K, ERK, Akt and PKC-zeta pathways. Mol Cell Endocrinol 2012; 363: 46–61.
- 3.
Urbonavicius S, Urbonaviciene G, Honore B, Henneberg EW, Vorum H, Lindholt JS. Potential circulating biomarkers for abdominal aortic aneurysm expansion and rupture: A systematic review. Eur J Vasc Endovasc Surg 2008; 36: 273–280; discussion 281–282.
- 4.
Pyo R, Lee JK, Shipley JM, Curci JA, Mao D, Ziporin SJ, et al. Targeted gene disruption of matrix metalloproteinase-9 (gelatinase B) suppresses development of experimental abdominal aortic aneurysms. J Clin Invest 2000; 105: 1641–1649.
- 5.
Longo GM, Xiong W, Greiner TC, Zhao Y, Fiotti N, Baxter BT. Matrix metalloproteinases 2 and 9 work in concert to produce aortic aneurysms. J Clin Invest 2002; 110: 625–632.
- 6.
Lu H, Aikawa M. Many faces of matrix metalloproteinases in aortic aneurysms. Arterioscler Thromb Vasc Biol 2015; 35: 752–754.
- 7.
Liu J, Lu H, Howatt DA, Balakrishnan A, Moorleghen JJ, Sorci-Thomas M, et al. Associations of apoAI and apoB-containing lipoproteins with AngII-induced abdominal aortic aneurysms in mice. Arterioscler Thromb Vasc Biol 2015; 35: 1826–1834.
- 8.
Daugherty A, Manning MW, Cassis LA. Angiotensin II promotes atherosclerotic lesions and aneurysms in apolipoprotein E-deficient mice. J Clin Invest 2000; 105: 1605–1612.
- 9.
Dominska K, Ochedalski T, Kowalska K, Matysiak-Burzynska ZE, Pluciennik E, Piastowska-Ciesielska AW. A common effect of angiotensin II and relaxin 2 on the PNT1A normal prostate epithelial cell line. J Physiol Biochem 2016; 72: 381–392.
- 10.
Haase N, Rugor J, Przybyl L, Qadri F, Muller DN, Dechend R. Relaxin does not improve Angiotensin II-induced target-organ damage. PLoS One 2014; 9: e93743.
- 11.
Uchida HA, Poduri A, Subramanian V, Cassis LA, Daugherty A. Urokinase-type plasminogen activator deficiency in bone marrow-derived cells augments rupture of angiotensin II-induced abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol 2011; 31: 2845–2852.
- 12.
Vu TH, Shipley JM, Bergers G, Berger JE, Helms JA, Hanahan D, et al. MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell 1998; 93: 411–422.
- 13.
Galis ZS, Johnson C, Godin D, Magid R, Shipley JM, Senior RM, et al. Targeted disruption of the matrix metalloproteinase-9 gene impairs smooth muscle cell migration arterial remodeling and geometrical. Circulation Research 2002; 91: 852–859.
- 14.
Deguchi JO, Huang H, Libby P, Aikawa E, Whittaker P, Sylvan J, et al. Genetically engineered resistance for MMP collagenases promotes abdominal aortic aneurysm formation in mice infused with angiotensin II. Lab Invest 2009; 89: 315–326.
- 15.
Carmo M, Colombo L, Bruno A, Corsi FR, Roncoroni L, Cuttin MS, et al. Alteration of elastin, collagen and their cross-links in abdominal aortic aneurysms. Eur J Vasc Endovasc Surg 2002; 23: 543–549.



