Abstract
Background:
According to troponin-based criteria of myocardial infarction (MI), patients without elevation of creatine kinase (CK), formerly classified as unstable angina (UA), are now diagnosed as non-ST-elevation MI (NSTEMI), but little is known about their outcomes.
Methods and Results:
Between July 2012 and March 2014, 3,283 consecutive patients with MI were enrolled. Clinical follow-up data were obtained up to 3 years. The primary endpoint was a composite of all-cause death, non-fatal MI, non-fatal stroke, cardiac failure and urgent revascularization for UA. There were 2,262 patients with ST-elevation MI (STEMI), 563 NSTEMI with CK elevation (NSTEMI+CK) and 458 NSTEMI without CK elevation (NSTEMI-CK). From day 0, Kaplan-Meier curves for the primary endpoint began to diverge in favor of NSTEMI-CK for up to 30 days. The 30-day event rate was significantly lower in patients with NSTEMI-CK (3.3%) than in STEMI (8.6%, P<0.001) and NSTEMI+CK (9.9%, P<0.001). Later, the event curves diverged in favor of STEMI. The event rate from 31 days to 3 years was significantly lower in patients with STEMI (19.8%) than in NSTEMI+CK (33.6%, P<0.001) and NSTEMI-CK (34.2%, P<0.001). Kaplan-Meier curves from 31 days to 3 years were almost identical between NSTEMI+CK and NSTEMI-CK (P=0.91).
Conclusions:
Despite smaller infarct size and better short-term outcomes, long-term outcomes of NSTEMI-CK after convalescence were as poor as those for NSTEMI+CK and worse than for STEMI.
Non-ST-elevation acute coronary syndrome (ACS) consists of non-ST-elevation myocardial infarction (NSTEMI) and unstable angina (UA).1
A diagnosis of NSTEMI is given if cardiac biomarkers are elevated. Formerly, MI was diagnosed by elevation of creatine kinase (CK). Over the past decades, cardiac troponin (cTn) has been used as the preferred biomarker because of its higher sensitivity and specificity for myocardial injury.2
In addition to patients with CK elevation (NSTEMI+CK), a large number of patients formerly classified by CK as UA are now ruled-in by cTn as NSTEMI (NSTEMI-CK).3–5
We have previously reported the clinical presentation, treatment and in-hospital outcomes of patients with ST-elevation MI (STEMI), NSTEMI+CK and NSTEMI-CK.6
In-hospital mortality rates were comparable between STEMI and NSTEMI+CK, but patients with NSTEMI-CK had favorable outcomes despite their complicated background. Previous studies have reported that NSTEMI is associated with worse long-term outcomes than STEMI. However, it remains unclear whether long-term outcomes after NSTEMI-CK are comparable to those after NSTEMI+CK. The purpose of this study was to investigate long-term outcomes of patients with MI in the contemporary cTn era, especially focusing on NSTEMI-CK.
Methods
The Japanese registry of acute myocardial infarction diagnosed by universal definition (J-MINUET) is a prospective observational multicenter study conducted at 28 Japanese medical institutions (UMIN000010037). Consecutive patients who were hospitalized within 48 h of the onset of MI were enrolled between July 2012 and March 2014. The study protocol has been previously reported.6
In brief, diagnosis of MI was based on the European Society of Cardiology (ESC)/American College of Cardiology (ACC) Foundation/American Heart Association/World Heart Federation Task Force for the Universal Definition of Myocardial Infarction.7
Only type 1 (spontaneous MI related to ischemia due to primary coronary event) and type 2 (MI secondary to ischemia due to either increased oxygen demand or decreased supply) were included in this registry. Type of cTn measured (cTnT or cTnI) depended on the attending physician, and the cutoff value of each institution was applied. In patients in whom CK was elevated more than twice the upper limit of normal, cTn measurement may not be required. Frequency and time interval data of cTn and CK measurements were not prespecified but left to the physicians’ decision.
Patients were evaluated at baseline for demographic and clinical characteristics. STEMI was diagnosed in the presence of new ST-elevation at the J point in at least 2 contiguous leads. New or presumably new left bundle branch block has been considered a STEMI equivalent. Data on the treatment and in-hospital clinical events were collected at the time of hospital discharge. Clinical follow-up after the index MI was performed through a review of medical records, telephone contact and mailed questionnaire.
This study was conducted in accordance with the Declaration of Helsinki. The protocol was approved by the ethics committees of each participating institution.
Outcomes
The primary endpoint was the composite of all-cause death, non-fatal MI, non-fatal stroke, cardiac failure and urgent revascularization for UA. Non-fatal MI included only types 1 and 2. Cardiac failure was defined as heart failure requiring hospital admission. Key secondary endpoints included (1) death, (2) composite of death and non-fatal MI, (3) death, non-fatal MI and non-fatal stroke and (4) composite of death, non-fatal MI, non-fatal stroke and cardiac failure.
Statistical Analysis
All continuous variables are presented as median (25–75th percentiles) and unpaired t-tests were used to compare groups. If the variables were not distributed normally, signed-rank tests were used. Non-continuous and categorical variables are presented as percentages and were compared using the chi-square test.
Event curves were constructed using the Kaplan-Meier method and compared using the log-rank test. Landmark analyses were done from 0 to 30 days and from 31 days to 3 years of follow-up. Univariate and multivariate Cox proportional hazards models were used to calculate hazard ratios for all events and 95% confidence intervals. Multivariable analyses were performed using covariates that established prognostic risk factors for cardiac events. Multiple imputation was used to replace each missing value with 2 or more acceptable values, representing a distribution of possibilities of covariates.8
The multiple imputation method is a more sophisticated imputation method than either the case-wise deletion method, which analyzes the cases with complete information, or the single imputation method.9
All statistical tests were 2-sided and P-values <0.05 were regarded as significant. Statistical analysis was performed with JMP, version 11.0.0 (SAS Institute Inc., Cary, NC, USA) and STATA, version 12 (StataCorp LP, College Station, TX, USA).
Results
A total of 3,283 patients were enrolled in the study and included in the analysis. There were 2,262 patients (68.9%) with STEMI, 563 patients (17.1%) with NSTEMI+CK and 458 patients (14.0%) with NSTEMI-CK (Figure 1).
Baseline Patient Characteristics
Baseline clinical characteristics and management of the study patients are shown in
Table 1. Patients with STEMI were younger, more likely male, had less concomitant disease, more current smokers, less previous cardiovascular disease, shorter time from onset to admission and less type 2 MI as compared with NSTEMI+CK and NSTEMI-CK. Primary percutaneous coronary intervention (PCI) was more frequently performed for patients with STEMI and urgent bypass surgery for those with NSTEMI+CK. MaxCK was significantly higher in patients with STEMI than in those with NSTEMI+CK.
Table 2
shows prescriptions before the onset of MI and at the time of hospital discharge. Patients with NSTEMI were taking more cardioprotective drugs, including antiplatelet agents, antihypertensive drugs and statins, before the onset of MI. At the time of hospital discharge, evidence-based medicines were prescribed for most of the patients. However, despite having more comorbid factors, patients with NSTEMI, especially those with NSTEMI-CK, took less β-blockers, angiotensin-converting enzyme inhibitors and statins.
Table 1.
Baseline Characteristics of the Patients in J-MINUET Study
| |
STEMI
(n=2,262) |
NSTEMI+CK
(n=563) |
NSTEMI-CK
(n=458) |
P value |
STEMI vs.
NSTEMI+CK |
STEMI vs.
NSTEMI-CK |
NSTEMI+CK vs.
NSTEMI-CK |
| Age (years) |
68
(60–77) |
71
(62–80) |
73
(63–80) |
<0.001 |
<0.001 |
0.26 |
| Male |
76.8% |
72.3% |
71.2% |
0.026 |
0.011 |
0.69 |
| Concomitant diseases |
| Hypertension |
63.4% |
70.3% |
76.8% |
0.002 |
<0.001 |
0.020 |
| Diabetes |
35.0% |
39.8% |
39.1% |
0.033 |
0.098 |
0.81 |
| Dyslipidemia |
49.9% |
53.9% |
59.4% |
0.094 |
<0.001 |
0.077 |
| CKD |
41.9% |
50.1% |
52.4% |
<0.001 |
<0.001 |
0.46 |
| eGFR |
66
(49–83) |
61
(43–77) |
61
(43–77) |
0.003 |
<0.001 |
0.29 |
| Current smoking |
37.3% |
26.9% |
26.9% |
<0.001 |
<0.001 |
0.98 |
| Previous history |
| Previous MI |
9.3% |
16.0% |
21.2% |
<0.001 |
<0.001 |
0.034 |
| Previous PCI |
11.2% |
21.1% |
28.3% |
<0.001 |
<0.001 |
0.034 |
| Previous CABG |
1.8% |
4.2% |
6.6% |
<0.001 |
<0.001 |
0.010 |
| Stroke |
10.4% |
13.5% |
13.3% |
0.044 |
0.080 |
0.93 |
| PAD |
3.2% |
7.5% |
8.2% |
<0.001 |
<0.001 |
0.69 |
| Killip class ≥2 |
25.6% |
29.0% |
12.9% |
0.006 |
<0.001 |
<0.001 |
| Time from onset to admission (min) |
140
(66–334) |
215
(84–513) |
180
(75–557) |
<0.001 |
<0.001 |
0.81 |
| ≤720 min (12 h) |
88.6% |
82.4% |
80.3% |
<0.001 |
<0.001 |
0.40 |
| Type 2 MI |
3.0% |
7.0% |
13.6% |
<0.001 |
<0.001 |
<0.001 |
| Urgent coronary angiography |
96.9% |
88.6% |
79.9% |
<0.001 |
<0.001 |
<0.001 |
| Initial TIMI 0/1 flow |
70.5% |
48.9% |
14.9% |
<0.001 |
<0.001 |
<0.001 |
| Multivessel disease |
41.5% |
53.1% |
43.7% |
<0.001 |
0.43 |
0.006 |
| Primary PCI |
93.1% |
72.6% |
60.8% |
<0.001 |
<0.001 |
<0.001 |
| Door to balloon time (min) |
66
(49–92) |
125
(80–236) |
171
(100–399) |
<0.001 |
<0.001 |
<0.001 |
| Stent use† |
90.7% |
89.3% |
92.1% |
0.40 |
0.42 |
0.21 |
| DES use‡ |
59.6% |
71.2% |
78.9% |
<0.001 |
<0.001 |
0.032 |
| Final TIMI 3 flow |
91.0% |
92.1% |
97.4% |
0.45 |
<0.001 |
0.004 |
| Urgent CABG |
1.1% |
6.0% |
2.0% |
<0.001 |
0.11 |
0.001 |
| Max CK (IU/L) |
2,017
(927–3,848) |
1,151
(702–2,076) |
161
(101–254) |
<0.001 |
<0.001 |
<0.001 |
Data given as % or median (25–75th percentiles). †Among patients treated with primary PCI; ‡among patients treated with stent. CABG, coronary aorta bypass graft; CK, creatine kinase; CKD, chronic kidney disease; DES, drug-eluting stent; eGFR, estimated glomerular filtration rate; MI, myocardial infarction; NSTEMI, non-ST-elevation MI; PAD, peripheral arterial disease; PCI, percutaneous coronary intervention; STEMI, ST-elevation MI.
Table 2.
Prescriptions for the Patients in J-MINUET Study
| |
STEMI |
NSTEMI+CK |
NSTEMI-CK |
P value |
STEMI vs.
NSTEMI+CK |
STEMI vs.
NSTEMI-CK |
NSTEMI+CK vs.
NSTEMI-CK |
| Before MI |
(n=2,262) |
(n=563) |
(n=458) |
|
|
|
| Aspirin |
20.5% |
33.0% |
47.4% |
<0.001 |
<0.001 |
<0.001 |
| P2Y12 antagonists |
5.8% |
10.5% |
17.0% |
<0.001 |
<0.001 |
0.002 |
| OAC |
3.4% |
5.3% |
8.1% |
0.046 |
<0.001 |
0.098 |
| Calcium blockers |
31.3% |
38.2% |
45.0% |
0.002 |
<0.001 |
0.030 |
| β-blockers |
10.5% |
19.0% |
25.1% |
<0.001 |
<0.001 |
0.022 |
| ACEIs |
5.7% |
8.3% |
9.0% |
0.024 |
0.011 |
0.74 |
| ARBs |
22.9% |
29.8% |
38.6% |
<0.001 |
<0.001 |
0.003 |
| Diuretics |
6.7% |
13.5% |
14.2% |
<0.001 |
<0.001 |
0.78 |
| Nitrates |
4.5% |
9.2% |
14.6% |
<0.001 |
<0.001 |
0.008 |
| Nicorandil |
3.1% |
6.0% |
9.6% |
0.002 |
<0.001 |
0.043 |
| Statins |
18.9% |
30.4% |
37.1% |
<0.001 |
<0.001 |
0.027 |
| Oral hypoglycemic drugs |
15.5% |
19.7% |
21.4% |
0.018 |
0.003 |
0.53 |
| Insulin |
3.7% |
7.5% |
5.7% |
<0.001 |
0.050 |
0.31 |
| At discharge |
(n=2,102) |
(n=518) |
(n=450) |
|
|
|
| Aspirin |
96.6% |
96.1% |
91.1% |
0.58 |
<0.001 |
0.002 |
| P2Y12 antagonists |
83.0% |
71.3% |
70.7% |
<0.001 |
<0.001 |
0.88 |
| OAC |
13.2% |
13.0% |
9.5% |
0.94 |
0.037 |
0.12 |
| Calcium blockers |
16.7% |
26.7% |
46.9% |
<0.001 |
<0.001 |
<0.001 |
| β-blockers |
71.2% |
68.1% |
55.3% |
0.20 |
<0.001 |
<0.001 |
| ACEIs |
58.0% |
47.5% |
30.3% |
<0.001 |
<0.001 |
<0.001 |
| ARBs |
24.8% |
31.9% |
41.4% |
0.002 |
<0.001 |
0.003 |
| Diuretics |
1.6% |
1.5% |
0.3% |
>0.99 |
0.032 |
0.078 |
| Nitrates |
7.5% |
13.3% |
24.2% |
<0.001 |
<0.001 |
<0.001 |
| Nicorandil |
19.6% |
22.1% |
27.3% |
0.22 |
<0.001 |
0.082 |
| Statins |
88.5% |
83.8% |
83.2% |
0.007 |
0.004 |
0.86 |
| Oral hypoglycemic drugs |
24.0% |
24.4% |
22.0% |
0.86 |
0.42 |
0.43 |
| Insulin |
4.3% |
6.7% |
4.8% |
0.041 |
0.69 |
0.25 |
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin-receptor blocker; MI, myocardial infarction; OAC, oral anticoagulant.
At the 3-year follow-up, the primary endpoint had occurred in 801 patients. The 3-year cumulative incidence of the primary endpoint was significantly higher in patients with NSTEMI+CK (40.2%) than in those with STEMI (26.7%, P<0.001) and NSTEMI-CK (36.4%, P=0.039) (Figure 2). The same trends were observed when only patients without chronic kidney disease (CKD), those with CKD or those with type 1 MI were analyzed. The 3-year incidences of each component of the primary endpoint are shown in
Table S1. Kaplan-Meier curves for key secondary endpoints are shown in
Figure 3. The 3-year mortality rate was significantly higher in patients with NSTEMI+CK (20.0%) than in those with STEMI (13.6%, P=0.002) or NSTEMI-CK (14.3%, P=0.006). Stroke occurred in 122 patients: ischemic stroke in 93 (76.2%), hemorrhagic stroke in 25 (20.5%) and stroke of unknown type in 4 (3.3%). Urgent revascularization for UA was performed in 185 patients and of these, 168 procedures (90.8%) were PCI.

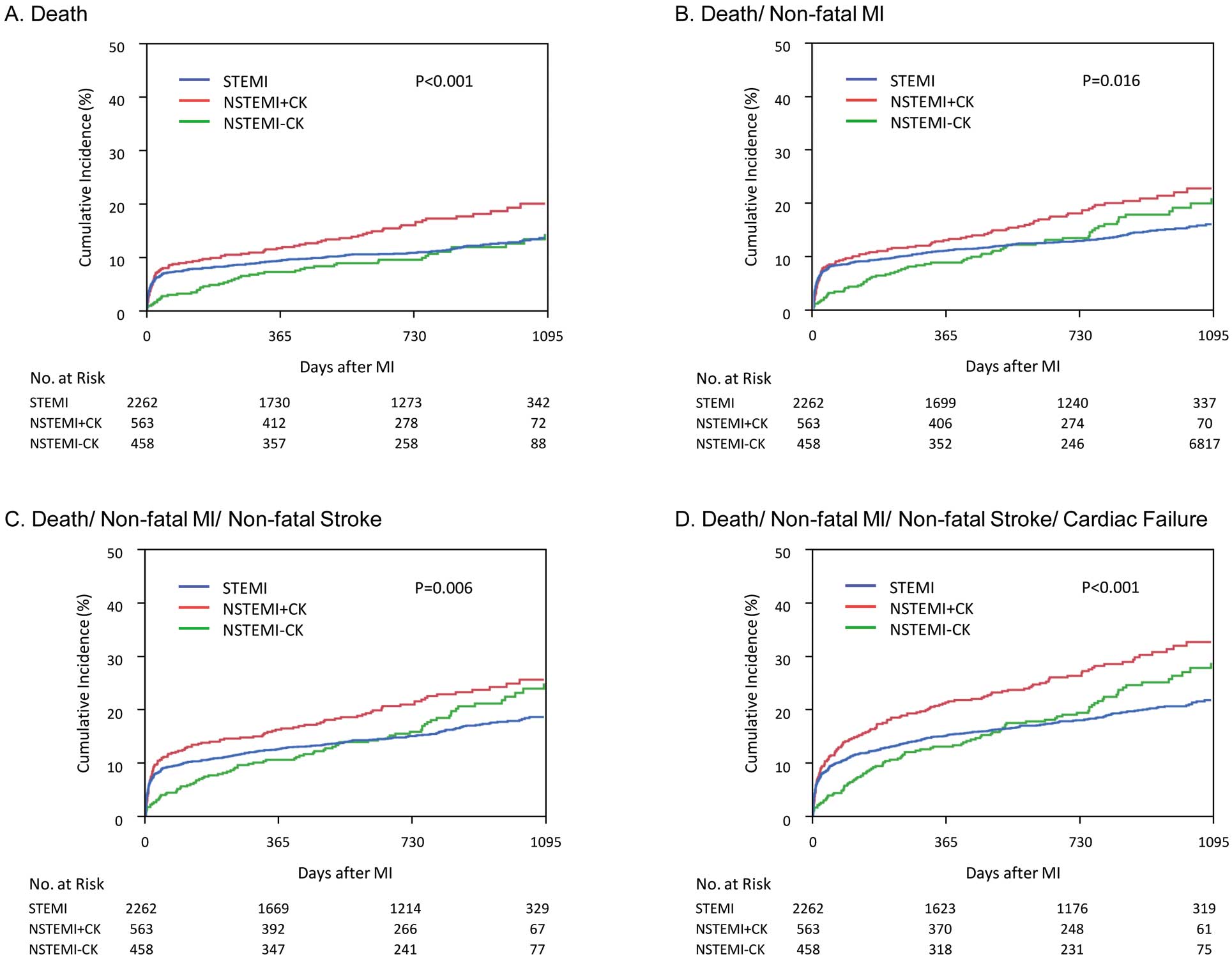
Figure 4
shows landmark Kaplan-Meier analyses for 0–30 days and 31 days to 3 years. From day 0, the Kaplan-Meier curves began to diverge for the primary endpoint in favor of NSTEMI-CK for up to 30 days. The 30-day event rate was significantly lower in patients with NSTEMI-CK (3.3%) than in those with STEMI (8.6%, P<0.001) or NSTEMI+CK (9.9%, P<0.001). The event curves diverged in favor of STEMI for up to 3 years. The event rate from 31 days to 3 years was significantly lower in patients with STEMI (19.8%) than in those with NSTEMI+CK (33.6%, P<0.001) or NSTEMI-CK (34.2%, P<0.001). Kaplan-Meier curves of the primary endpoint from 31 days to 3 years were almost identical for NSTEMI+CK and NSTEMI-CK (P=0.91). Interval incidence of the primary endpoint after 30 days is shown as 30 days to 6 months and each half-year thereafter in
Figure S1. After 1 year for STEMI and half-year for NSTEMI+CK and NSTEMI-CK, the interval incidence of the primary endpoint became constant: approximately 2%/0.5 year for STEMI and 5%/0.5 year for NSTEMI+CK and NSTEMI-CK.

Multivariate analysis showed that NSTEMI-CK was associated with a lower incidence of the primary endpoint from 0 to 30 days, whereas both NSTEMI+CK and NSTEMI-CK were independent predictors of the primary endpoint from 31 days to 3 years (Table 3).
Table 3.
Hazard Ratios of Primary Endpoint in J-MINUET Study
| |
NSTEMI+CK vs. STEMI |
NSTEMI-CK vs. STEMI |
NSTEMI-CK vs. NSTEMI+CK |
| Univariate analysis |
| 0 days to 3 years |
1.61 (1.35–1.91), P<0.001 |
1.27 (1.04–1.56), P=0.019 |
0.78 (0.62–0.99), P=0.037 |
| 0 days to 30 days |
1.40 (1.02–1.93), P=0.040 |
0.37 (0.20–0.68), P=0.001 |
0.26 (0.14–0.50), P<0.001 |
| 31 days to 3 years |
1.71 (1.38–2.10), P<0.001 |
1.69 (1.36–2.11), P<0.001 |
0.99 (0.76–1.28), P=0.91 |
| Multivariate analysis |
| 0 days to 3 years |
1.42 (1.17–1.72), P<0.001 |
1.25 (1.00–1.58), P=0.054 |
0.89 (0.69–1.13), P=0.33 |
| 0 days to 30 days |
1.22 (0.86–1.72), P=0.27 |
0.37 (0.19–0.73), P=0.004 |
0.39 (0.20–0.77), P=0.007 |
| 31 days to 3 years |
1.50 (1.20–1.89), P<0.001 |
1.62 (1.27–2.08), P<0.001 |
1.05 (0.80–1.37), P=0.74 |
Data given as hazard ratio (95% confidence interval). Multivariate model adjusted for age, sex, hypertension, diabetes, dyslipidemia, CKD, current smoking, previous MI, previous stroke, Killip class, type of MI, logarithm of time from onset to admission, urgent coronary angiography, primary PCI and urgent bypass surgery. Abbreviations as in Table 1.
Discussion
The major findings of this study were (1) almost half of patients with NSTEMI do not have elevation of CK (NSTEMI-CK), (2) short-term (0–30 days) outcomes of STEMI and classical NSTEMI (NSTEMI+CK) were similar but significantly worse than NSTEMI-CK, and (3) after convalescence, long-term (31 days to 3 years) outcomes of NSTEMI-CK were almost identical to those for NSTEMI-CK and worse than for STEMI.
In the past, MI was clinically diagnosed by criteria proposed by World Health Organization that consisted of symptoms, ECG abnormalities and a rise in cardiac enzymes.10
CK and CK-MB were used for the diagnosis of MI until cTnI and -T were developed as more sensitive and specific biomarkers. In 2000, ESC and ACC proposed a new definition of MI, a universal definition, in which cTn was adopted as the preferred cardiac biomarker. The change in diagnostic biomarker and subsequent improvement in diagnostic sensitivity significantly affected the number of MI diagnoses. Numerous patients who were formerly diagnosed as having UA by CK-based criteria are now diagnosed as having NSTEMI (NSTEMI-CK). In the current study, nearly half of the NSTEMI cases diagnosed by cTn-based criteria did not have elevation of CK (NSTEMI-CK). Although most contemporary studies enroll patients with MI based on cTn-based criteria, little is known about the presentation, management and outcomes of patients with NSTEMI-CK. In the initial report of J-MINUET study, we described the presentation, management and in-hospital outcomes of patients with spontaneous MI diagnosed by the universal definition, focusing on NSTEMI-CK.6
The current study is a follow-up of the J-MINUET registry that investigated long-term outcomes of patients with STEMI, NSTEMI+CK and NSTEMI-CK.
Short-term (0–30 days) incidence of the primary endpoint was 9.9% for patients with NSTEMI+CK, which was comparable to that for STEMI (8.6%) but higher than for NSTEMI-CK (3.3%). These findings are consistent with earlier studies. In the CK-based criteria era, the Global Registry of Acute Coronary Events reported that in-hospital mortality rates for STEMI, NSTEMI and UA were 7%, 6% and 3%, respectively.11
More recent studies have shown that NSTEMI diagnosed by cTn-based criteria has favorable short-term outcomes compared with STEMI. In these studies, NSTEMI-CK, formerly diagnosed as UA, was include in NSTEMI.12,13
In patients with NSTEMI, less extensive infarct, undetectable by serum CK measurement, was associated with favorable short-term outcomes.
Previous studies, mostly from Western countries other than Japan, have consistently demonstrated that long-term outcomes of NSTEMI are worse than for STEMI, both in the CK-based criteria era and with the cTn-based criteria.13,14
However, there is little data on the long-term outcomes of Japanese patients with NSTEMI. The Prevention of AtherothrombotiC Incidents Following Ischemic Coronary Attack (PACIFIC) registry is a representative Japanese multicenter registry. It reported no significant difference in the cumulative incidence of major adverse cardiac and cerebrovascular events and death from hospital discharge to 1 year or from 1 to 2 years between STEMI and NSTE-ACS.15
However, NSTE-ACS in the PACIFIC registry included cTn-negative UA. The J-MINUET registry is the latest multicenter registry of Japanese patients with acute MI diagnosed by the universal definition. We showed that long-term outcomes of NSTEMI were worse than for STEMI in Japanese patients, consistent with patients in other Western countries.
More importantly, no previous study has investigated long-term outcomes of NSTEMI patients in whom only cTn but not CK was elevated. The current study first demonstrated that not only NSTEMI+CK but also NSTEMI-CK was associated with worse long-term outcomes than STEMI. Although event rates from 0 to 30 days were lower in NSTEMI-CK than STEMI, the interval incidence of the primary endpoint was doubled for NSTEMI-CK thereafter. Kaplan-Meier curves of NSTEMI-CK and of STEMI crossed during follow-up. Compared with NSTEMI+CK, the event rate of NSTEMI-CK was lower during the first 30 days but the Kaplan-Meier curves became parallel thereafter. Landmark analyses clearly showed that Kaplan-Meier curves from 31 days to 3 years for the primary endpoint were mostly identical between NSTEMI+CK and NSTEM-CK. These findings provide a clinical rationale for the cTn-based criteria of MI (universal definition).
Elevation of CK is a measure of extensive myocardial damage and is an important determinant of short-term outcomes. However, long-term outcomes after convalescence were as poor for NSTEMI-CK as for NSTEMI+CK and worse than for STEMI. Although the incidence of cardiac failure was higher in NSTEMI+CK than in NSTEMI-CK, patients with NSTEMI-CK suffered from more non-fatal MI during follow-up. Extensive myocardial damage increases the risk of cardiac failure after NSTEMI+CK, whereas a residue of viable myocardium may be associated with a higher incidence of non-fatal MI after NSTEMI-CK.
Although CK elevation was associated with poor short-term outcome, factors other than CK elevation played important roles in long-term outcomes. Compared with STEMI, the incidence of urgent revascularization for UA was almost doubled after NSTEMI in both patients with and without CK elevation. These findings suggested that NSTEMI+CK and NSTEMI-CK have a common background that is different from that of STEMI. First, patients with NSTEMI have more comorbid factors than STEMI patients.6
Multivariate analysis showed that NSTEMI was independently associated with worse long-term outcomes. However, the severity, duration, management and multiplication of these risk factors could not be fully adjusted. Second, patients with NSTEMI have more extensive coronary artery disease.6,13
Asakura et al16
studied patients who underwent coronary angioscopy in all 3 coronary arteries at 1 month after MI. They showed all 3 major coronary arteries, both infarct-related and non-related arteries, were widely diseased and had multiple yellow plaques, suggesting a pan-coronary process of vulnerable plaque development in patients with MI. It has been reported that patients with multiple yellow plaques have a higher risk of ACS.17
Using intracoronary imaging, it has been reported that coronary lesions are qualitatively and quantitatively different between STEMI and NSTEMI,18
which is one of the possible explanations for the higher incidence of coronary events after NSTEMI.
Reflecting the higher incidence of concomitant disease and previous history of cardiovascular disease, patients with NSTEMI were taking more cardioprotective drugs, including antiplatelet agents, β-blockers, angiotensin-converting enzyme inhibitors and statins, before the onset of MI. At the time of hospital discharge, however, the prescription rate was significantly lower for patients with NSTEMI than for those with STEMI. Because the short-term outcomes of patients with NSTEMI-CK were favorable, physicians might have paid less attention to the post-hospital management. Intensive secondary prevention at least equal to that applied to STEMI patients is imperative for patients with NSTEMI, regardless of CK elevation.
In this study, the proportion of NSTEMI was 31%, which was lower than in recent reports from other Western countries including the USA, but consistent with previous studies from Japan.15,19,20
In the early 1990s, the proportion of NSTEMI was less than 30% in the USA. Over the past 2 decades, the incidence of MI has consistently declined.21,22
It was more obvious for STEMI, while the incidence of NSTEMI has transiently increased since the introduction of cTn-based criteria. During the same period, there was a trend of steady increase of MI in Japan, presumably caused by rapid westernization of lifestyle.23,24
This is one possible explanation for the higher proportion of STEMI in this study. However, it is noteworthy that the patients with MI received contemporary management. Primary PCI was performed for 93.1% of patients with STEMI and urgent coronary angiography for 84.7% of patients with NSTEMI. Medication, including aspirin, P2Y12 inhibitors, β-blockers, angiotensin-converting enzyme inhibitors and statins, were prescribed for most of the patients.
Study Limitations
This study has several limitations. Because only patients who were admitted within 48 h of MI onset were enrolled, patients who came to hospital after 48 h were excluded, which may be in part attributable for the lower incidence of NSTEMI patients. Participating institutions are regional core centers capable of advanced medical management. There might have been a selection bias for enrolled patients. Because this was a multicenter study that investigated clinical practice for Japanese patients with acute MI diagnosed by the universal definition, the frequency and timing of cTn and CK measurements were not prespecified but left to the physicians’ decision.
Conclusions
NSTEMI patients without CK elevation had favorable short-term outcomes compared with STEMI patients or NSTEMI patients with CK elevation. However, long-term outcomes after convalescence were as poor for NSTEMI without CK elevation as for NSTEMI with CK elevation, and worse than for STEMI. These findings provide a clinical rationale for the cTn-based criteria of MI (universal definition). Intensive secondary prevention no less than for STEMI is imperative for patients with NSTEMI, regardless of CK elevation.
Acknowledgments
The authors thank all the enrolled patients, participating cardiologists, medical and other staff who contributed to this study.
This work was supported by the Intramural Research Fund, grant number 23-4-5, for Cardiovascular Diseases of the National Cerebral and Cardiovascular Center.
Disclosures
Masaharu Ishihara received lecture fees from Bayer, MSD and Astra Zeneka, and a scholarship fund from Abbott Vascular, Boston Scientific, Sanofi, MSD, Astellas, Bayer, Pfizer, Daiichi Sankyo, MID and Goodman.
Appendix
J-MINUET Investigators
Masaharu Ishihara, Hyogo College of Medicine (Chairperson); Hisao Ogawa, National Cerebral and Cardiovascular Center; Nobuaki Kokubu, Sapporo Medical University; Tadaya Sato, Akita Medical Center; Teruo Inoue, Dokkyo Medical University; Shigeru Oshima, Gunma Prefectural Cardiovascular Center; Hiroshi Funayama, Saitama Medical Center Jichi Medical University; Ken Kozuma, Hiroyuki Kyono, Teikyo University; Wataru Shimizu, Nippon Medical School; Satoru Suwa, Juntendo University Shizuoka Hospital; Kengo Tanabe, Mitsui Memorial Hospital; Tetsuya Tobaru, Sakakibara Heart Institute; Kazuo Kimura, Yokohama City University Medical Center; Junya Ako, Kitasato University; Mafumi Owa, Suwa Red Cross Hospital; Takahito Sone, Yasuhiro Morita, Ogaki Municipal Hospital; Yukio Ozaki, Fujita Health University; Satoshi Yasuda, Teruo Noguchi, Masashi Fujino, Yoshihiro Miyamoto, Kunihiko Nishimura, National Cerebral and Cardiovascular Center; Junichi Kotani, Osaka University Graduate School of Medicine; Takashi Morita, Osaka General Medical Center; Atsunori Okamura, Sakurabashi Watanabe Hospital; Yoshihiko Saito, Hiroyuki Okura, Nara Medical University; Masaaki Uematsu, Kansai Rosai Hospital; Shirou Uemura, Kawasaki Medical School; Atsushi Hirohata, The Sakakibara Heart Institute of Okayama; Yasuharu Nakama, Hiroshima City Hospital; Keijiro Saku, Fukuoka University School of Medicine; Kenichi Tsujita, Graduate School of Medical Sciences, Kumamoto University; Koichi Nakao, Saiseikai Kumamoto Hospital Cardiovascular Center; Kazuteru Fujimoto, National Hospital Organization Kumamoto Medical Center; Yoshisato Shibata, Miyazaki Medical Association Hospital; Kazuhito Hirata, Okinawa Chubu Hospital.
Supplementary Files
Supplementary File 1
Figure S1.
Interval incidence of the primary endpoint shown as 30 days to half-year and each half-year thereafter.
Table S1.
Three-year incidence of each component of the endpoints in J-MINUET Study
Please find supplementary file(s);
http://dx.doi.org/10.1253/circj.CJ-17-0033
References
- 1.
Braunwald E, Antman EM, Beasley JW, Califf RM, Cheitlin MD, Hochman JS, et al. ACC/AHA guidelines for the management of patients with unstable angina and non-ST-segment elevation myocardial infarction: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on the Management of Patients With Unstable Angina). J Am Coll Cardiol 2000; 36: 970–1062.
- 2.
The Joint European Society of Cardiology/American College of Cardiology Committee. Myocardial infarction redefined: A consensus document of the Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. J Am Coll Cardiol 2000; 36: 959–969.
- 3.
Rogers WJ, Frederick PD, Stoehr E, Canto JG, Ornato JP, Gibson CM, et al. Trends in presenting characteristics and hospital mortality among patients with ST elevation and non-ST elevation myocardial infarction in the National Registry of Myocardial Infarction from 1990 to 2006. Am Heart J 2008; 156: 1026–1034.
- 4.
Roger VL, Weston SA, Gerber Y, Killian JM, Dunlay SM, Jaffe AS, et al. Trends in incidence, severity, and outcome of hospitalized myocardial infarction. Circulation 2010; 121: 863–869.
- 5.
D’Souza M, Sarkisian L, Saaby L, Poulsen TS, Gerke O, Larsen TB, et al. Diagnosis of unstable angina pectoris has declined markedly with the advent of more sensitive troponin assays. Am J Med 2015; 128: 852–860.
- 6.
Ishihara M, Fujino M, Ogawa H, Yasuda S, Noguchi T, Nakao K, et al; J-MINUET investigators. Clinical presentation, management and outcome of Japanese patients with acute myocardial infarction in the troponin era: Japanese registry of acute myocardial infarction diagnosed by universal definition (J-MINUET). Circ J 2015; 79: 1255–1262.
- 7.
Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD; Writing Group on behalf of the Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction. Third universal definition of myocardial infarction. J Am Coll Cardiol 2012; 60: 1581–1598.
- 8.
Little RJA, Rubin DB. Likelihood-based approaches to the analysis of missing data: Applications to some common models. In: Statistical analysis with missing data, 2nd edn. New Jersey: Wiley-InterScience, 2002; 65–83.
- 9.
Rubin, DB. Inference and missing data. Biometrika 1972; 63: 581–592.
- 10.
Nomenclature and criteria for diagnosis of ischemic heart disease. Report of the Joint International Society and Federation of Cardiology/World Health Organization task force on standardization of clinical nomenclature. Circulation 1979; 59: 607–609.
- 11.
Steg PG, Goldberg RJ, Gore JM, Fox KA, Eagle KA, Flather MD, et al; GRACE Investigators. Baseline characteristics, management practices, and in-hospital outcomes of patients hospitalized with acute coronary syndromes in the Global Registry of Acute Coronary Events (GRACE). Am J Cardiol 2002; 90: 358–363.
- 12.
Abbott JD, Ahmed HN, Vlachos HA, Selzer F, Williams DO. Comparison of outcome in patients with ST-elevation versus non-ST-elevation acute myocardial infarction treated with percutaneous coronary intervention (from the National Heart, Lung, and Blood Institute Dynamic Registry). Am J Cardiol 2007; 100: 190–195.
- 13.
Chan MY, Sun JL, Newby LK, Shaw LK, Lin M, Peterson ED, et al. Long-term mortality of patients undergoing cardiac catheterization for ST-elevation and non-ST-elevation myocardial infarction. Circulation 2009; 119: 3110–3117.
- 14.
McManus DD, Gore J, Yarzebski J, Spencer F, Lessard D, Goldberg RJ. Recent trends in the incidence, treatment, and outcomes of patients with STEMI and NSTEMI. Am J Med 2011; 124: 40–47.
- 15.
Daida H, Miyauchi K, Ogawa H, Yokoi H, Matsumoto M, Kitakaze M, et al; PACIFIC investigators. Management and 2-year long-term clinical outcome of acute coronary syndrome in Japan: Prevention of atherothrombotic incidents following ischemic coronary attack (PACIFIC) registry. Circ J 2013; 77: 934–943.
- 16.
Asakura M, Ueda Y, Yamaguchi O, Adachi T, Hirayama A, Hori M, et al. Extensive development of vulnerable plaques as a pan-coronary process in patients with myocardial infarction: An angioscopic study. J Am Coll Cardiol 2001; 37: 1284–1288.
- 17.
Ohtani T, Ueda Y, Mizote I, Oyabu J, Okada K, Hirayama A, et al. Number of yellow plaques detected in a coronary artery is associated with future risk of acute coronary syndrome: Detection of vulnerable patients by angioscopy. J Am Coll Cardiol 2006; 47: 2194–2200.
- 18.
Lee SY, Mintz GS, Kim SY, Hong YJ, Kim SW, Okabe T, et al. Attenuated plaque detected by intravascular ultrasound: Clinical, angiographic, and morphologic features and post-percutaneous coronary intervention complications in patients with acute coronary syndromes. JACC Cardiovasc Interv 2009; 2: 65–72.
- 19.
Ogawa H, Kojima S. Modern state of acute myocardial infarction in the interventional era: Observational case–control study: Japanese Acute Coronary Syndrome Study (JACSS). J Cardiol 2009; 54: 1–9.
- 20.
Yamamoto T, Yasutake M, Takagi H, Akutsu K, Fujita N, Kasagami Y, et al. Impact of the revised criteria for acute myocardial infarction using cardiac troponins in a Japanese population with acute coronary syndromes. Circ J 2005; 69: 774–779.
- 21.
Yeh RW, Sidney S, Chandra M, Sorel M, Selby JV, Go AS. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med 2010; 362: 2155–2165.
- 22.
Rosamond WD, Chambless LE, Heiss G, Mosley TH, Coresh J, Whitsel E, et al. Twenty-two-year trends in incidence of myocardial infarction, coronary heart disease mortality, and case fatality in 4 US communities, 1987–2008. Circulation 2012; 125: 1848–1857.
- 23.
Takii T, Yasuda S, Takahashi J, Ito K, Shiba N, Shirato K, et al. Trends in acute myocardial infarction incidence and mortality over 30 years in Japan: Report from the MIYAGI-AMI Registry Study. Circ J 2010; 74: 93–100.
- 24.
Hata J, Ninomiya T, Hirakawa Y, Nagata M, Mukai N, Gotoh S, et al. Secular trends in cardiovascular disease and its risk factors in Japanese: Half-century data from the Hisayama Study (1961–2009). Circulation 2013; 128: 1198–1205.





