2017 Volume 81 Issue 7 Pages 920-928
2017 Volume 81 Issue 7 Pages 920-928
Cardiovascular disease (CVD) is the greatest cause of death, accounting for nearly one-third of all deaths worldwide. The increase in obesity rates over 3 decades is widespread and threatens the public health in both developed and developing countries. Obesity, the excessive accumulation of visceral fat, causes the clustering of metabolic disorders, such as type 2 diabetes, dyslipidemia, and hypertension, culminating in the development of CVD. Adipose tissue is not only an energy storage organ, but an active endocrine tissue producing various biologically active proteins known as adipokines. Since leptin, a central regulator of food intake and energy expenditure, was demonstrated to be an adipose-specific adipokine, attention has focused on the identification and characterization of unknown adipokines to clarify the mechanisms underlying obesity-related disorders. Numerous adipokines have been identified in the past 2 decades; most adipokines are upregulated in the obese state. Adipokines such as tumor necrosis factor (TNF)-α, interleukin (IL)-6, IL-1β, and resistin are pro-inflammatory, and exacerbate various metabolic and cardiovascular diseases. However, a small number of adipokines, including adiponectin, are decreased by obesity, and generally exhibit antiinflammatory properties and protective functions against obesity-related diseases. Collectively, an imbalance in the production of pro- and antiinflammatory adipokines in the obese condition results in multiple complications. In this review, we focus on the pathophysiologic roles of adipokines with cardiovascular protective properties.
Cardiovascular disease (CVD) is the greatest cause of death, accounting for nearly one-third of all deaths worldwide.1–3 The increase in obesity rates over 3 decades is widespread and threatens the public health in both developed and developing countries.4 Obesity, the excessive accumulation of visceral fat, causes the clustering of metabolic disorders, such as type 2 diabetes, dyslipidemia, and hypertension, culminating in the development of CVD.5 Recent evidence indicates that obese states (e.g., excess adiposity, adipocyte dysfunction) cause chronic low-grade inflammation, which contributes to the development of obesity-linked disorders, including insulin resistance and atherosclerosis.6,7
Adipose tissue is not only an energy storage organ, but an active endocrine tissue producing various biologically active proteins known as adipokines (also known as adipocytokines).6 The term “adipokine” refers to any bioactive product of adipose tissue origin. Since leptin, a central regulator of food intake and energy expenditure, was demonstrated to be an adipose-specific adipokine, great attention has been given to the identification and characterization of unknown adipokines to clarify the mechanisms underlying obesity-related disorders.8 Numerous adipokines have been identified in the past 2 decades; most adipokines are upregulated in the obese state.9 Adipokines such as tumor necrosis factor (TNF)-α, interleukin (IL)-6, IL-1β, and resistin are pro-inflammatory, and exacerbate various metabolic and cardiovascular diseases. However, a small number of adipokines, including adiponectin (APN), are decreased by obesity, and generally exhibit antiinflammatory properties and protective functions against obesity-related diseases.6 Collectively, an imbalance in the production of pro- and antiinflammatory adipokines in the obese conditions results in multiple complications. In this review, we focus on the pathophysiologic roles of adipokines with cardiovascular protective properties.
APN is the most abundantly expressed adipokine of adipose origin, identified by systematic analysis of adipose-expressed genes.10 APN regulates cellular function via activation of its specific receptors, APN receptor 1 (AdipoR1) and APN receptor 2 (AdipoR2), each encoded by their respectively named genes. The APN receptors contain 7 transmembrane domains topologically distinct from G protein-coupled receptor proteins. APN binds to the C-terminal extracellular domain of the APN receptor, whereas the N-terminal cytoplasmic domain interacts with an adaptor protein (phosphotyrosine interacting with PH domain and leucine zipper 1, APPL1). Whereas AdipoR1 is abundantly expressed in muscular tissues (including the heart) and endothelial cells, AdipoR2 is predominantly expressed in the liver.11 As such, the relevant importance of AdipoR1 vs. AdipoR2 concerning APN’s biologic effects is largely determined by their expression profile in different tissues.
Abundantly present in human plasma, APN plasma levels negatively correlate with body mass index (BMI).12,13 The plasma APN concentration is significantly lower in coronary artery disease (CAD) patients compared with healthy subjects. Moreover, hypoadiponectinemia (plasma APN <4.0 µg/mL) is significantly associated with CAD prevalence, even after adjustment for well-known CAD risk factors such as diabetes mellitus (DM), dyslipidemia, hypertension, tobacco use, and BMI in male Japanese subjects.14 In prospective studies, high plasma APN levels have predicted lower risk of acute myocardial infarction (MI) in subjects with CAD and type 2 DM.15,16 Hypoadiponectinemia is also associated with complexity of coronary lesions in the patients with stable CAD.17,18 Consistently, higher APN levels are significantly associated with lower risk of CAD in the Framingham Offspring Study.19 Thus, circulating APN levels may be a valuable biomarker of CAD prevalence. In contrast, several reports indicated no association between APN concentration and CAD prevalence.20–22 The APN level is not a predictor of CAD in the American Indian population.21 High APN levels are only associated with reduced risk of non-fatal MI in men, and no association has been observed between APN levels and fatal coronary events in both men and women.20 A meta-analysis indicated that the association of APN level with CAD risk is comparatively weak.22 These inconsistent results might stem from differences in the study populations (e.g., sex, race, comorbidities). Therefore, further studies are warranted to elucidate the significance of APN as a CAD biomarker.
Experimental studies have indicated that APN modulates various vascular disorders. Adenovirus-mediated APN overexpression reduces atherosclerotic lesion formation in atherosclerosis-prone apolipoprotein E (ApoE) knockout (KO) mice, which is accompanied by decreased expression levels of TNF-α and scavenger receptor (SR)-A.23 Furthermore, ApoE/APN double-KO mice exhibit more severe atherosclerosis progression (accompanied by increased T-lymphocyte accumulation in atherosclerotic lesions) compared with ApoE-single KO mice.24 Additionally, APN knockout (APN-KO) mice demonstrate enhanced neointimal formation after vascular wire injury.25 APN-KO mice fed an atherogenic diet manifest reduced endothelium-dependent vasodilation in aortic ring samples.26 APN-deficiency leads to salt-sensitive hypertension, whereas APN administration reduces increased blood pressure in obese mice.27 APN is detected on the endothelial surface of the normal aorta of wild-type mice; it is present on endothelial cells, synthetic smooth muscle cells, and monocytes adherent to endothelial cells.28 By associating with the tethering molecule T-cadherin, APN accumulates in the vascular wall.29 These in vivo data suggest that APN protects against various obesity-inducible vascular diseases such as atherosclerosis, vascular dysfunction, and hypertension by its interaction with vascular component cells.
In vitro, treatment with APN protein attenuates lipopolysaccharide (LPS)-induced expression of TNF-α in cultured macrophages via inhibition of nuclear factor (NF)-κB signaling (Figure 1). APN treatment also reduces SR-A expression and prevents transformation of macrophages to foam cells30 (Figure 1). APN increases the tissue inhibitor of metalloproteinase-1 via IL-10 expression in human monocyte-derived macrophages, thereby contributing to stabilization of atherosclerotic plaques.31 Additionally, APN promotes the polarization of macrophages to their antiinflammatory M2 state32,33 (Figure 1). Collectively, APN modulates the phenotype and function of macrophages in its target organs, thereby attenuating the vascular inflammatory response.
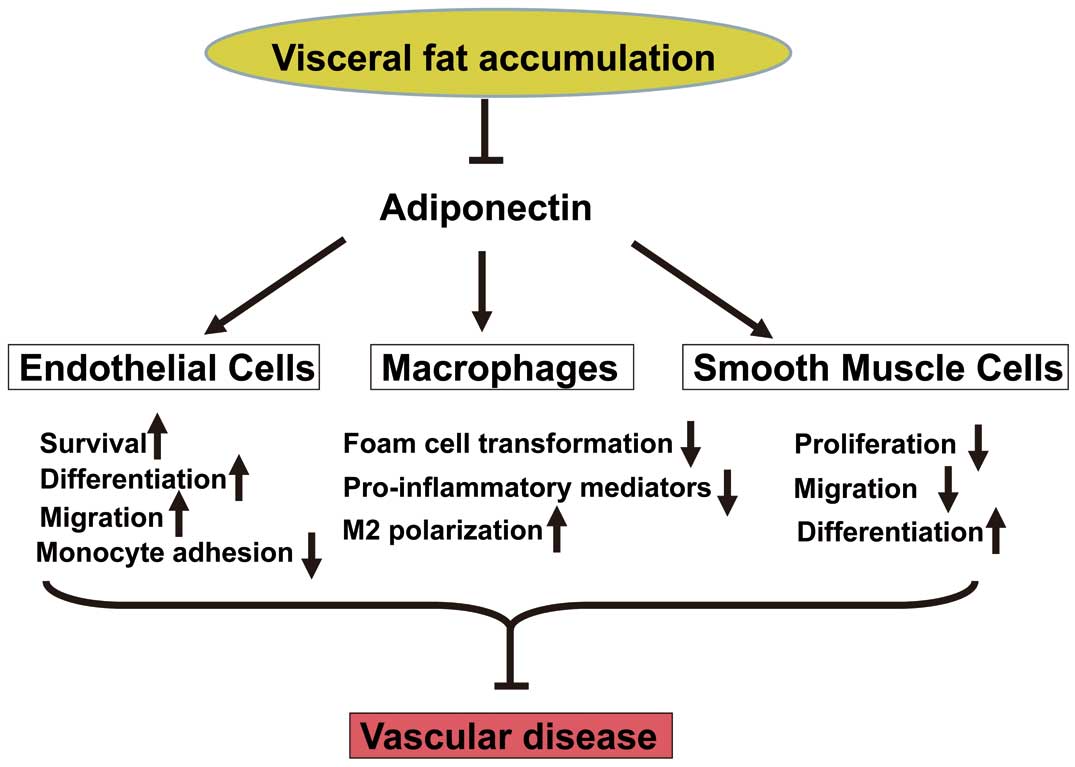
Protective functions of adiponectin in obesity-related vascular disease. Adiponectin has beneficial effects in vascular disease by directly acting on vascular component cells such as endothelial cells, macrophages, and smooth muscle cells. Adiponectin promotes endothelial cell survival, differentiation and migration. Adiponectin suppresses monocyte adhesion to endothelial cells. In macrophages, adiponectin reduces foam cell formation and the inflammatory response. Adiponectin promotes the polarization of macrophages toward antiinflammatory M2. Adiponectin decreases the proliferation and migration, and increases the differentiation of vascular smooth muscle cells.
Treatment of endothelial cells with APN promotes differentiation into capillary-like structures and migration, and reduces apoptosis under conditions of serum starvation partly via the AMP-activated protein kinase (AMPK)-eNOS-dependent pathway34,35 (Figure 1). APN also enhances endothelial cell differentiation, migration, and survival through a cyclooxygenase (COX)-2 dependent mechanism.36 APN inhibits monocyte adhesion to TNF-α-stimulated endothelial cells37 (Figure 1). It reduces TNF-α-stimulated expression of vascular cell adhesion molecule (VCAM)-1 in cultured endothelial cells via cyclic AMP (cAMP)/protein kinase A (PKA) signal-mediated inhibition of NF-κB activation.38 APN also inhibits the TNF-α-stimulated inflammatory response in cultured endothelial cells by recruitment and activation of ceramidase in an AdipoR1/caveolin-1 dependent manner.39 Furthermore, APN suppresses reactive oxygen species production stimulated by high glucose in cultured endothelial cells by a cAMP/PKA pathway.40 As outlined above, APN promotes endothelial cell health, attenuates endothelial inflammation, and ultimately inhibits atherogenesis initiation.
Treatment of vascular smooth muscle cells with APN attenuates growth factor induced-proliferation and migration by inhibiting extracellular signal-regulated kinase activation41 (Figure 1). APN reduces cytokine-stimulated expression of heparin-binding EGF-like growth factor (HB-EGF) in endothelial cells.25 Moreover, APN promotes vascular smooth muscle cell differentiation via AMPK-mediated inhibition of mammalian target of rapamycin complex 142 (Figure 1). Collectively, APN prevents atherosclerosis progression by directly affecting the behavior of vascular component cells, including macrophages, endothelial cells, and smooth muscle cells. APN therefore represents a target molecule for the prevention or treatment of atherosclerotic disease.
CTRP FamilyIn 2004, a highly conserved family of APN paralogs, designated C1q/TNF-related proteins (CTRPs), was reported.43 Each of the 15 known members (CTRP1–CTRP15) consists of 4 distinct domains, comprising an N-terminal signal peptide, a short variable domain, a collagen-like domain, and a C-terminal C1q-like globular domain.43,44 Both CTRPs and APN belong to the C1q/TNF protein superfamily. Investigated for its structural similarity to APN, the CTRP family members exhibit broadly diverse functions.
CTRP9CTRP9 shares the greatest amino acid overlap with APN in its globular C1q domain, sharing 51% amino acid identity with the globular domain of APN.45 Plasma CTRP9 levels are decreased in rodent models of obesity.46 Interestingly, CTRP9 forms heterotrimers with APN, and appears to share the AdipoR1 in both cultured endothelial cells and cardiomyocytes.45–47 CTRP9 stimulates eNOS phosphorylation and nitric oxide production in endothelial cells via AdipoR1-dependent activation of AMPK, and enhances endothelium-dependent vasorelaxation of aortic rings47 (Figure 2). CTRP9 attenuates inflammatory responses to TNF-α in cultured endothelial cells via AMPK activation48 (Figure 2). CTRP9 also reduces high glucose-inducible oxidative damage in cultured endothelial cells through the AdipoR1-dependent pathway49 (Figure 2). CTRP9 therefore serves a protective function in endothelial cells. Furthermore, systemic delivery of CTRP9 via adenoviral vector expression system reduced neointimal formation in a mouse model of vascular injury. CTRP9 reduces the proliferation of cultured vascular smooth muscle cells via the cAMP/PKA-dependent pathway (Figure 2). Of importance, CTRP9 exerts an antiproliferative effect upon vascular smooth muscle cells, which is independent of AdipoR1 or AMPK. CTRP9 reduces the inflammatory response to oxidized low-density lipoprotein in cultured macrophages in an AMPK-dependent mechanism.50 Furthermore, administration of CTRP9 by the lentiviral vector system reduced the macrophage and lipid content of carotid plaques in ApoE-KO mice, indicating CTRP9 may be an antiatherogenic factor.51 Taken together, it is likely CTRP9 possesses vascular protective effects, but future studies using transgenic animal models are necessary to dissect the role endogenous CTRP9 plays in vascular disease.

Vascular protection by CTRP9. CTRP9 promotes the activation of AMP-activated protein kinase (AMPK) and endothelial nitric oxide synthetase (eNOS) in endothelial cells via adiponectin receptor 1 (AdipoR1)-dependent signaling, enhancing endothelium-dependent vasorelaxation and reducing endothelial inflammation and damage. CTRP9 decreases the proliferation of vascular smooth muscle cells via cAMP/PKA signaling, independent of AdipoR1. PKA, protein kinase A.
Omentin-1 was originally identified as a soluble galactofuranose-binding lectin.52 Omentin-1 is abundantly expressed in human visceral fat, and its circulating concentration is decreased in obese subjects.53 Furthermore, plasma omentin-1 levels negatively correlate with carotid intima media thickness, an established marker of early atherosclerosis in healthy men and type 2 DM patients.54,55 Omentin-1 levels are negatively associated with the prevalence and angiographic severity of CAD in patients with metabolic syndrome.56 Furthermore, serum omentin-1 levels are lower in patients with acute coronary syndrome than in subjects with stable angina pectoris.57 These findings support the utility of omentin-1 as a biomarker of CAD.
In experimental studies, omentin-1 exerted protective functions against vascular diseases. Omentin-1 transgenic (OMT-Tg) mice on an ApoE-KO background (ApoE-KO/OMT-Tg mice) exhibited attenuated atherosclerotic areas in the aortic sinus compared with ApoE-single KO mice.58 ApoE-KO/OMT-Tg mice manifested significantly reduced macrophage infiltration and expression of pro-inflammatory cytokines (such as TNF-α, IL-6 and monocyte chemoattractant protein 1) in the aorta compared with ApoE-KO mice. In human monocyte-derived macrophages, omentin-1 protein significantly reduced lipid droplets and cholesteryl ester contents. Omentin-1 also reduced LPS-induced expression of pro-inflammatory genes (such as TNF-α) in macrophages via Akt activation (Figure 3). Consistently, administration of omentin-1 in ApoE-KO mice decreased atherosclerosis.59 Omentin-1 treatment also enhanced vasodilation in isolated aortic rings of mice in an eNOS-dependent pathway60 (Figure 3). Omentin-1 enhances endothelial cell differentiation into capillary-like structures, and decreases endothelial cell apoptosis in an AMPK/eNOS-dependent fashion (Figure 3). Systemic delivery of omentin-1 promoted blood flow recovery and capillary formation in a mouse model of vascular insufficiency via eNOS signaling.61 Omentin-1 has also inhibited TNF-α-induced expression of intracellular adhesion molecule-1 and VCAM-1 in cultured endothelial cells.62 Thus, omentin-1 exerts salutary effects on endothelial cell function and activation. Furthermore, systemic delivery of omentin-1 attenuated neointimal thickening and vascular cell proliferation in the injured arteries of mice.63 Omentin-1 reduces the growth of vascular smooth muscle cells in an AMPK-dependent mechanism (Figure 3). Transgenic overexpression of omentin-1 reduces neointimal formation in response to injury, accompanied by attenuated inflammatory response in injured vessels. Taken together, the evidence shows that omentin-1 functions as an adipokine with antiatherogenic and antiinflammatory properties, similar to both APN and CTRP9.

Protective effects of omentin-1 in atherosclerosis. Omentin-1 promotes endothelium-dependent vasorelaxation and endothelial cell survival via AMPK/eNOS-dependent signaling. Omentin-1 suppresses the inflammatory response of macrophages by Akt activation. Omentin-1 reduces the proliferation of vascular smooth muscle cells via AMPK signaling. Abbreviations as in Figure 2.
Clinical studies have demonstrated significantly reduced APN levels in type 2 diabetic patients.64 Furthermore, total plasma APN concentrations are inversely correlated with MI risk.14,15 In 2002, the metabolic effects of an APN-deficient state in mice were reported (delayed clearance of free fatty acid in plasma, decreased muscle fatty acid-transport protein-1 expression, and increased TNF-α concentration).65 In 2005, exacerbated myocardial ischemic injury was reported in APN-KO animals, the first direct evidence that APN was an intrinsic protein protecting the heart against ischemia/reperfusion (I/R) injury.66 Since then, numerous animal experiments have demonstrated that exogenous recombinant APN significantly protects the heart against I/R injury.67,68
Mechanistic studies have been performed to determine the manner by which APN is cardioprotective against I/R injury. APN exerts its metabolic-regulating, antiinflammatory, and vasculoprotective actions largely through AMP-dependent protein kinase (AMPK),69 a serine-threonine kinase that acts as an energy sensor that can be activated by hypoxic stress.70 APN exerts its cardioprotective effect via several important signaling pathways. APN increases nitric oxide (NO) production by activation of the AMPK-Akt-eNOS phosphorylation pathway.35,71 APN is also cardioprotective by inhibiting both oxidative stress (by decreasing gp91phox overexpression in I/R cardiac tissue) and nitrative stress (by preventing NO overproduction by inhibiting iNOS expression during pathologic states).67 Furthermore, this antioxidative/antinitrative cardioprotective effect of APN is largely AMPK independent,72,73 and largely mediated via PKA-dependent NFκB inhibition.74
Despite being traditionally viewed as an adipocyte-specific endocrine molecule with cardioprotective effects, APN is also expressed locally in adult cardiomyocytes, its production increased by activation of peroxisome proliferator-activated receptor-γ (PPARγ).75–77 Moreover, cardiomyocyte-derived APN is biologically active in protecting against myocardial I/R injury, achieving the protective effect primarily via paracrine/autocrine activation of APN receptors.78 Additionally, in a related fashion, the cardioprotective effects of PPAR-γ agonists are critically dependent on intact APN expression. PPARγ agonists improve insulin sensitivity by upregulating APN expression.79 During pathologic conditions in which plasma APN levels are markedly reduced and/or the APN stimulatory action of PPAR-γ agonists is impaired (e.g., advanced DM or elderly DM patients), PPAR-γ agonists may upregulate superoxide production, culminating in unfavorable cardiovascular outcomes.80
In a recent study investigating the direct mechanisms underlying both APN signaling and APN resistance, GRK2 phosphorylated APN receptor 1 post-MI impaired the cardioprotective functions of APN.81 Inhibition of GRK2-mediated AdipoR1 phosphorylation represents a completely novel manner of restoring APN cardioprotective signaling in the post-MI injury period.
Adiponectin, Heart Disease, and DMIschemic heart disease (IHD) is the leading cause of death in patients with DM. Hyperglycemia and hyperlipidemia directly adversely effect ischemic cardiomyocytes, initiating larger infarct size and more severe heart failure after myocardial ischemia.82 Significant research has been done to find the molecular basis linking DM and IHD to identify modalities that may decrease cardiovascular mortality in diabetic patients. Hypoadiponectinemia in type-2 diabetic patients contributes to increased cardiomyocyte death and the patient’s death after MI.83 Significant work elucidating the transmembrane signaling mechanisms responsible for APN’s cardiomyocyte-protective effect has yielded important information. A signaling complex has been demonstrated to be essential for the anti-ischemic/cardioprotective effects of APN (Figure 4).39 It is composed of the structural protein caveolin (Cav), AdipoR1, APPL1 (the most important molecule in APN’s AMPK-dependent signaling), and adenylate cyclase and neutral ceramidase (2 important molecules in APN’s AMPK-independent signaling).84 The interaction between AdipoR1 and caveolin is required for proper APN-mediated signaling. Treatment with high glucose/high lipid disrupts the AdipoR1/Cav signalosome, leading to APN resistance.85

AMPK-dependent and AMPK-independent signaling pathways mediating adiponectin cardiac protetction. Adipoenctin protects the heart via the AMPK-mediated Akt-eNOS-NO pathway and PPARγ-adiponectin pathway. However, the antiinflammatory and antiapoptotic effects of adiponectin are largely AMPK-independent, and are mediated primarily by nCeramidase activation and reactive oxidative/nitrative stress suppression. iNOS, inducibe nitric oxide synthase; PPARγ, peroxisome proliferator-activated receptor-γ. Other abbreviations as in Figure 2.
The diabetic condition itself may cause cardiac APN resistance, diminishing the cardioprotective effects of APN. High glucose/high lipid treatments impair APN activation of AMPK-dependent ACC (Acetyl-CoA Carboxylase) signaling, as well as AMPK-independent antioxidative/antinitrative protection, severely inhibiting the cardioprotective effect of APN.84
As mentioned before, the efficacy of recombinant exogenous APN in ameliorating myocardial I/R disease has been demonstrated in multiple animal studies. The practical clinical application of exogenous recombinant APN is limited for multiple reasons such as APN’s complex quaternary structure, rapid turnover, and the high cost of production to generate the amounts required for clinical care. The recently discovered APN receptor agonist, AdipoRon, is a synthetic molecule that is orally active, binds both APN receptors 1 and 2, ameliorates insulin resistance and type 2 DM, and prolongs the shortened lifespan of genetic diabetic mice.86 AdipoRon attenuates post-ischemic cardiac injury and possesses potent antinitrative and antioxidative effects, and may represent a novel therapeutic approach to treating cardiovascular complications from obesity-related disorders such as DM.87 In a recent work by Wang and colleagues, AdipoRon restored deranged autophagic flux in the diabetic state. Hypoadiponectinemia impairs autophagic flux, which contributes to enhanced myocardial I/R injury in the diabetic state. APN receptor activation by AdipoRon restored PRKAA-mediated autophagosome formation and antioxidant-mediated autophagosome clearance, a novel intervention effective against myocardial I/R injury in the diabetic condition (accepted, but yet unpublished data at the time of writing).
CTRP3CTRP3 (also known as cartducin) is a growth plate secretory protein of primarily cartilaginous origin, but monocytic and osteosarcoma cells also secrete it.88 Initially identified during a search for genes responsible for chondrocyte differentiation induction, CTRP3 regulates APN secretion from adipocytes.89,90 CTRP3 additionally regulates glucose homeostasis,91 and stimulates in vitro endothelial cell proliferation and migration.92 MI decreases the circulating levels of CTRP3. CTRP3 replenishment improves survival and restores cardiac function after MI and attenuates post-ischemic pathologic remodeling (evidenced by echocardiographic and histologic parameters).93 Preventing post-MI CTRP3 inhibition or CTRP3 supplementation may aid in the restoration of cardiac function and mitigate heart failure sequelae (Figure 5).

Major cardioprotective CTRPs. A total of 15 CTRPs have been identified to date. It is now clear that different CTRP members have distinct functions, some of which are completely biologically opposite. CTRP3, CTRP6, and CTRP9 have been demonstrated to be cardioprotective by multiple investigators utilizing various animal models. AMPK, AMP-activated protein kinase; CF, cardiac fibroblast; CTRP, C1q/TNF-related protein; ECM, extracellular matrix; TNF, tumor necrosis factor.
Expressed in adipose tissue, brain, heart, and placenta, the adipokine CTRP6 regulates metabolism and inflammation.94,95 Additionally, CTRP6 improves cardiac function and ameliorates ventricular remodeling post-MI, protecting against cardiac fibrosis by inhibiting myofibroblast differentiation, extracellular matrix production, and cardiac fibroblast migration via Smad-independent RhoA/MRTF-A (myocardin-related transcription factor-A) signaling (Figure 5).96
CTRP9As mentioned earlier in this review, of the entire known CTRP family, CTRP9 shares the most homology and amino acid overlap (51%) with APN.45 In comparison with its family members, CTRP9 has been the subject of significantly more published research concerning its CVD implications. Similar to APN, total plasma CTRP9 levels are significantly reduced in diabetic animals.97 However, in dissimilar fashion to APN-deficient mice (which lack phenotypic changes under physiologic conditions), CTRP9-deficient mice are obese, insulin resistant, and exhibit hepatic steatosis with reduced skeletal muscle AMPK activation and mitochondrial content.84 Such results are genetic evidence for a physiologic role of CTRP9, suggesting that CTRP9 may play a significant role in cardiovascular homeostasis. Additionally, CTRP9 primarily circulates in the plasma as the gCTRP9 isoform. This is different from APN, which circulates in the plasma as multimers containing full-length APN.98
The cellular secretome’s important influence on the local microenvironment continues to gain recognition.99 The heart itself produces secretomes (known as “cardiokines”), essential regulators of cardiac physiology and pathology.100 CTRP9, unlike APN, is not a typical adipokine and functions as a cardiokine. The initial CTRP tissue distribution study (using semiquantitative PCR) demonstrated that the heart is the third most abundant CTRP9-expressing organ.45 CTRP9 is the most abundantly expressed adipokine in the heart, exceeding local APN expression more than 100-fold, with local cardiac CTRP9 levels exceeding plasma CTRP9 levels more than 2-fold.97
CTRP9 is a potent cardioprotective molecule capable of attenuating acute I/R injury.46,97,98 Additionally, CTRP9 mitigates pathologic ventricular remodeling post-MI and ischemic heart failure in nondiabetic and diabetic animals, largely via a PKA-dependent pathway.101 In the diabetic condition, TNF-α-initiated oxidative PPARγ suppression downregulates CTRP9, which contributes to exacerbated cardiac injury (Figure 5).97
The concept that adipose tissue is a biologically active organ secreting proteins of significant metabolic consequence is a dramatic paradigm shift in the past 2 decades. In this review, we have discussed the pathophysiologic roles of adipokines in both the vascular and cardiac systems. As our understanding of adipokines continues to grow, we continue to discover novel therapeutic modalities mitigating both vascular and cardiac injury exacerbated by metabolic disorders such as type 2 DM, dyslipidemia, and hypertension.
APN is an adipocyte-derived protein with diverse biologic function in multiple organ systems, including vascular tissue and the heart. Moreover, similar to insulin resistance, APN resistance develops in obesity, DM, and heart failure. However, the molecular mechanisms underlying APN resistance development in different cell types under different pathologic conditions require clarification. The underlying molecular mechanisms responsible for APN receptor downregulation (demonstrated in basic and clinical observations) remain unknown. Successful restoration of APN receptor expression may ameliorate the clinical cardiac and vascular complications of I/R injury. Additionally, AdipoR1 phosphorylation inhibits APN signaling, albeit via unknown molecular mechanisms.81 It is important to determine whether pharmacologic or genetic interventions capable of blocking AdipoR1 phosphorylation will re-establish the cardiometabolic regulatory, antiinflammatory, and cardioprotective effects of APN.
The practical clinical application of exogenous recombinant APN is limited by multiple factors discussed in this review. The promising studies discussed in this review concerning AdipoRon, the first orally active APN receptor agonist, may circumvent the limitations of APN for clinical applicability.
The relatively newly discovered CTRP family consists of adipokines with exciting therapeutic potential. Several CTRPs (including CTRP1, CTRP4, CTRP7, and CTRP9) are expressed in the heart at levels significantly greater than APN. CTRP9 remains the most abundantly expressed adipokine in the adult mouse heart. Cardiac-produced CTRP9 levels significantly exceed those in plasma circulation, suggesting that alteration of cardiac-produced CTRP9 may significantly contribute to cardiac pathophysiology.97 The closest APN paralog, CTRP9, shares functions (such as aging and sex regulation) with APN.45 Evidence supports important metabolic,97 vasoprotective,47 and cardioprotective roles for CTRP9 in diabetic97 and nondiabetic46 mice. Preservation of CTRP9 expression or augmentation of CTRP9-initiated signaling mechanisms may be potential avenues of ameliorating ischemic cardiovascular injury in metabolic disorders.
In the past decade, the comprehension of adipokine biology and signaling in the cardiovascular system has seen great advances. Knowledge gaps remain, and represent opportunities for novel work seeking improved therapeutics against the cardiovascular complications of metabolic diseases.
This work was supported by following grants: Grant-in-Aid for Scientific Research (#26293185) and grants from Takeda Science Foundation and the Uehara Memorial Foundation (N.O.), American Diabetes Association 1-14-BS-218 (Y.W.), National Institutes of Health (HL-96686 and HL-123404), and the American Diabetes Association 1-15-BS-122 (X.-L.M.).
Research grants were received from Astellas Pharma Inc., Daiichi Sankyo Co. Ltd. and Takeda Pharma Co. Ltd. (to N.O.). However, the research topics of these grants were not restricted. Lecture fees were received from Kowa Pharmaceutical Co. Ltd. (to N.O.). Molecular Cardiovascular Medicine was endowed by Kowa Pharmaceutical Co. Ltd. (to N.O. and K.O.).