2017 Volume 81 Issue 9 Pages 1246-1253
2017 Volume 81 Issue 9 Pages 1246-1253
Selection of patients with atherosclerotic carotid stenosis for revascularization is mainly based on the degree of luminal narrowing of the carotid artery. However, identification of other features of plaque apart from the degree of stenosis could enable better selection for intervention if they are also associated with the occurrence of stroke. Before these risk factors can possibly play a role in treatment decisions, their prognostic value needs to be proven. The purpose of this narrative review is to summarize current knowledge regarding the risk factors for stroke in patients with carotid stenosis, how they can be determined, and to what extent they predict stroke, based on recent literature. References for this review were identified by searches of PubMed between 1995 and October, 2016 and references from relevant articles. For each topic in this review different relevant search terms were used. The main search terms were ‘carotid stenosis’, ‘atherosclerosis’, ‘stroke risk’, and ‘vulnerable plaque’. Language was restricted to English. The final reference list was generated on the basis of relevance to the topics covered in this review.
Carotid artery stenosis is the presumed cause of approximately 15% of ischemic strokes.1 In patients with carotid stenosis, the risk of stroke after a transient ischemic attack (TIA) is reported to be 15–20% within the first 3 months after the initial event.2,3 Treatment consists of prophylactic medication, and in selected patients additional carotid revascularization (i.e., carotid endarterectomy (CEA) or stenting) to prevent emboli originating from the carotid plaque and in certain cases to improve cerebral perfusion pressure.
The first-choice method in most centers is surgical removal of the carotid plaque by means of CEA, which currently has a variable operative risk of stroke or death of 1–5% in symptomatic patients (i.e., patients with a recent ipsilateral ischemic event), and 1–3% in asymptomatic patients, depending on patient selection and source of data.4 Because of the risk of complications, it is important to carefully select patients who are most likely to benefit from surgery.
Furthermore, because of the substantial improvement in the quality of medical treatment of patients with carotid stenosis over the past decades, the future risk of stroke is generally low. The actual risk may be low enough to obviate carotid revascularization in many patients currently considered for CEA. This stresses the importance of stratifying risk in the context of modern medical treatment, enabling the selection of those patients who will benefit from revascularization.
Currently, initial screening and selection of patients for carotid revascularization is mainly based on the degree of luminal narrowing and identifying those with a severe degree of stenosis. However, factors other than luminal stenosis have been shown to determine the benefit from CEA, mainly because they influence the risk of recurrent stroke in patients who are treated medically. These include age, sex, timing of surgery, and the structural characteristics of the ipsilateral carotid plaque. However, most of the data reviewed in the next 2 paragraphs, demonstrating the clinical predictors of recurrent stroke (including stenosis severity), are based on randomized trials completed many years ago using catheter-based angiography, which is rarely used today. Also, most of the trials are based on medical treatment using outdated standards for best medical therapy. Moreover, techniques for imaging plaque characteristics in vivo have advanced considerably. Even some of the observational studies performed within the past 10 years may be out of date because of advances in imaging technology and medical therapy. Thus, much of the work needs to be repeated and tested in new clinical trials.
If the factors predicting stroke can be confirmed in prospective studies, it may be possible to more accurately identify patients at a high risk of stroke, enabling better selection for carotid intervention. The aim of this review is to provide an overview of the most important patient and plaque characteristics that predict stroke in patients with carotid stenosis, with an emphasis on imaging of plaque characteristics.
In subgroup analyses of pooled data from the 2 largest symptomatic carotid surgery trials, the NASCET and the European Carotid Surgery Trial (ECST), Rothwell et al showed that several clinical and angiographic variables other than the degree of carotid stenosis are associated with the risk of recurrent stroke.5,6 There was no relationship between these risk factors and surgical risk, thus there was also an increased benefit from surgery in the patients concerned. A risk prediction model including these variables was derived from the patients randomized to medical treatment in ECST and was validated in NASCET.6 Table 1 shows the different risk factors included in the model and associated hazard ratios (HRs). The model estimates the 1- and 5-year risk of recurrent ipsilateral stroke in medically treated patients with recent (<6 months) symptomatic carotid disease. The risk model has been used as an aid to clinical decision-making. However, the accuracy of the model has not been confirmed in prospective studies. Because the risk estimates are based on subgroup analyses only, they should be used with caution. Also, the model is unlikely to represent the up-to-date risk because medical therapy has improved since the CEA trials. Although antiplatelet treatment was standard during ECST and NASCET, medical treatment is currently more extensive with the use of statins and intensive blood pressure treatment. Newer studies have shown that the risk of stroke after medical intervention has fallen in recent years, which can be explained by the improvements in medical therapy.7,8 Therefore, the model may overestimate recurrent stroke risk in view of current clinical practice. The model has recently been recalibrated to take account of improvements in medical therapy and renamed the Carotid Artery Risk (CAR) score. The scoring tool is available for use as a clinical tool on smart phones or on-line,9 but it should be borne in mind that the predictive value of the score is uncertain and needs to be tested in clinical trials.
| Risk factor | HR (95% CI) | P value |
|---|---|---|
| Stenosis (per 10%) | 1.18 (1.10–1.25) | <0.0001 |
| Near occlusion | 0.49 (0.19–1.24) | 0.1309 |
| Male sex | 1.19 (0.81–1.75) | 0.3687 |
| Age (per 10 years) | 1.12 (0.89–1.39) | 0.3343 |
| Time since last event (per 7 days) | 0.96 (0.93–0.99) | 0.0039 |
| Presenting event | ||
| Ocular | 1.000 | 0.0067 |
| Single TIA | 1.41 (0.75–2.66) | |
| Multiple TIAs | 2.05 (1.16–3.60) | |
| Minor stroke | 1.82 (0.99–3.34) | |
| Major stroke | 2.54 (1.48–4.35) | |
| Diabetes | 1.35 (0.86–2.11) | 0.1881 |
| Previous myocardial infarction | 1.57 (1.01–2.45) | 0.0471 |
| Peripheral vascular disease | 1.18 (0.78–1.77) | 0.4368 |
| Treated hypertension | 1.24 (0.88–1.75) | 0.2137 |
| Irregular/ulcerated plaque | 2.03 (1.31–3.14) | 0.0015 |
ECST (n=3,024) is one of the 2 largest symptomatic carotid surgery trials that recruited patients with some degree of carotid stenosis and recent (<6 months) ipsilateral transient or mild symptomatic ischemic event between 1981 and 1994. Hazard ratios (HRs) calculated from data on the patients who were randomized to medical treatment (derived from a Cox model). Near-occlusion refers to a severe carotid stenosis with reduction in the distal internal carotid artery caliber because of failure to maintain sufficient flow in the artery. Many near-occlusions will ultimately progress to complete occlusion. In contrast to high-grade stenosis otherwise, near-occlusion is associated with a lower risk of stroke. Note that one of the risk factors is an irregular or ulcerated plaque. This definition is based on conventional angiography, which was used during the carotid surgery trials, but not in routine current practice. Data derived from Rothwell et al.6 CI, confidence interval; TIA, transient ischemic attack.
In cardiology, the finding that a low-grade coronary artery stenosis frequently leads to myocardial infarction raised the hypothesis that not only luminal stenosis, but also properties of the atherosclerotic plaque are related to clinical ischemic events. In 1989 the American cardiologist James E. Muller first described this theory as the ‘vulnerable plaque concept’.10 This concept has also been applied to carotid atherosclerosis. The hallmark of a vulnerable or high-risk plaque is a composition that makes it prone to rupture, resulting in precipitation of thrombus on the plaque and distal emboli. The vulnerable carotid plaque concept was supported by histopathology studies that found specific constituents of carotid artery plaques after an ischemic stroke.11,12 These include a thin or ruptured fibrous cap, a large lipid-rich necrotic core (NC), intraplaque hemorrhage (IPH), and macrophage infiltration. Interestingly, these pathological characteristics can also occur in plaques that do not cause significant luminal stenosis, either because the plaque is small or, for example, in plaques that have grown outwardly, expanding towards the outside boundary instead of narrowing the lumen.13 Despite the severity of the carotid stenosis not being identified as significant on angiographic imaging, these plaques may have a high risk of rupture and embolization in some cases.14
Evidence that plaque morphology was a risk factor for ischemic stroke was found in the subgroup analyses in ECST, which led to the aforementioned risk prediction tool. An irregular or ulcerated plaque surface, visualized with conventional angiography, the standard imaging method at the time of ECST, was associated with the risk of recurrent stroke compared with a smooth plaque surface (HR 2.03; Table 1).
Different methods of identifying high-risk plaques in vivo with non-invasive imaging have been studied over the past years. The next sections will describe the most important imaging modalities and summarize the existing evidence for specific imaging parameters as predictors of stroke, focusing on results from prospective studies that looked at plaque features in relation to stroke during follow-up. Additionally, PET/CT is discussed in the Supplementary File 1. It is important to note that prognostic studies are often performed separately in symptomatic and asymptomatic carotid stenosis patients and in lower and higher grades of stenosis. However, although the risk of stroke is lower from an asymptomatic low-grade stenosis than from a symptomatic high-grade stenosis, both have the same underlying pathology of carotid atheroma, with the same potential vulnerable plaque features. Therefore, it is important to look in symptomatic as well as asymptomatic patients.
Magnetic resonance imaging (MRI) is known for its excellent soft tissue contrast at a typical resolution of 0.5–2 mm. Plaque burden and atherosclerotic plaque components, such as IPH, lipid-rich NC (LRNC) and calcification can therefore be distinguished using various dedicated vessel wall MRI sequences.15 The use of MR contrast agents can further increase sensitivity for measuring the integrity of the fibrous cap.16
The most commonly used method of MR plaque imaging consists of a series of images with different contrast weightings. In this ‘multi-contrast’ protocol (Figure 1), each plaque component shows a unique combination of image contrasts, usually considered with respect to the adjacent sternocleidomastoid muscle. Comparative studies have shown good correlation of plaque MRI with histopathology of endarterectomy specimens.17,18 However, it should be noted that these were relatively small series with variable imaging protocols and histological methods.19,20

Multisequence carotid 3-Tesla MRI. Coregistered T1-weighted turbo spin echo (TSE), contrast-enhanced (CE) T1 W TSE, time-of-flight (TOF), and T1 W transient field echo (TFE). External carotid artery (e) and internal carotid artery (i). (A) CE T1 TSE shows a lipid-rich necrotic core (*), demarcated by a fibrous cap. (B) Hyperintense area in the wall of the internal carotid artery (*) on the T1 TFE image represents intraplaque hemorrhage.
In recent years, many studies have investigated the association between MRI plaque characteristics and subsequent cerebrovascular events. IPH in particular is thought to be a crucial parameter related to plaque rupture and therefore studied extensively. Cohort studies that investigated the risk of stroke associated with the presence of IPH were included in several meta-analyses.21–23 Their results are summarized in Table 2. In the analysis by Saam et al,23 the prevalence of IPH varied between 28% (asymptomatic patients) and 67% (symptomatic patients). Their meta-analysis (n=689) showed that ipsilateral IPH was associated with subsequent TIA and stroke in symptomatic (HR 11.71; 95% confidence interval (CI) 5.18–26.48) and in asymptomatic patients (HR 3.50; 95% CI 2.59–4.73). The overall annualized event rate was 17.7% in patients with IPH, compared with 2.4% in those without. There was a significant between-study heterogeneity and evidence of a moderate publication bias. In a second meta-analysis (n=678) that included most of the same studies, but also some different studies, a HR of 5.86 (95% CI 2.90–11.85) was found in symptomatic, and 3.66 (95% CI 2.70–4.95) in asymptomatic patients.21 The difference in HRs between the 2 meta-analyses in symptomatic patients can be explained by the inclusion of a recent study that showed a HR for TIA or ischemic stroke of 3.54 (95% CI 1.06–11.86), which was included in the second meta-analysis.24 Evidence for other vulnerable plaque characteristics as predictors of stroke was also summarized. Four studies examined LRNC in a total of 403 patients with a mean follow-up of 24 months. A thin or ruptured fibrous cap was studied in a total of 363 patients. Both characteristics were associated with TIA and stroke, with HRs of 3.00 (95% CI 1.51–5.95) and 5.93 (95% CI 2.65–13.29), respectively.22
| No. of studies |
Total population |
Follow-up period (mean) |
HR/OR [95% CI] |
|
|---|---|---|---|---|
| Intraplaque hemorrhage | ||||
| Saam et al 2013 (HR)23 | 8 | 689 | 1–38 (20) months | 5.7 [3.0–10.9] |
| Gupta et al 2013 (HR)21 | 7 | 678 | 9–38 (20) months | 4.6 [2.9–7.2] |
| Hosseini et al 2013 (OR)22 | 7 | 667 | 9–38 months | 10.0 [5.5–18.4] |
| Lipid-rich necrotic core | ||||
| Gupta et al 2013 (HR)21 | 4 | 403 | 12–38 (24) months | 3.0 [1.5–5.9] |
| Thin or ruptured fibrous cap | ||||
| Gupta et al 2013 (HR)21 | 4 | 363 | 12–38 (22) months | 5.9 [2.7–13.2] |
*Combined data on symptomatic and asymptomatic stenosis. NB: the studies included in the 3 meta-analyses largely overlap. CI, confidence interval; HR, hazard ratio; OR, odds ratio.
Although the interest in using carotid MRI to assess atherosclerotic burden is increasing, the technique is still being continuously improved in terms of image resolution, image contrast, and scan time. A good example is the application of 3D isotropic resolution carotid MRI.25,26 In most studies to date, multislice 2D images of the carotid arteries were obtained and although this typically allows for a high spatial resolution (0.5 mm) within each imaging slice, the slice thickness is generally large (2–3 mm). Small structures might therefore remain undetected because of signal averaging along the slice direction. Additionally, overestimation of wall thickness may occur in tortuous arteries, which are frequently found in the elderly population. Conversely, 3D imaging has equal resolution (<1 mm) in all dimensions, providing images with improved anatomical detail and the facility to generate reconstructions in arbitrary planes (Figure 2). More importantly, the increased scan efficiency of 3D measurements allows full coverage of the complete cervical and intracranial parts of the carotid arteries.

3D isotropic resolution 3-Tesla MRI of the carotid arteries of a patient with a recent right-sided stroke. Because of the isotropic resolution (0.7 mm), reconstruction in all orientations can be made, allowing visualization of the plaque burden in the entire length of the artery. Coronal view (A). A large atherosclerotic plaque, causing luminal narrowing is seen in the right internal carotid artery (arrow). The red line shows the position of the axial reconstruction (B).
Despite major advances in carotid MRI techniques, as well as an increasing amount of evidence supporting the potential of plaque MRI for stroke risk stratification, general consensus on the optimal, clinically feasible scan protocol is lacking and will require careful identification of the most essential features governing plaque vulnerability, as well as agreement on the most sensitive MR sequence for detection or parameter quantification.
Additionally, the quantitative analysis of plaque MR images, which is still mainly dependent on manual segmentation, is time consuming and subject to intra- and interobserver variability.27 Automatic plaque characterization techniques could potentially overcome these problems.28
TCD US can be used to detect embolic signals in the intracranial arteries (Figure 3). These signals, called microembolic signals (MES) or high-intensity transient signals (HITS), are thought to represent microemboli that originate from a more proximal source. If they are detected in the middle cerebral artery ipsilateral to an atherosclerotic plaque in the carotid artery, MES could be a marker of a vulnerable plaque.
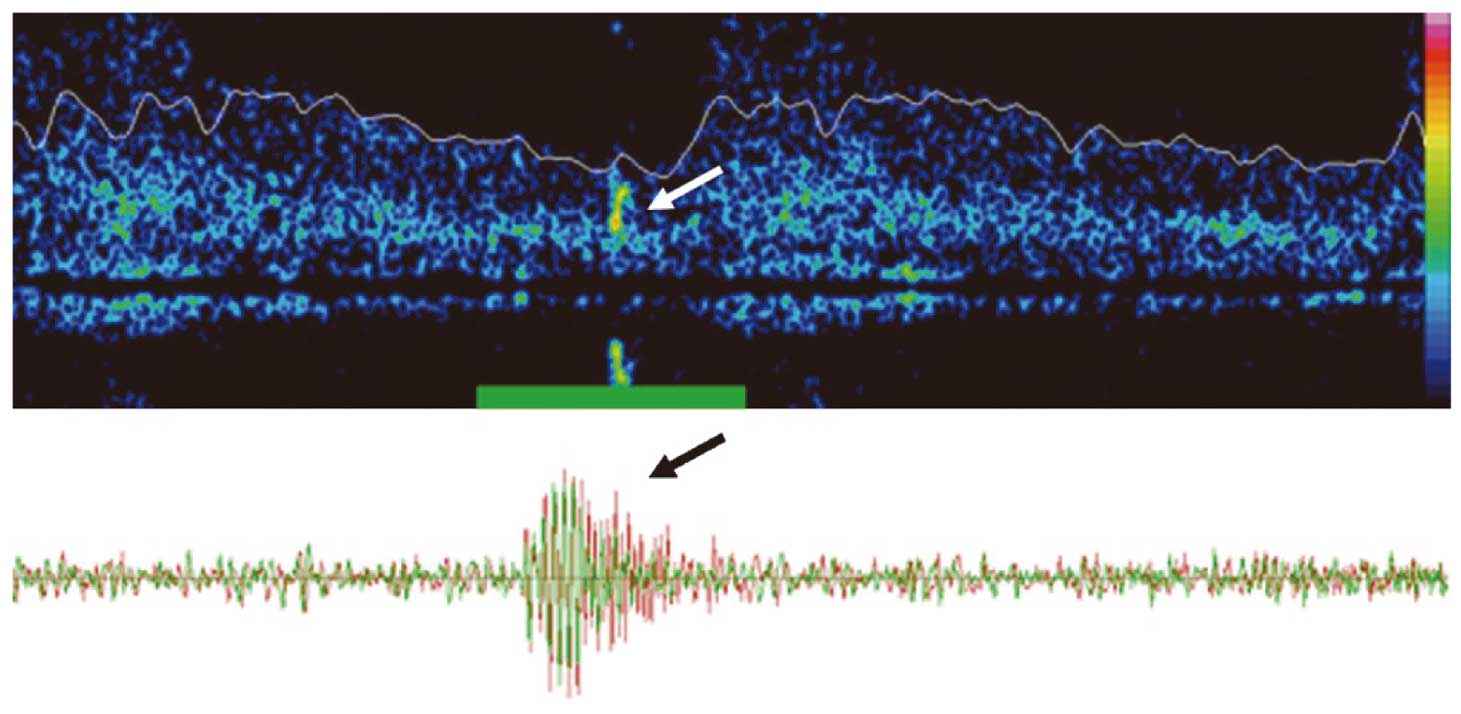
Microembolic signals (MES) on transcranial Doppler recording of the middle cerebral artery. Sound reflection of an embolus is seen as a MES in frequency (Upper) and time (Lower) domains. MES can be heard as a snapping or chirping sounds on the audible output.
Several studies have looked at the prognostic value of MES in symptomatic and asymptomatic patients. A meta-analysis showed that the presence of MES is associated with TIA and ischemic stroke in symptomatic (odds ratio (OR) 6.36; 95% CI 2.90–13.96) and asymptomatic carotid stenosis patients (OR 7.57; 95% CI 2.32–24.69).29,30 In a meta-analysis for symptomatic stenosis, 2 of the 4 studies recruited less than 15 patients and the results were mainly based on the 1 study that included 200 patients.31 In the asymptomatic stenosis studies, there was high between-study heterogeneity. A more recent systematic review of TCD as a predictor of cerebral events concluded there is a generally weak level of evidence and that definitive recommendations on its utility in the clinical setting cannot be made.32 Results from the studies that included ≥50 patients are summarized in Table 3.33–38
| Study | Year | n | MES positive | Follow-up | No. of events | OR (95% CI) |
|---|---|---|---|---|---|---|
| Symptomatic patients | ||||||
| Markus et al31 | 2005 | 200 | 89 (44.5%) | 3 months | 31 (15.5%) | 4.67 (1.99–11.01) |
| Censori et al33 | 2000 | 50 | 20 (40%) | 0.6 months | 7 (14%) | 12.43 (1.36–113.41) |
| Altaf et al34 | 2014 | 123 | 46 (37.4%) | Median 36 days (IQR 15–87) |
37 (30%) | HR 3.28 (1.68–6.42) |
| Molloy et al35 | 1999 | 67 | 20 (42%) | 0.8 months | 9 (13%) | 12.12 (2.24–65.55) |
| Asymptomatic patients | ||||||
| Markus et al30 | 2010 | 467 | 77 (16.5%) | 24 months | 32 (6.9%) | 2.50 (1.13–5.51) |
| Abbott et al36 | 2005 | 231 | 60 (26%) | 70.8 months | 18 (7.8%) | 1.47 (0.4–4.48) |
| Spence et al37 | 2005 | 319 | 32 (10%) | 12 months | 16 (5%) | 15.65 (5.34–45.88) |
| Siebler et al38 | 1995 | 64 | 8 (12.5%) | 16.8 months | 5 (7.8%) | 31 (3–302) |
MES, microembolic signals. Other abbreviations as in Tables 1,2.
Although the hypothesis that MES denote solid emboli from a carotid plaque is physiologically plausible, it is not possible to prove this histologically, nor is it possible to know if an embolic signal originates from the carotid artery or from another embolic source, such as the aorta or the heart, unless one records simultaneously above and below the culprit plaque or multiple emboli are detected bilaterally, which makes a proximal source more likely. Nevertheless, if the presence of MES is a prognostic factor for ischemic stroke, TCD could be useful for identifying patients who are at increased risk. The question of whether patients with MES have a greater benefit from carotid intervention than those without MES can only be answered in a randomized controlled trial.
MES have been detected in approximately 40% of symptomatic carotid stenosis patients and are less frequently found (10–25%) in patients with asymptomatic carotid stenosis. To our knowledge, the prevalence of MES in a healthy population is unknown. The duration of TCD recording differs between studies: most perform a 1-h recording, but 30-min to 4-h recordings are reported. This variability limits the comparability of results in different studies, because the test is generally considered positive when ≥1 embolic signals occur, regardless of the moment of occurrence. Long recordings make the performance, as well as analysis, time consuming. To save time, user-friendly alternatives such as an ambulatory TCD device and automated emboli detection software have been developed. Besides saving of time, automated detection software also facilitates easier analysis and wider implementation of TCD emboli detection, which is currently limited because there is no gold standard for the detection of microemboli and detection is based on human observation.
Since the mid-1980s, research groups have been using US imaging in an attempt to determine the morphology and hence vulnerability of carotid atherosclerotic plaque. US imaging offers many advantages over CT and MRI, mainly being non-invasive, relatively inexpensive and does not expose patients to ionizing radiation.39 It is also suitable for patients with contraindications to MRI or contrast media. However, US has poor soft tissue contrast in comparison with CT and MRI and is highly operator dependent. US can give information regarding plaque burden, texture and echogenicity but is less valuable in detecting surface ulceration. It can determine the echogenicity of plaque material by measuring the ability of plaque components to create an echo. Soft plaques, including lipid and thrombus, have low echogenicity or are echolucent. Fibrous tissue and calcification are echogenic.
Two recent meta-analyses (1in symptomatic,40 1 in asymptomatic stenosis41) have summarized the literature on the risk of stroke based on the sonographic characteristics of carotid plaques. For symptomatic stenosis, 23 studies were included with 6,706 carotid plaques. Plaque neovascularity, complex plaque, plaque ulceration, plaque echolucency and intraplaque motion all had a higher prevalence in symptomatic than in asymptomatic plaques.40 To our knowledge, no prospective cohort studies with recurrent stroke as the primary endpoint were included in this meta-analysis. The meta-analysis in asymptomatic stenosis looked at only echogenicity as a predictor of ipsilateral stroke; 8 prospective observational studies looking at clinically defined first-time stroke were included. In a total of 7,557 patients, with a mean follow-up of 37 months, predominantly echolucent plaques had a significantly increased risk of ipsilateral stroke compared with predominantly echogenic plaques (relative risk (RR) 2.31; 95% CI 1.58–3.39; cumulative incidence of ipsilateral stroke 5.7% vs. 2.4%, respectively).41 In general, it is remarkable that many different non-standardized definitions of vulnerable plaque characteristics are used across studies, which precludes a meaningful pooled analysis.
Other important factors in US plaque imaging that appear to predict risk of future events include plaque volume and area. Spence’s group in Canada has published many papers on these factors. A large study of 1,686 patients showed that 77% of events occurred in patients in the top quartile of plaque area.42 Plaque area of 1.19–6.73 cm2 conferred a RR of 3.5 (P<0.001) for the combined outcome of stroke, myocardial infarction and vascular death.42
Newly developed 3D acquisition and reconstruction of US images are being used to analyze the carotid plaque.43 This technique has been proven feasible for measuring plaque and artery volumes with good interobserver reproducibility and therefore can be used to monitor disease progression.44 Another new tool is contrast-enhanced US, which can visualize plaque neovascularization.45 US has not been widely adopted for plaque imaging, in part because of concern about operator variability and lack of independent validation of the various methods developed for plaque analysis.
Apart from accurately visualizing luminal degree of stenosis, CT axial tissue imaging acquired as part of CT angiography (CTA) after intravenous contrast injection enables distinguishing of the arterial wall from the lumen and to identify fatty, calcified and mixed plaques, based on CT densities.46,47 CT can provide information on wall volume, ulceration and lipid content. However, visualization of plaque characteristics by CT has major restrictions as a result of significant overlap between the densities associated with the different plaque components. IPH cannot be identified, except when the hemorrhage is very recent. Also, artefacts as a result of severe plaque calcification can make it difficult to identify the different plaque characteristics.
To our knowledge, the association between plaque morphology assessed with CT and risk of stroke has not been studied prospectively. In several cross-sectional studies, differences between symptomatic and asymptomatic plaques were evaluated with CT.48,49 The first study compared CT of 40 ‘carotid stroke’ patients with 50 ‘non-carotid stroke’ patients. Larger wall volume, thinner fibrous cap, higher lipid content and lower amount of calcium were significantly associated with symptomatic plaques.48 A second study compared the CTA images of 315 asymptomatic plaques with those of 14 symptomatic plaques. Wall thickness, plaque ulceration, fibrous cap thickness, LRNC, and calcification were compared, but only maximal carotid wall thickness was significantly greater in symptomatic than in asymptomatic plaques. Interestingly, in the latter study calcification was more abundant in symptomatic arteries, which is in contrast to the common idea that calcification stabilizes a plaque.49 A recent study by Gupta et al50 looked at the association between soft (non-calcified) and hard (calcified) plaque thickness measurements and symptomatic stenosis in patients with moderate (50–69%) stenosis. They found that soft-plaque thickness was significantly higher in patients with symptomatic stenosis, while hard-plaque thickness was significantly higher in asymptomatic stenosis.50 Because of the retrospective design of these studies, no assessment of risk associated with specific plaque characteristics could be done.
Recent literature on the risk factors for stroke in patients with carotid stenosis is abundant, highlighting the current interest in this topic. Many potential biomarkers and techniques to identify these factors have been investigated. In this review we have made a distinction between identification of risk factors based on clinical patient characteristics and plaque-related features. The evidence for the patient-related risk factors derived from the carotid surgery trials is robust, because of the large numbers of patients included in the trials, but might not apply today because of changes in risk factor profiles and medical therapy in recent years. The available literature indicates that plaque characteristics certainly might play a role in identifying high-risk plaques. In fact, all studies discussed showed an association between the studied parameter and stroke risk, which is promising, but also suggestive of publication bias. Apart from the imaging parameters and techniques discussed in this paper, others have been investigated, but to our knowledge not in cohort studies with clinical outcome.
Taking into account the amount and quality of evidence, and the strengths and weaknesses of the different techniques, in our opinion the most promising imaging parameters are plaque morphology assessed with MRI (IPH in particular), and MES on TCD. MRI is currently more feasible than TCD because of wider availability and the fact that plaque imaging can be combined with routine brain and vascular imaging.
Despite the evidence that plaque morphology is a risk factor for ischemic stroke, it is currently not taken into account in treatment guidelines for carotid stenosis. Because the current diagnostic stroke work-up is focused on finding luminal stenosis of ≥50%, potentially high-risk plaques that do not cause significant luminal stenosis but might benefit from CEA are missed. These plaques may be the cause of a substantial proportion of cryptogenic strokes.51,52 Conversely, many recently symptomatic patients with severe carotid stenosis may remain free of stroke recurrence while on medical therapy.
The imaging techniques of plaque-related features are subject to continuous development. Comparison between studies is limited because of large study heterogeneity with regard to study design, patient populations, imaging protocols, definitions of parameters, and analysis methods. Moreover, it is hard to draw definite conclusions from the generally small studies with variable methodological quality. In our opinion, before plaque characteristics can be taken into account in new guidelines, their value must first be confirmed in large prospective studies, preferably clinical trials, that use uniform predefined definitions of plaque imaging parameters.
One ongoing prospective study is the Plaque At Risk (PARISK) study, which evaluates and compares the ability of plaque MRI, TCD US, CTA, duplex US, and molecular biomarkers to identify patients with carotid stenosis with an increased risk of recurrent TIA or ischemic stroke (NCT01208025).53 In a similar study, the Carotid Plaque Imaging in Acute Stroke (CAPIAS) study, the predictive value of MRI plaque imaging and molecular biomarkers will be assessed (NCT01284933).54
The Oxford risk prediction tool, based on clinical variables, already showed good accuracy in its validation of NASCET data. However, it needs to be externally validated in a clinical trial before it can be used as a decision model. A pilot study for a new large, international carotid surgery trial, the Second European Carotid Surgery Trial (ECST-2), with the objective of confirming the accuracy of the risk assessment model and its role in the selection of patients for carotid intervention, in the context of modern medical management, is currently recruiting patients (ISRCTN97744893).9 The main hypothesis for the pilot stage of ECST-2 is that patients who have clinical characteristics that predict a 5-year risk of future ipsilateral stroke of <20%, when treated with modern optimized medical treatment (OMT) alone, will not benefit from early revascularization in addition to OMT. The recalibrated version of the risk model, the CAR score, taking into account the estimated effect of improved medical therapy, will be used to select patients for this trial (see above). Patients will be randomized between OMT and OMT plus early carotid intervention. In ECST-2, plaque MRI and TCD, as well as molecular biomarkers, will be investigated in a substudy. Although imaging parameters do not currently influence selection for carotid intervention in this trial, information on imaging parameters in 2 groups with randomly assigned treatment can clarify whether patients with certain plaque characteristics benefit more from either treatment.
If future studies can provide us with reliable values of plaque imaging parameters predictive of future stroke, it is likely that selection of patients with carotid stenosis for carotid revascularization will change to involve incorporation of plaque imaging findings with other clinical features into more sophisticated scoring tools.
P.J.N.: research funding: Netherlands Heart Foundation, Fonds NUTS-OHRA, The Netherlands Organisation for Health Research and Development. M.M.B.: research funding: grant from the Stroke Association UK. B.F.C.: research funding: VENI grant from the Dutch Technology Foundation STW. L.H.B.: research funding: Swiss National Science Foundation and Swiss Heart Foundation. Scholarship from Swiss National Science Foundation and the University of Basel.
Supplementary File 1
Current Practice Guidelines Based on Stenosis Severity
Imaging Plaque Inflammation With PET/CT
Please find supplementary file(s);
http://dx.doi.org/10.1253/circj.CJ-16-1284