2018 Volume 82 Issue 1 Pages 71-77
2018 Volume 82 Issue 1 Pages 71-77
Background: Atrial fibrillation (AF) can be initiated from arrhythmogenic foci within the muscular sleeves that extend not only into the pulmonary veins but also into both vena cavae. The superior vena cava (SVC) is a key target site for catheter ablation. Patients with SVC-derived AF often lack the clinical risk factors of AF.
Methods and Results: We conducted a meta-analysis of the clinical and genetic factors of 2,170 AF patients with and without SVC arrhythmogenicity. In agreement with previous reports, the left atrial diameter was smaller in AF patients with SVC arrhythmogenicity. Among 6 variants identified in a previous genome-wide association study in Japanese patients, rs2634073 and rs6584555 were associated with SVC arrhythmogenicity. This finding was confirmed in our meta-analysis using independent cohorts. We also found that SVC arrhythmogenicity was conditionally dependent on age, body mass index, and left ventricular ejection fraction.
Conclusions: Both clinical and genetic factors are associated with SVC arrhythmogenicity.
Atrial fibrillation (AF) can be initiated from arrhythmogenic foci derived from muscular sleeves that extend not only into the pulmonary veins (PVs) but also into both vena cavae.1,2 Since the first recognition that AF triggers exist in the PVs, and subsequent trials of their electrical isolation with catheters,1 PV isolation has become one of the standard treatments for AF. Subsequently, intracardiac foci in sites other than the PVs have been found to trigger and/or drive AF,3 including the superior vena cava (SVC).4–6 Patients with arrhythmogenic SVC have been reported to have a smaller left atrial (LA) diameter and to display coexistence of spontaneous common atrial flutter compared with AF patients with PV foci only.6 Interestingly, patients with arrhythmogenic SVC have long myocardial sleeves around the SVC and high amplitude electrical potentials within them.5 These common phenotypes in arrhythmogenic SVC suggest the possible existence of genetic factors, a topic that has not been researched.
Genome-wide association studies of AF have identified 15 associated loci in international meta-analysis studies and 6 loci in Japanese patients to date.7,8 In particular, chromosome 4q25 variants have been repeatedly confirmed as a crucial locus for the prediction of success of PV isolation and cardioversion in people of European ancestry, whereas it failed to show that association in those of Asian ancestry.9–11 This study aimed to investigate the clinical characteristics and genetic factors associated with SVC arrhythmogenicity in consecutive AF patients undergoing catheter ablation including PV isolation.
We evaluated 2,170 AF patients who underwent PV isolation and catheter ablation for the first time at Saitama Red Cross Hospital (Study 1), the National Disaster Medical Center (Study 2), and Tsuchiura Kyodo Hospital (Study 3). All patients provided written informed consent to participate. The study protocol was approved by the ethical committees of the 3 cohorts above and of Tokyo Medical and Dental University. The study complied with the Declaration of Helsinki.
In Study 1, 1,534 consecutive participants who underwent catheter ablation for AF were recruited from 2011 to 2014; among them, 1,334 participants were evaluated for SVC arrhythmogenicity. No SVC arrhythmogenicity was found in 1,028 patients (non-SVC patients), and SVC arrhythmogenicity was found in 306 patients (SVC patients). The numbers of patients with paroxysmal AF and persistent AF were 919 and 353, respectively. The criteria used to define arrhythmogenic SVC are described later.5,6
The additional cohorts for the meta-analysis of SVC study were Study 2 and Study 3. All of the 268 paroxysmal AF patients who underwent PV isolation in Study 2 were recruited from 2012 to 2014. All participants in Study 3 were recruited as previously described for another clinical study on SVC arrhythmogenicity.6 The 568 patients gave written informed consent for genetic testing.
Mapping and Ablation ProtocolCatheter ablation was performed in all 3 cohorts using a common protocol.5,6,12 In brief, all anti-arrhythmic agents were discontinued ≥5 half-lives prior to the procedure. Transesophageal echocardiography was performed to exclude atrial thrombi after at least 1 month of anticoagulant administration. Surface electrocardiography and bipolar intracardiac electrography were continuously monitored and recordings were stored in a computer-based recording system (Labsystem Pro, Boston Scientific Inc., Boston, MA, USA Study 1; and Bard Electrophysiology, Lowell, MA, USA Studies 2 and 3). The intracardiac electrograms were filtered from 30 to 500 Hz. A 7-Fr 20-pole 3-site (7-Fr 14-pole 2-site in Study 3) mapping catheter was inserted through the right jugular vein for pacing and recording; 4-electrode catheters were positioned in the SVC, right atrium, coronary sinus (CS) and right ventricle throughout the procedure.
Assessment of SVC ArrhythmogenicitySVC arrhythmogenicity was conventionally defined as ectopy in the SVC initiating AF despite repeated PV isolation. In addition, SVC arrhythmogenicity was assessed with drug challenge and pacing maneuvers. A mapping catheter was placed in the SVC to map the circumferential SVC region using CT or transesophageal echocardiography as a reference (Figure 1). After PV isolation, the induction of atrial arrhythmias was attempted by infusion of high-dose isoproterenol (ISP), followed by atrial burst pacing from the pacing catheter in the CS. We defined an arrhythmogenic SVC as follows: (1) ectopy in the SVC initiating AF, (2) frequent ectopy from the SVC, or (3) AF in the SVC.5,6,12 We also defined it as (4) ectopy in the SVC initiating AF after repeated PV isolation and AF ablation.

Outline of mapping procedure for superior vena cava (SVC) arrhythmogenicity. (Upper) Anterior-posterior and left anterior oblique views of the configuration of the multi-electrode catheters. (Lower) Representative case. A circular mapping catheter was placed in the SVC. Atrial fibrillation initiation originated from the SVC. APC, atrial premature contraction; CS, coronary sinus; ETP, esophageal temperature probe; LPV, left pulmonary vein; RA, right atrium; SR, sinus rhythm; TA, tricuspid annulus.
All patients were continuously monitored with in-hospital ECG for several days after the procedure. Patients were followed up at the outpatient clinic at least every 3 months and evaluated with ECG and 24-h Holter monitoring. No anti-arrhythmic agents were prescribed after a 3-month blanking period. Recurrence was generally defined if AF could be documented by ECG.
SNP GenotypingGenomic DNA was extracted from white blood cells and purified in accordance with the standard manufacturer’s protocol (Wizard® SV Genomic DNA Purification System; Promega, Madison, WI, USA). After DNA was amplified in multiple primer sets, genotyping of single-nucleotide polymorphisms (SNP) was performed with an Invader assay (Third Wave Molecular Diagnostics®, Madison, WI, USA) using an ABI7300 (Applied Biosystems, Foster City, CA, USA) for 6 loci: at 1q24 in PRRX1 (rs593479), 4q25 near PITX2 (rs2634073), 7q31 in CAV1 (rs1177384), 10q25 in NEURL1 (rs6584555), 12q24 in CUX2 (rs649002), and 16q22 in ZFHX3 (rs12932445). All of these have been previously demonstrated to be associated with AF in the Japanese population.7,8 We validated all genotyping results for the 6 loci with capillary sequencing for 24 AF individuals in this study. The result of the Invader assay coincided completely with that of sequencing. To guarantee the quality control for SNP genotyping, we calculated the Hardy-Weinberg equilibrium, comparing the genotypic frequency of the AF cases with previously published data.7,8
Statistical AnalysisThe associations of SVC arrhythmogenicity with the variants were analyzed using logistic regression modeling (SPSS version 19, Chicago, IL, USA). We assessed whether the loci associated with SVC arrhythmogenicity in the univariate logistic analysis were also significant after adjustment for each parameter such as age, sex, body mass index (BMI), hypertension, and LA diameter. The P-value of the SNP association study for statistical significance was corrected with Bonferroni’s correction (corrected P-value <0.00833). Continuous data are expressed as the mean and standard deviation for normally distributed variables or as the median and 25th and 75th percentiles for non-normally distributed variables, and were compared using Student’s t-test or Mann-Whitney U-test, respectively. Categorical variables were analyzed using a logistic regression model. P<0.05 was considered statically significant.
We computed the weighted genetic risk score (GRS) for each individual by multiplying risk allele dosages weighted with the respective natural logarithm of the odds ratio of SNPs. We analyzed the association study of GRS with univariate and multivariate logistic regression models.
Meta-analysis was performed for both categorical and continuous data using the R statistical software environment with “metafor”. To evaluate the heterogeneity of meta-analysis, we calculated I2, and regarded I2 >25% and/or P<0.05 as the criteria for heterogeneity.
Of the 1,539 consecutive patients who underwent PV isolation and catheter ablation for AF, 1,334 were recruited as patients with SVC or non-SVC arrhythmogenicity after ISP challenge (22.9% and 77.1%, respectively). The PV isolation and catheter procedure were successfully performed for all participants. SVC isolation was carried out successfully for these patients (Figure 1).
The clinical characteristics of the 2 groups are summarized in Table 1 for the 3 individual cohorts. Compared with patients without SVC arrhythmogenicity, those with SVC arrhythmogenicity had fewer risk factors for AF, such as male sex, hypertension, diabetes, and congestive heart failure. AF onset age was younger, BMI and LA diameter were smaller, and ejection fraction (EF) was higher. Recurrence after catheter ablation was also observed more frequently in AF patients with SVC arrhythmogenicity.
| AF without SVCA | AF with SVCA | P value | |
|---|---|---|---|
| Study 1 | n=1,028 | n=306 | |
| Sex (female %) | 24.4 | 32.8 | 0.003 |
| Age (years) | 61.9±13.4 | 60.1±15.7 | 0.04 |
| Age at onset (years) | 57.3±14.0 | 54.7±16.0 | 0.006 |
| BMI (kg/m2) | 24.01±3.22 | 23.16±3.72 | 0.0004 |
| HTN (%) | 51.2 | 43 | 0.007 |
| DM (%) | 11.2 | 7.4 | NS |
| CHF (%) | 10.4 | 5.5 | 0.008 |
| AF (Paf/Pef/Caf) (%) | 68.7/26.0/5.3 | 69.5/28.0/2.5 | NS |
| Exposure duration (year) | 4.38±5.01 | 5.03±5.57 | NS |
| LAD (mm) | 37.12±6.91 | 35.64±7.059 | 0.003 |
| EF (%) | 64.31±11.4 | 66.1±10.4 | 0.019 |
| Recurrence (%) | 10 | 16.5 | 0.002 |
| Study 2 | n=239 | n=29 | |
| Sex (female %) | 30.2 | 39.4 | NS |
| Age (years) | 64.9±10.1 | 64.3±12.1 | NS |
| Age at onset (years) | NA | NA | |
| BMI (kg/m2) | 23.40±3.15 | 23.1±4.96 | NS |
| HTN (%) | NA | NA | |
| DM (%) | NA | NA | |
| CHF (%) | NA | NA | |
| AF (Paf/Pef) (%) | 98.5/1.5 | 96.9/3.1 | NS |
| Exposure duration (year) | 5.02±11.28 | 17.42±38.66 | 0.086 |
| LAD (mm) | 37.40±5.72 | 34.45±5.53 | 0.005 |
| EF (%) | 65.00±8.22 | 67.72±6.24 | NS |
| Recurrence (%) | NA | NA | |
| Study 3 | n=546 | n=22 | |
| Sex (female %) | 23 | 31.8 | NS |
| Age (years) | 62.6±9.9 | 60.6±9.5 | NS |
| Age at onset (years) | NA | NA | |
| BMI (kg/m2) | 24.52±3.58 | 23.59±3.76 | NS |
| HTN (%) | 45.7 | 36.3 | NS |
| DM (%) | 9.5 | 9.1 | NS |
| CHF (%) | 8.6 | 9 | NS |
| AF (Paf/Pef/longPsAF) (%) | 63.4/20.8/15.8 | 72.7/13.6/13.6 | NS |
| Exposure duration (year) | NA | NA | |
| LAD (mm) | 40.51±6.15 | 38.09±4.67 | 0.075 |
| EF (%) | 63.84±8.81 | 65.47±6.71 | NS |
| Recurrence (%) | NA | NA |
AF, atrial fibrillation; BMI, body mass index; Caf, chronic atrial fibrillation; CHF, chronic heart failure; DM, diabetes mellitus; EF, ejection fraction; HTN, hypertension; LAD, left anterior descending; longPsAF, long standing atrial fibrillation; Paf, paroxysmal atrial fibrillation; Pef, persistent atrial fibrillation; SVCA, superior vena cava arrhythmogenicity.
Table 2 shows the genotypic frequency in Study 1of the 6 SNP loci that were significantly associated with AF in the Japanese population in previous reports.7,8 We obtained the genotyping data of 1,334 patients and achieved a successful genotyping rate of 98.7%. The genotypic frequencies were similar to those previously reported.7,8 There were 2 SNPs, rs2634073 in chromosome 4q25 and rs6584555 in NEURL1, that were significantly associated with SVC arrhythmogenicity (Study 1, P=0.00046 and 0.0030, respectively; Table 2). We also performed a subanalysis of the association between the 2 SNPs and SVC arrhythmogenicity in males and females. Both SNPs were significant in males (rs2634073, P=0.00051, β=1.78 and rs6584555, P=0.022, β=0.72), but neither was significant in females (rs2634073, P=0.089, β=1.4 and rs6584555, P=0.21, β=0.78).
| Study 1 | Study 2 | Study 3 | ||||
|---|---|---|---|---|---|---|
| β | P value | β | P value | β | P value | |
| PRRX1 | 1.17 | 0.073 | – | – | – | – |
| CAV1 | 1.05 | 0.6 | – | – | – | – |
| CUX2 | 0.95 | 0.62 | – | – | – | – |
| NEURL | 0.72 | 0.003 | 0.73 | 0.26 | 0.66 | 0.26 |
| ZFHX3 | 1.11 | 0.25 | – | – | – | – |
| 4q25 | 1.56 | 0.00046 | 1.87 | 0.13 | 1.42 | 0.41 |
| GRS | 9.93 | 1.59×10−6 | 10.31 | 0.075 | 1.66 | 0.52 |
SNP, single-nucleotide polymorphism; SVCA, superior vena cava arrhythmogenicity.
To calculate GRS, we combined the data for the 2 SNP genotypes. GRS was also significantly associated with SVC arrhythmogenicity (P=1.59×10−6). GRS was also associated with SVC arrhythmogenicity in the multivariate logistic analysis adjusted for parameters such as age, sex, BMI, hypertension, and LA diameter (P=0.0020, β=1.696).
Meta-Analysis of LA Diameter and Genotype FrequencyWe performed a meta-analysis of all clinical (Figure 2A–E) and genetic factors (Figure 3A–C) available in the 3 cohorts (Table 3). LA diameter correlated negatively with SVC arrhythmogenicity.6 This meta-analysis confirmed the association of LA diameter with SVC arrhythmogenicity (Figure 2E, Table 3; P=5.72×10−5, I2=15.6%). Otherwise, we found a significant difference in sex, age, BMI, and EF between the 2 groups (Figure 2A–D). As for the 2 possible variants, although the genotypic frequency of rs2634073 varied in the 3 cohorts, the overall association was significant (Figure 3A, Table 3; P=1.27×10−4, I2=0.0%). The genotypic frequency of rs6584555 was consistent in the 3 cohorts and significantly associated with SVC arrhythmogenicity (Figure 3B, Table 3; P=5.80×10−4, I2=0.0%). After combining the 2 variants, the calculated GRS was also significantly associated (Figure 3C, Table 3; P=5.2×10−7, I2=0.0%).

Meta-analysis of clinical factors. Forest plots of (A) sex, (B) age, (C) body mass index, (D) left ventricular ejection fraction, and (E) left atrial diameter. We performed logistic regression analysis with a random effect (RE) model.
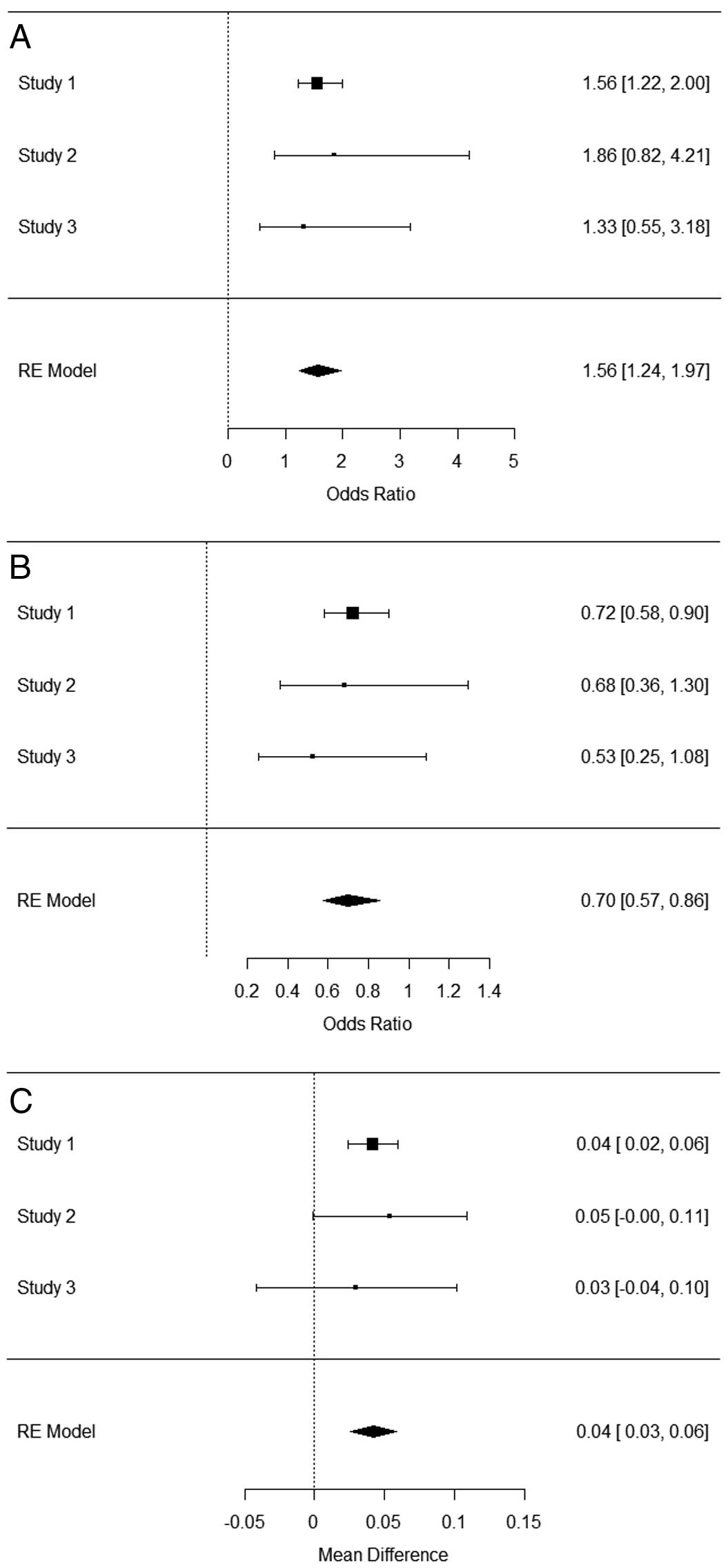
Meta-analysis of SNP genotypes. Forest plots of rs2634073 (A), rs6584555 (B) and genetic risk score (C). We performed logistic regression analysis with a random effect (RE) model. SNP, single-nucleotide polymorphism.
| P value | Estimate | SE | I2 | Test for heterogeneity | |
|---|---|---|---|---|---|
| Sex | 8.47×10−4 | 0.415 | 0.124 | 0.00 | Q=0.0231, P=0.988 |
| Age | 0.0328 | −1.651 | 0.773 | 0.00 | Q=0.320, P=0.851 |
| BMI | 5.57×10−5 | −0.805 | 0.2 | 0.00 | Q=0.641, P=0.725 |
| EF | 2.12×10−3 | 1.918 | 0.624 | 0.00 | Q=0.311, P=0.855 |
| LA diameter | 5.72×10−5 | −1.897 | 0.471 | 0.16 | Q=1.954, P=0.376 |
| rs2634073 | 1.27×10−4 | 0.447 | 0.116 | 0.00 | Q=0.305, P=0.858 |
| rs6584555 | 5.80×10−4 | −0.354 | 0.103 | 0.00 | Q=0.692, P=0.707 |
| GRS | 5.20×10−7 | 0.0424 | 0.0084 | 0.00 | Q=0.294, P=0.863 |
LA, left atrium. Other abbreviations as in Table 1.
To investigate the effect of these variants on the closest gene expression, we sorted the eQTL in the GTEx portal site (https://www.gtexportal.org) and found 10 eQTLs that change NEURL1 expression. Among them, rs12253987 and rs7900994 were in linkage disequilibrium with rs6584555 (D Prime 0.889 and 1.0, respectively) in the HapMap project and their risk allele decreased NEURL1 expression (effect size −0.35, -value 0.0000059; effect size −0.46, -value 0.0000072; Table S1).
Recent studies have shown that the anatomical5 and clinical6 characteristics of patients with arrhythmogenic SVC are different from those without arrhythmogenic SVC; the former have a longer myocardial SVC sleeve and smaller LA diamter. In the current study, we tested the hypothesis that patients with arrhythmogenic SVC would also have a genetic fingerprint different from the others. Therefore, we conducted a meta-analysis of 3 cohorts to investigate the association between SNP variants identified in genome-wide association studies for AF and SVC arrhythmogenicity. Using data from subjects who underwent catheter ablation for AF, we found 2 SNP variants that were significantly associated with SVC arrhythmogenicity.
The genes closest to the 2 SVC variants we found were PITX2 in 4q25 and NEURL1 in 10q24. PITX2c was originally reported as a left-right determinant in cardiac development.13 In animal studies, Pitx2 expression can be observed in the heart regions arising from the secondary heart field. Although it is downregulated in the ventricular chambers, high and robust expression is maintained in the atrial chambers and in discrete components of the inflow tract, including the LA chamber.14,15 More recent studies have revealed that PITX2 plays a crucial role in regulating a variety of ion channels and gap junction channels as a transcription factor.16,17 Two atrial-specific conditional knockout mice lines (Sox2-Cre-Pitx2 and Nppa-Cre-Pitx2) affected their arrhythmogenicity through the calcium-handling pathway and WNT signaling.18 By contrast, the function of NEURL1, an ubiquitin E3 ligase in cardiomyocytes, remains unknown. The suppression of NEURL1 expression in zebrafish with morpholino leads to shortening of the action potential (AP) duration. NEURL1 has been suggested to regulate the metabolism of ion transporters. The closest genes, PITX2 for rs2634073 and NEURL1 for rs6584555, interact with each other as previously described.8 These proteins regulate the profile of ion transporters or change AP duration. Hence, we speculate that reduced PITX2 and NEURL1 expression in patients with genetic risk for SVC arrhythmogenicity could cause AF susceptibility by AP duration shortening and disorganized profiles of genes and proteins.
We also studied the effect of genotype on gene expression using GTEx. NEURL1 includes 10 eQTLs, 2 of which are in linkage disequilibrium with rs6584555. On the other hand, no eQTL of PITX2 has ever been reported. According to published data, NEURL1 expression is decreased with the risk allele of rs6584555.
Study LimitationsThis study has 2 limitations. First, the protocols for the assessment of SVC arrhythmogenicity were not completely identical in the 3 cohorts. Nevertheless, we identified at least 2 SNPs that were associated with SVC arrhythmogenicity. Second, we had no data about the properties of the SVC, such as the length of the myocardial sleeves or the amplitude of electrical potentials. Analysis of the association between such SVC properties and genotype variants may provide important clues to the mechanism of AF onset.
Among SNP variants identified previously in genome-wide association studies of AF in Japan, we found rs2634073 and rs6584555 to be associated with SVC arrhythmogenicity. Meta-analysis of 3 patient cohorts showed that those with SVC arrhythmogenicity had normal LA diameter and high frequency of risk alleles rs2634073 and rs6584555. These variants are located in the PITX2 and NEURL1 loci. NEURL1 expression was downregulated in the left atrial appendage according to the number of risk allele of rs6584555 in the GTEx portal site. We speculate that AP duration dispersion caused by the suppression of NEURL1 in SVC tissue of patients with the risk genotype could be the cause of arrhythmogenic SVC.
We thank Koji Higuchi at Hiratsuka Kyosai Hospital and Tatsuhiko Tsunoda at Tokyo Medical and Dental University for their advice on SVC properties and statistical methods. We also express our gratitude to the study participants and the doctors and research staff at the three study sites.
No conflicts of interest to disclose.
This work was supported by a Grant from Tailor-made Medical Treatment Program (1K157) and a Grant-in-Aid (23790841) from Ministry of Education, Culture, Sports, Science and Technology of Japan.
Supplementary File 1
Table S1. NEURL1 eQTL in GTEx portal site
Please find supplementary file(s);
http://dx.doi.org/10.1253/circj.CJ-17-0350