2017 Volume 81 Issue 9 Pages 1354-1359
2017 Volume 81 Issue 9 Pages 1354-1359
Background: In Ebstein’s anomaly (EA) current surgical criteria may not translate into better long-term survival. The aim of this study was therefore to determine if surgical treatment for EA increases survival, and to analyze factors associated with mortality.
Methods and Results: A retrospective study was carried out involving 103 patients with surgical indication using current criteria, comparing operated (n=49; 47.5%) and non-operated patients (n=54; 52.4%); the severity of disease was similar in all cases. Overall follow-up was 12 years (range, 1–49 years). There were no differences in mortality: in the surgical and non-surgical groups, survival at 10 years was 92.8% vs. 90.7%; 20 years, 85.7% vs. 81.0%; and 30 years, 78.5% vs. 72.2%, respectively. On multivariate analysis right ventricular fractional shortening (RVFS) was associated with mortality in both groups. Decreasing RVFS was associated with worse survival according to severity: when RVFS was <20%, survival at 20, 40 and 60 years was 58%, 39%, and 12.5%, respectively (P<0.0013). Left ventricular ejection fraction also correlated with survival (P<0.0013).
Conclusions: Surgery did not translate into benefit in terms of survival, and this was clearly associated with RV function; therefore this should be a key factor in the surgical decision making.
Ebstein’s anomaly (EA) accounts for <1% of all congenital heart disease. There is general consensus that EA affects not only the tricuspid valve but also the whole right ventricle (RV). This results in a particular type of right-sided cardiomyopathy.1 The characteristic features of this anomaly are: (1) predominant septal and inferior tricuspid leaflet attachment to the underlying myocardium due to failure of delamination; (2) anterior and apical rotational displacement of the functional annulus; (3) dilation of the “atrialized” portion of the RV with variable degrees of hypertrophy and wall thinning; (4) redundancy, fenestration and tethering of the anterior leaflet; (5) dilation of the right atrioventricular junction (the true tricuspid annulus); and (6) variable degree of ventricular myocardial dysplasia and dysfunction.1–3
These anatomical and functional abnormalities cause important tricuspid regurgitation (TR) in many patients that contributes to right chamber dilation and atrial or ventricular arrhythmias. Hemodynamics and clinical patterns depend on anatomical severity, degree of right-to-left atrial shunt, and age at presentation.4,5
Long-term outcome is usually good for patients who survive the neonatal period and for those with no symptoms following the first years of life.3 Therefore, many patients will develop symptoms and surgical treatment may be necessary. Current surgical criteria according to European or US guidelines include: (1) presence of symptoms or worsening functional class; (2) cyanosis (oxygen saturation <90%); (3) paradoxical embolism; (4) progressive cardiomegaly on chest X-ray; and (5) progressive RV dilation or RV systolic dysfunction.6 Other authors recommend surgical repair in patients with more than moderate TR in the presence of symptoms (New York Heart Association [NYHA] class >II or arrhythmias) or worsening exercise capacity.7 Optimal timing for surgical management in this patient subset remains controversial. Badiu et al found that NYHA functional class >II and cardiothoracic index >0.6 are important prognostic factors associated with long-term mortality.8 They suggested that, even in an asymptomatic or minimally symptomatic patient, early surgery must be performed before cardiomegaly develops and functional class worsens. In contrast, other authors consider that surgery should be delayed until symptoms appear, cyanosis becomes evident or paradoxical embolism occurs.9
Currently, there are insufficient data on the evolution of EA after surgical treatment, and, even though many studies report good outcome after surgery, general consensus on the timing of surgical management is lacking. The aim of this study was therefore to investigate the long-term prognosis of EA with and without surgical treatment, and analyze the factors associated with mortality.
We performed a retrospective, observational study in patients with EA in the outpatient clinic of the National Institute of Cardiology Ignacio Chávez, Mexico City. Patients treated with palliative or corrective surgery and patients who, for different reasons (economic, social, religious or cultural condition) did not accept surgery, were followed up. We included patients with the diagnosis of EA, who had surgical indications according to current guidelines, with similar anatomic and functional characteristics, without other congenital heart defects other than atrial septal defect (ASD) or patent foramen ovale. Patients who had surgery at institutions other than the present one, and for whom data on the surgery, or echocardiography or clinical records were not available, were excluded. The present study was approved by the institutional ethics committee.
Indication for surgical management was based on 1 or more of the following criteria: presence of symptoms or worsening functional class; cyanosis (oxygen saturation <90%); paradoxical embolism; severe tricuspid valve regurgitation; progressive cardiomegaly on chest X-ray; and progressive RV systolic dysfunction.
EA was defined as the attachment of the tricuspid septal valve >8 mm/m2.1 Severity was classified according to echocardiography by measuring valve attachment percentage at end diastole.10 Tricuspid septal valve attachment was measured as the distance between the anterior mitral valve insertion and tricuspid septal valve attachment to the interventricular septum. To measure attachment percentage, the total interventricular septal distance was measured and the following formula applied: (total interventricular septal distance-tricuspid septal valve attachment distance)/total interventricular septal distance. All measurements were performed at end diastole. EA was considered mild for tricuspid valve attachment percentage <44%; moderate, 45–60%; and severe, >0.61%. Tricuspid valve regurgitation was graded depending on jet length. RV function was evaluated using RV fractional shortening area (RVFS) and tricuspid annulus plane systolic excursion (TAPSE).
Surgical TechniquePatients included in the surgical group received either a corrective or a palliative procedure. Corrective procedures were tricuspid valve repair or replacement. Tricuspid valve repair was performed using Carpentier’s technique due to surgeon preference. When anatomical conditions of the tricuspid valve were not favorable for repair, tricuspid valve replacement was performed using a biological Carpentier Edwards Perimount mitral prosthesis, with preservation of all subvalvular apparatus, or at least the posterior papillary muscle in order to prevent RV dysfunction. In cases of impaired RV function, palliative procedures were done, favoring a one and a half surgery. One and a half surgery preserved RV anterograde flow, and included a tricuspid valve repair or replacement (depending on the anatomical status of the tricuspid valve), in addition to a superior vena cava to pulmonary artery Glenn’s anastomosis. Surgical procedures were performed under cardiopulmonary bypass, with or without aortic cross clamping and cardiac arrest.
Follow-upClinical follow-up was conducted to assess mortality. Mortality was evaluated in the first 30 days after surgery or until hospital discharge (early mortality). Late postoperative events were defined as complications occurring after the previously stipulated period of time, until the end of follow-up. The primary endpoint was global mortality in the surgical and non-surgical groups. Early mortality was assessed in the surgical group. The secondary endpoint was identification of factors associated with mortality.
Statistical AnalysisThe clinical and echocardiographic records in the present institution were analyzed. Information was stored in an electronic Excel spreadsheet and processed using STATA 12.1. Continuous variables are expressed as mean±SD, or median (IQR) depending on distribution; categorical variables are given as number and percentage. Student’s t-test or Wilcoxon’s rank sum test were used to compare continuous variables, and chi-squared test was used for categorical variables. Overall survival was plotted according to the Kaplan-Meier method and compared using log-rank test. Logistic regression was performed to identify the factors associated with mortality in each group. A multivariate model was constructed with the factors that showed statistical correlation on bivariate analysis to identify factors that were independently associated with mortality. P<0.05 was considered statistically significant.
A total of 183 patients were included, and 103 met the inclusion criteria: 49 in the surgical group (47.5%), and 54 in the non-surgical group (52.4%). Overall long term clinical follow-up was 12 years (range, 1–49 years). Table 1 lists the clinical and echocardiographic characteristics. Age at diagnosis was significantly different between the 2 groups, and cyanosis was significantly more prevalent in the surgical group. In the surgical group, the procedures used were as follows: corrective surgery, n=36 (73.4%; Carpentier’s valve repair, n=16; biological valve replacement, n=20); one and a half palliative procedure, n=10 (22.4%); fistula repair, n=1 (0.97%); and Fontan procedure, n=2 (4.08%).
| Surgery (n=49) |
No surgery (n=54) |
P value | |
|---|---|---|---|
| Female | 30 (61.2) | 30 (55.5) | 0.560 |
| Age at diagnosis (years) | 6 (0.36–15.7) | 13.6 (1.5–21) | 0.049 |
| Death | 11 (22.45) | 13 (24.07) | 0.849 |
| Cause of death | 0.131 | ||
| C heart failure | 10 (91) | 7 (53.8) | |
| VT | 1 (9) | 5 (38.5) | |
| Stroke | 0 | 1 (7.7) | |
| EA severity | 0.856 | ||
| Moderate | 19 (38.8) | 20 (37.1) | |
| Severe | 30 (61.2) | 34 (62.9) | |
| LVEDD (mm) | 31.5±7 | 33.5±7 | 0.187 |
| LVESD (mm) | 20.7±6 | 21.6±7 | 0.510 |
| RVEDD (mm) | 56.0±12 | 54.76±14 | 0.654 |
| TAPSE (mm) | 13.7±5 | 13.2±4 | 0.593 |
| RVFS (%) | 26.5 (14–33) | 21 (12–28) | 0.252 |
| TR severity | 0.333 | ||
| Moderate | 17 (34.7) | 14 (25.9) | |
| Severe | 32 (65.3) | 40 (74.1) | |
| PSP (mmHg) | 25 (21–32) | 30 (21–35) | 0.262 |
| PFO | 12 (24.5) | 15 (27.8) | 0.705 |
| ASD | 31 (63.3) | 27 (50) | 0.175 |
| Tricuspid annulus (mm) | 45.4±15.6 | 50.2±15.6 | 0.239 |
| LVEF (%) | 55 (50.5–60) | 55.5 (50–60) | 0.620 |
| Cyanosis | 34 (69.3) | 24 (44.4) | 0.006 |
| NYHA Class | 0.067 | ||
| 1 | 16 (40.0) | 4 (13.3) | |
| 2 | 20 (50.0) | 22 (73.4) | |
| 3 | 3 (7.5) | 4 (13.3) | |
| 4 | 1 (2.5) | 0 | |
| WPW syndrome | 14 (28.5) | 20 (37.1) | 0.362 |
| AF/flutter | 14 (28.5) | 9 (16.7) | 0.147 |
| VT | 4 (8.16) | 3 (5.52) | 0.599 |
| AV block | 13 (27.1) | 7 (12.9) | 0.073 |
| Heart pacing | 5 (10.2) | 5 (9.29) | 0.872 |
| Ablation | 11 (22.5) | 14 (26) | 0.681 |
Data given as n (%), mean±SD or median (range). AF/flutter, atrial fibrillation/flutter; ASD, atrial septal defect; AV, atrioventricular; EA, Ebstein’s anomaly; LVEDD, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction; LVESD, left ventricular end-systolic diameter; NYHA, New York Heart Association; PFO, patent foramen ovale; PSP, pulmonary systolic pressure; RVEDD, right ventricular end-diastolic diameter; RVFS, right ventricular fractional shortening; TAPSE, tricuspid annulus plane systolic excursion; TR, tricuspid regurgitation; VT, ventricular tachycardia; WPW, Wolff-Parkinson-White.
Early mortality in the surgical group was 18.36% and average RVFS was 11±3.4%. In the early mortality surgical group, there were 3 palliative surgeries (1 Glen, 1 Fontan and 1 fistula), and 6 corrective surgeries (2 tricuspid valve replacements and 4 tricuspid valve repairs). Seven of these patients (77.7%) had RV dysfunction that did not respond to medical therapy.
There were no differences in regard to long-term mortality between the surgical and non-surgical groups (P=0.564, log rank test; Figure 1); the survival at 10, 20 and 30 years was 92.8%, 85.7% and 78.5% in the surgical group, vs. 90.7%, 81.0%, and 72.2% in the non-surgical group, respectively. Waiting time till surgery, from the start of follow-up to surgery, was 1.2 years (IQR, 0.22–5.3 years), on logistic regression analysis there was no association between waiting time for surgery and mortality (OR, 0.95; 95% CI: 0.818–1.109, P=0.594). On bivariate analysis, in the surgical group RVFS, ASD, left ventricular ejection fraction (LVEF), and early post-surgical complications were associated with mortality, but after multivariate analysis only RVFS remained significant (Table 2). In contrast, in the non-surgical group, on bivariate analysis RVFS, LVEF and cyanosis were associated with mortality, but after multivariate analysis only RVFS was independently associated with mortality (cyanosis showed a tendency toward, but did not reach, statistical significance; Table 2).
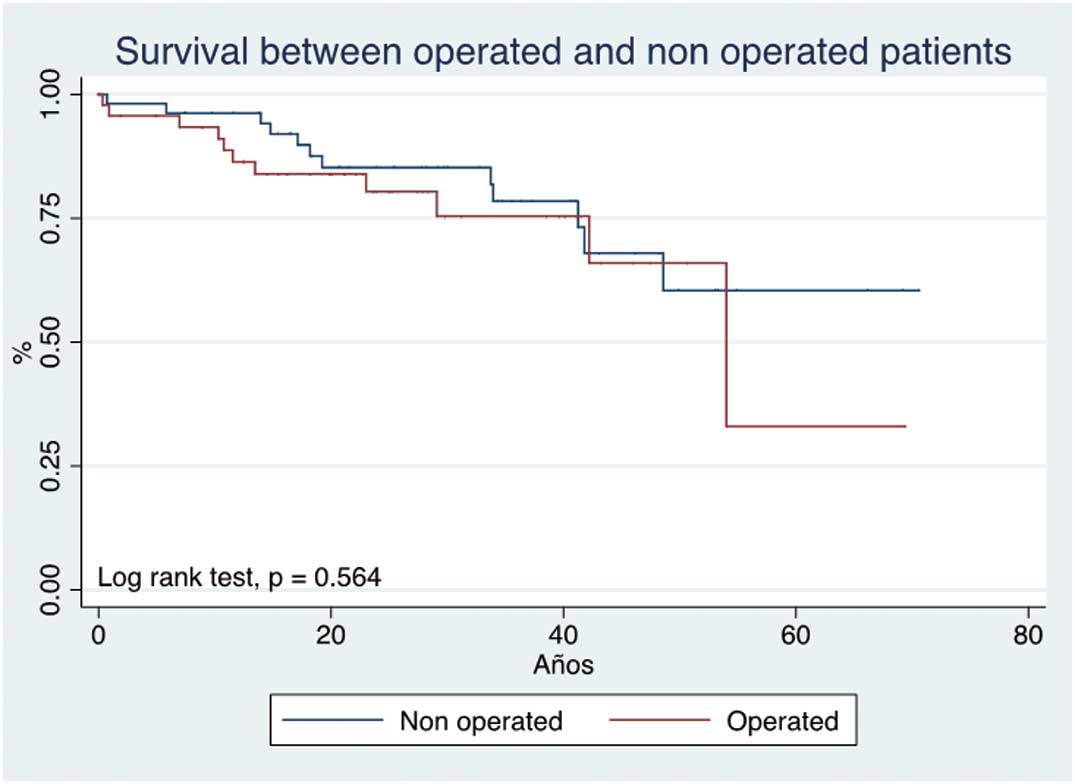
Long-term mortality according to use of surgery for Ebstein’s anomaly.
| Bivariate (OR) |
P value | Multivariate (OR) |
P value | |
|---|---|---|---|---|
| Surgical group | ||||
| Gender | 1.411 | 0.845 | – | – |
| Age at diagnosis | 1.009 | 0.728 | – | – |
| Age group | 0.755 | 0.534 | – | – |
| EA severity | 1.939 | 0.378 | – | – |
| RVFS | 0.792 | 0.001 | 0.612 | 0.033 |
| Severe TR | 2.931 | 0.205 | – | – |
| PSP | 1.004 | 0.932 | – | – |
| ASD | 0.232 | 0.043 | 0.173 | 0.299 |
| LVEF | 0.763 | 0.008 | 0.732 | 0.204 |
| Associated anomalies | 0.931 | 0.513 | – | – |
| Cyanosis | 3.103 | 0.311 | – | – |
| AF/flutter | 0.192 | 0.135 | – | – |
| VT | 1.166 | 0.899 | – | – |
| Blocks | 1.012 | 0.987 | – | – |
| CTI | 2.072 | 0.269 | – | – |
| Surgical technique | 1.166 | 0.491 | – | – |
| Corrective surgery | 2.407 | 0.221 | – | – |
| Early complications | 14.22 | 0.001 | 7.031 | 0.228 |
| Late complications | 0.481 | 0.343 | – | – |
| Non-surgical group | ||||
| Gender | 0.607 | 0.436 | – | – |
| Age at diagnosis | 0.984 | 0.523 | – | – |
| Age group | 0.572 | 0.158 | – | – |
| EA severity | 2.361 | 0.246 | – | – |
| RVFS | 0.844 | 0.008 | 0.629 | 0.028 |
| Severe TR | 1.222 | 0.708 | – | – |
| PSP | 1.001 | 0.995 | – | – |
| ASD | 0.816 | 0.751 | – | – |
| LVEF | 0.826 | 0.001 | 0.907 | 0.184 |
| Associated defects | 0.918 | 0.724 | – | – |
| Cyanosis | 7.021 | 0.019 | 13.16 | 0.053 |
| AF/flutter | 1.751 | 0.481 | – | – |
| VT | 7.277 | 0.119 | – | – |
| Bundle branch block | 0.486 | 0.524 | – | – |
| CTI | 2.115 | 0.149 | – | – |
CTI, cardiothoracic index. Other abbreviations as in Table 1.
With regard to the relationship between severity of RV dysfunction and survival, patients with RVFS <20% had a mean survival of 58%, 39%, 12.5%, at 20, 40 and 60 years of age, respectively (P<0.0013). In contrast, in patients with RVFS >40%, the survival at 20 and 40 years of age was 100% (Figure 2). LVEF was also related to survival: LVEF ≤54% was associated with lower survival at 20, 40 and 60 years (P<0.0045; Figure 3). In the surgical group, those with preserved RV function had better survival than their counterparts in the non-surgical group (P<0.032, P<0.002, respectively; Figure 4).
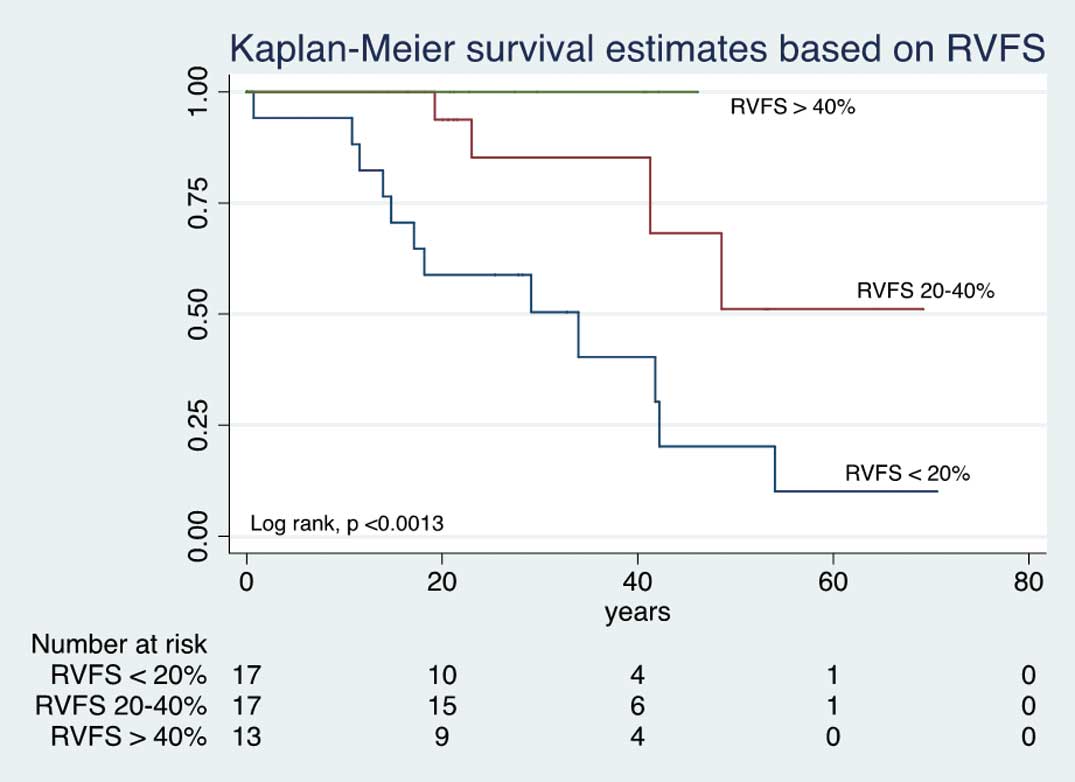
Kaplan-Meier survival vs. degree of right ventricular fractional shortening (RVFS) in Ebstein’s anomaly.
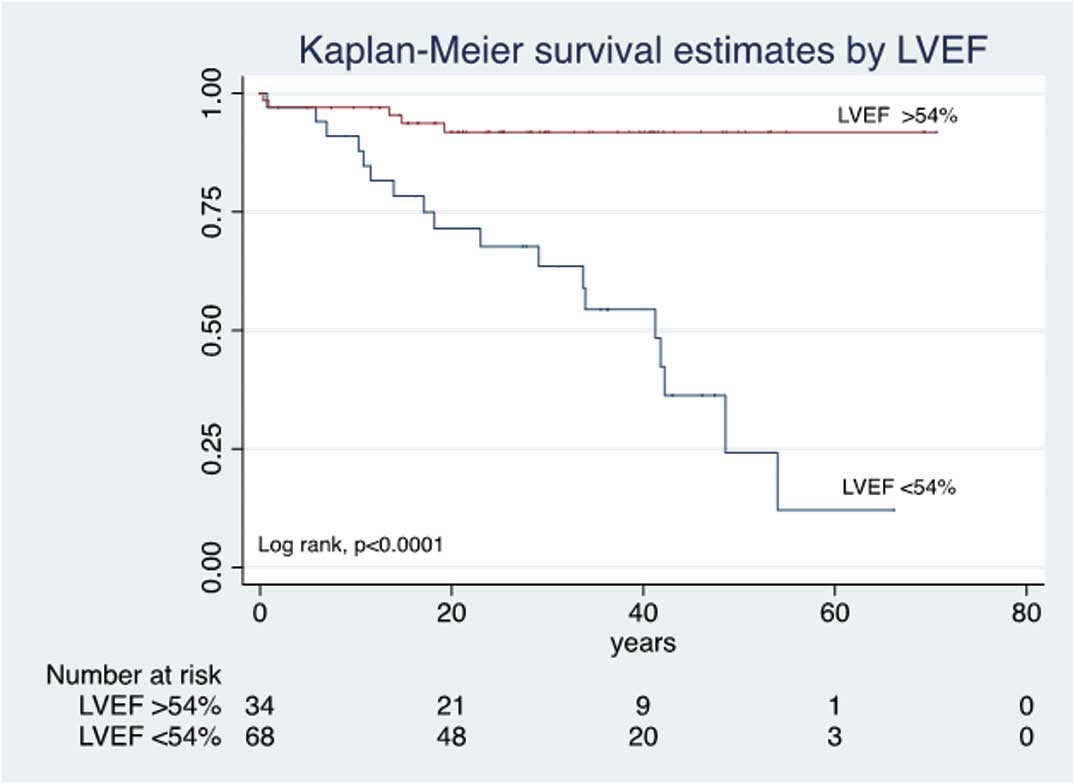
Kaplan-Meier survival vs. degree of left ventricular ejection fraction (LVEF) in Ebstein’s anomaly.
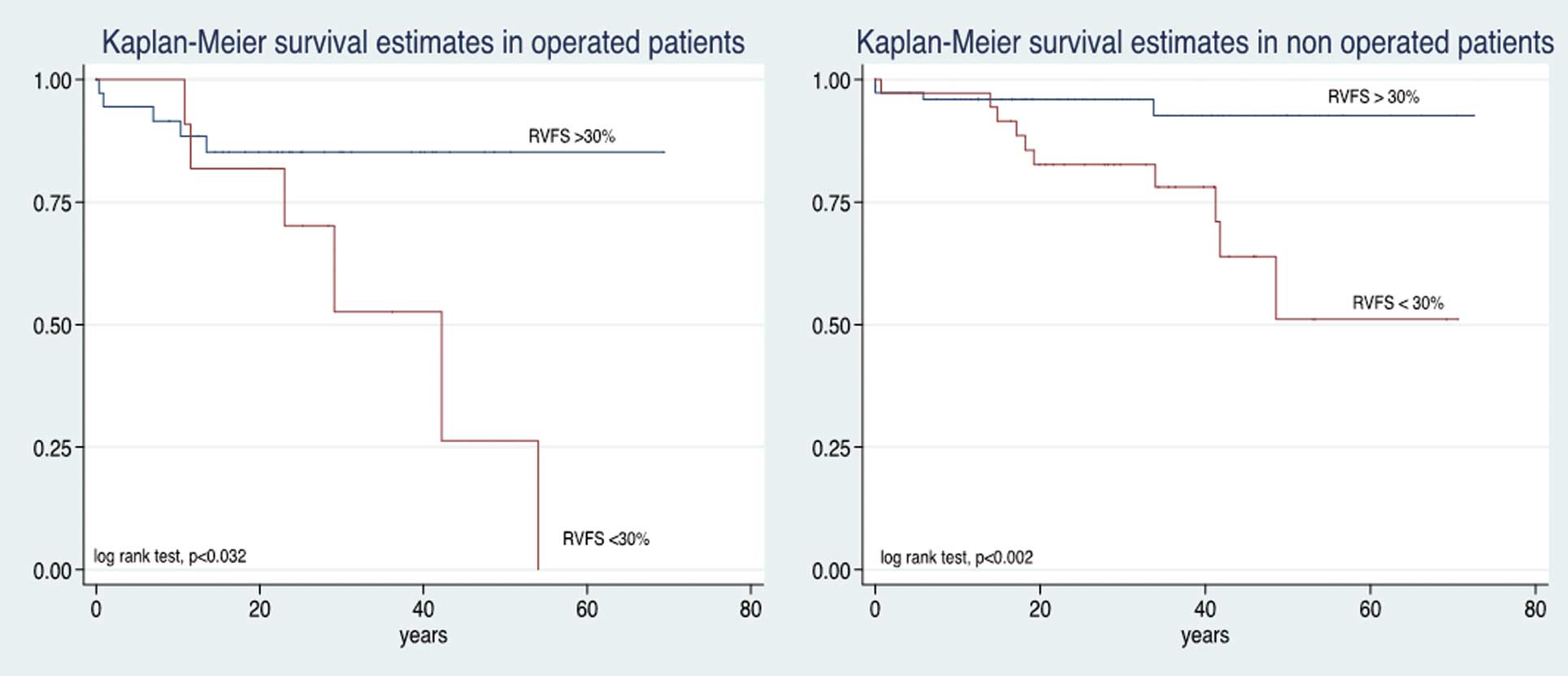
Kaplan-Meier survival vs. right ventricular function and presence of surgical treatment in Ebstein’s anomaly. RVFS, right ventricular fractional shortening.
In the present study, mortality in EA was analyzed according to the presence of surgical and non-surgical treatment. In order to be able to compare the groups, we included patients with similar severity, clinical and echocardiographic characteristics, and which fulfilled the currently accepted surgical criteria. At late follow-up there was no significant difference in mortality between the groups, but RV function was the main determinant of prognosis in both groups: when RV function was preserved, surgery improved survival. In contrast, in patients with significant RV dysfunction, early post-surgical mortality (first 30 days), was high.
Due to the low prevalence of EA it is difficult to have comparative groups, and even more to perform a prospective study. Therefore we decided to retrospectively collect data to compare 2 groups of patients with close clinical characteristics, who fulfilled current surgical criteria without prohibitive surgical risk. Some of the patients did not accept surgery, and they were included in the non-surgical group.
Surprisingly, the surgical and non-surgical group had similar long-term survival, meaning that surgery had no impact on survival, because in general it is expected that surgery prolongs life. In the literature no other studies have compared the impact of surgery on survival between these groups. The present study is one of the first to compare 2 apparently similar groups, to assess the real impact of surgery in terms of mortality. Other studies have shown improvement of symptoms, and better cardiac performance with an overall survival rate of 91.26% at 10 years in surgical patients.11,12 Brown et al noted a late survival of 84.7% and 71.2% at 10 and 20 years, respectively, in a big surgical group.9 Attenhofer Jost et al, in a study of 81 patients aged >50 years, found that this group had a good survival, but in comparison with the general population their survival was worse.12 In contrast, in a group with only clinical follow-up of adult patients, estimated cumulative overall survival was 76% at 10 years and 41% at 20 years of follow-up.10 In the present study, comparing patients with similar characteristics in surgical and non-surgical groups, survival at 10 years was 92.8% vs. 90.7%; and at 20 years, 85.7% vs. 81.0%, respectively.
Interestingly the surgical group had higher early mortality (18.36%) than in other series: 5.9% at 30 days.9 Chauvaud, in his series of 142 operated patients, noted an early mortality of 10%; and 5 patients with akinetic RV and a very thin ventricular wall had very early mortality (24 h).13 Early mortality in the present series was explained by RV failure in 77% of patients. Moreover, all of them had pre-surgical RV severe dysfunction, and surgical treatment was not aimed at reducing RV overload. Most of the surgeries were performed before 1995, and at that time surgical criteria were not clearly stated; we believe this contributed to the higher early mortality in the present series.
We investigated whether waiting time to surgery was associated with poor benefit of surgical treatment (i.e., that the longer time of volume overload would related to worse RV function), but on logistic regression analysis this was not the case: mortality was not associated with waiting time to surgery.
All of the present patients had complete closure of ASD, as was routine at the present institution; on bivariate analysis, ASD closure was associated with mortality but lost significance on multivariate analysis. ASD closure could be important because it can precipitate right-sided heart failure in some patients, given that it can function as an escape route to volume overload in severely damaged RV.14 This could have contributed to early mortality in the present surgical group.
In both groups, mortality on multivariate analysis was strongly associated with RV systolic function measured by RVFS. Due to the retrospective nature of this study, the more consistent measure on echocardiographic records was RVFS, therefore we selected this parameter to evaluate RV systolic function. Notably, when patients were divided into 3 groups according to RVFS, survival was worse with increasing RV dysfunction. In order to determine if RV systolic dysfunction was associated with the failure of surgical treatment to improve prognosis in terms of mortality, we compared patients according to presence of RV dysfunction. We found that worse RV function was associated with lower survival, independent of surgery.
The strong association of mortality with RVFS, supports the theory that EA is an RV myopathy.14 Survival differs from patient to patient due to the grade of myocardial damage. On histology, in most patients with EA, atrialized and functional RV is thin, fibrotic, and has an absolute decrease in the number of muscle fibers;15 this myocardial damage occurs in different grades, and is the basis of the wide spectrum of severity in this disease.
In other studies, many factors have been associated with mortality: age at diagnosis, cardiothoracic ratio, male sex, associated cardiac lesions, degree of TR, and residual RV function.10,16,17 We also found that low LVEF was associated with lower survival. Alterations in LV geometry cause changes in LV systolic function,18 and impaired LV systolic function is an independent predictor of mortality in EA (OR, 4.10; P=0.002),19 this is further supported by the present study.
Based on the present results, RV function should be the cornerstone for surgical decision making (time and type of surgery). Imaging should be performed to accurately assess RV function; Novel methods now exist to reproducibly and accurately assess RV function such as 3-D echocardiography, ventricular mechanics and cardiac magnetic resonance imaging.
Survival in EA is clearly associated with residual RV function. RV function should therefore be the key factor for decision making with regard to surgical technique and timing. Close monitoring of RV function is mandatory due to the absence of benefit in terms of mortality when RV systolic dysfunction occurs.
Study LimitationsWe included patients with moderate or severe EA, therefore the present results are not representative of the whole spectrum of disease. Due to its retrospective nature we did not have follow up on RV function; and last, novel echocardiographic techniques were not used to assess RV function due to their unavailability.
The authors declare no conflict of interest.