2018 Volume 82 Issue 10 Pages 2575-2583
2018 Volume 82 Issue 10 Pages 2575-2583
Background: The objective of the present analyses was to describe the baseline characteristics and treatment of the Japanese patients with HFrEF in THE PARALLEL-HF study.
Methods and Results: Key demographic, clinical and laboratory findings, along with treatment, were reported and compared with patients enrolled in the PARADIGM-HF trial and other contemporary randomized clinical trials and registries of Japanese patients with HFrEF. In addition, the MAGGIC and EMPHASIS-HF risk scores were calculated. A total of 225 Japanese patients were randomized in PARALLEL-HF with a mean age of 67.9 years and the majority of the patients being male (85.8%) and in NYHA Class II (93.8%). Key baseline characteristics in PARALLEL-HF were generally comparable with PARADIGM-HF, and other contemporary clinical trials and registries of Japanese HFrEF patients. Patients enrolled in PARALLEL-HF were well treated with conventional evidence-based therapy at baseline (angiotensin-converting enzyme inhibitor inhibitor/angiotensin receptor blocker, 62.7%/37.3%; β-blockers, 94.7%; mineralocorticoid receptor antagonist, 59.1%). Despite the evidence-based treatment and most patients being in NYHA Class II, these patients had a low LVEF (mean 28.1%) and were at high risk of cardiovascular mortality and morbidity as assessed by the MAGGIC and EMPHASIS-HF risk scores.
Conclusions: Overall, the patients in PARALLEL-HF were largely representative of contemporary ambulatory patients with HFrEF who are well treated with evidence-based therapies. PARALLEL-HF will determine whether sacubitril/valsartan provides similar improvements in clinical outcomes in Japanese HFrEF patients as observed in the PARADIGM-HF study.
The aging population and rising prevalence of various cardiovascular (CV) risk factors, such as hypertension and diabetes, in Japan imply that a considerable proportion of the population will continue to develop heart failure (HF). By the year 2025, the annual incidence of new-onset HF in Japanese individuals aged ≥65 years (≈31% of total population) is estimated to be >0.37 million, emphasizing the need for effective treatments in patients with HF.1–3 The “Phase III Prospective comparison of Angiotensin Receptor neprilysin inhibitor with Angiotensin-converting enzyme inhibitor to Determine Impact on Global Mortality and Morbidity in Heart Failure trial” (PARADIGM-HF) showed that sacubitril/valsartan, an angiotensin receptor neprilysin inhibitor (ARNI) was superior to the angiotensin-converting enzyme inhibitor (ACEI) enalapril in reducing the risk of CV and all-cause death, hospitalization for HF, and decline in quality of life in patients with HF and reduced ejection fraction (HFrEF).4,5 Burnett et al conducted a network meta-analysis of 57 randomized controlled trials published between 1987 and 2015, comparing the efficacy of ACEIs, angiotensin receptor blockers (ARBs), ARNIs, mineralocorticoid receptor antagonists (MRA), and β-blockers and their combinations in reducing all-cause death in patients with HFrEF.6 In that analysis, combination therapy with ARNIs (sacubitril/valsartan), β-blockers, and MRA was associated with the greatest reduction in all-cause death vs. placebo (hazard ratio [HR] 0.37; 95% confidence interval [CI]: 0.19–0.65) and was superior to all other drug classes or combination therapies that are known to reduce mortality rates in HFrEF.6 The “Phase III prospective comparison of ARNI with ACEI to determine the noveL beneficiaL trEatment vaLue in Japanese Heart Failure patients” (PARALLEL-HF) study has been designed to evaluate the clinical efficacy and safety of sacubitril/valsartan vs. enalapril in Japanese patients with HFrEF.7
Editorial p 2479
Data from global clinical trials and disease registries in the USA, Europe and the Asia-Pacific region highlighted several differences in the demographic and clinical characteristics of HF patients in the Asia-Pacific region compared with patients from Western countries; for example, patients from the Asia-Pacific region were younger, had greater comorbidity burden and received lower-than-recommended levels of treatment.2,8 The patient eligibility criteria of PARALLEL-HF were designed to largely follow those of PARADIGM-HF to select a representative sample of the larger population of Japanese patients with HFrEF. Therefore, the objective of the present analyses was to (1) describe the baseline characteristics of PARALLEL-HF participants; and (2) compare the study population of PARALLEL-HF with those of PARADIGM-HF and other randomized clinical trials and registries of Japanese patients with HFrEF. In addition, the risk profiles of patients enrolled in PARALLEL-HF was assessed at study entry using the MAGGIC and EMPHASIS-HF risk scores for death and was compared with those in PARADIGM-HF.
The objectives and design of PARALLEL-HF have been described in detail.7 In brief, PARALLEL-HF is a randomized, double-blind, parallel-group, active-controlled study of adult chronic HF patients (New York Heart Association [NYHA] Classes II–IV) with reduced left ventricular ejection fraction (LVEF ≤35%) and on stable doses of ACEIs or ARBs before study entry. The study has 3 phases: (1) screening, (2) single-blind treatment run-in, and (3) double-blind randomized treatment. At the screening visit, patients’ eligibility is assessed by demographics and medical history, LVEF, NYHA class, levels of N-terminal pro-brain natriuretic peptide (NT-proBNP), serum potassium, blood urea nitrogen, and serum creatinine, and estimated glomerular filtration rate (eGFR) (Table 1). Eligible patients then enter a single-blind active treatment run-in of sacubitril/valsartan 50 mg twice daily (b.i.d.) for 2 weeks. Patients who tolerate this dosage during the run-in are randomized (1:1) to receive sacubitril/valsartan 100 mg b.i.d. or enalapril 5 mg b.i.d. for 4 weeks followed by uptitration, if tolerated, to target doses of sacubitril/valsartan 200 mg b.i.d. or enalapril 10 mg b.i.d. in a double-blind manner. The primary objective of the trial is to assess the effect of sacubitril/valsartan compared with enalapril in addition to background HF treatments, in delaying the time to first occurrence of the composite endpoint of CV death or HF hospitalization, the primary endpoint. Assuming a 20% hazard reduction in the primary endpoint with sacubitril/valsartan over enalapril, approximately 57 primary endpoint events are required in 220 patients to ensure at least an 80% probability of observing a HR of sacubitril/valsartan over enalapril of <1 and to support the consistency in efficacy between the PARALLEL-HF and PARADIGM-HF studies.
| Inclusion criteria | PARALLEL-HF7 | PARADIGM-HF9 | Ivabradine Japanese Phase II trial11 |
J-EMPHASIS-HF10 |
|---|---|---|---|---|
| Age (years) | ≥20 | ≥18 | ≥20 | ≥55 |
| NYHA class | II–IV | II–IV | II–IV | II–IV |
| LVEF (%) | ≤35% | ≤40% (changed to ≤35%) |
≤35% | ≤30% (or ≤35%+QRS duration >130 ms on ECG) |
| Natriuretic peptide level |
NT-proBNP ≥600 pg/mL, or NT-proBNP ≥400 pg/mL+HF hospitalization within 12 months |
BNP ≥150 pg/mL (NT-proBNP ≥600 pg/mL) or BNP ≥100 pg/mL (NT-proBNP ≥400 pg/mL)+HF hospitalization within 12 months |
– | CV hospitalization 6 months before randomization or BNP ≥250 pg/mL or NT-proBNP ≥500 pg/mL (men) and ≥750 pg/mL (women) within 15 days of randomization |
| Resting HR | – | – | ≥75 beats/min in sinus rhythm |
– |
| eGFR (mL/min/1.73 m2) | ≥30 | ≥30 | ≥30 | ≥30 |
| SBP (mmHg) | ≥100 (screening visit); ≥95 (at end of run-in) |
≥100 (screening visit); ≥95 (at end of run-in) |
– | |
| Run-in | Yes (sacubitril/valsartan run-in) |
Yes (sequential enalapril and sacubitril/valsartan run-in) |
– | – |
| Baseline treatment | Stable dose of ACEI/ARB and β-blocker for 4 weeks; MRA as indicated |
Stable dose of ACEI/ARB (at least equivalent to enalapril 10 mg b.i.d.) and β-blocker for 4 weeks; MRA as indicated |
Optimal, stable treatment according to the Japanese Guideline for treatment of chronic HF |
ACE inhibitor, ARB, β-blocker, or diuretic |
| Comparison | Enalapril 10 mg b.i.d. Sacubitril/valsartan 200 mg b.i.d. |
Enalapril 10 mg b.i.d. Sacubitril/valsartan 200 mg b.i.d. |
Placebo Ivabradine 2.5 mg or 5 mg b.i.d. |
Placebo Eplerenone 25 mg or 50 mg o.d. |
| Enrollment period | 2015–2016 | 2009–2012 | 2013–2015 | 2010–2015a |
aStudy period. ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; eGFR, estimated glomerular filtration rate; HFrEF, heart failure and reduced ejection fraction; HR, heart rate; J-EMPHASIS-HF, Eplerenone in Japanese Patients with Chronic Heart Failure; MRA, mineralocorticoid receptor antagonist; NT-proBNP, N-terminal pro-brain natriuretic peptide; NYHA, New York Heart Association; PARADIGM-HF, Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure; PARALLEL-HF, Prospective comparison of ARNI with ACEI to determine the noveL beneficiaL trEatment vaLue in Japanese Heart Failure patients; SBP, systolic blood pressure.
We describe here the analysis of the baseline characteristics of the 225 randomized patients in PARALLEL-HF. In addition, we compare the characteristics of these patients with the patients with HFrEF in PARADIGM-HF,9 and in recent randomized clinical trials (J-EMPHASIS-HF10 and a Phase II study that evaluated the effects of ivabradine11) and in the CHART-212 and JCARE-CARD13 registries that included Japanese patients with HFrEF.
Finally, the patient risk scores for death were assessed using the MAGGIC and EMPHASIS-HF risk scores. The MAGGIC risk score was calculated for all of the 225 randomized patients using the screening visit data for 13 prognostic variables (age, LVEF, NYHA class, serum creatinine, diabetes, β-blocker prescription, systolic blood pressure [SBP], body mass index [BMI], time since HF diagnosis, current smoker, chronic obstructive pulmonary disease [COPD], male sex, and ACEI or ARB prescription), as previously described.14 The EMPHASIS-HF risk score was calculated for the 211 randomized patients in NYHA Class II, using the screening visit data for 10 prognostic variables (age, sex, SBP, eGFR, diabetes, BMI, hemoglobin, prior HF hospitalization, prior myocardial infarction (MI)/coronary artery bypass graft (CABG), and heart rate), as previously described.15 Similar calculations of the MAGGIC (for the complete randomized set) and EMPHASIS-HF risk scores (for the NYHA Class I/II randomized set) were conducted for PARADIGM-HF patients with screening visit data for all the required prognostic variables.
A total of 246 patients entered the run-in period to receive sacubitril/valsartan 50 mg b.i.d. for 2 weeks; of these, 225 were randomized in the study between June 2015 and December 2016 across 49 sites. In total, 21 patients (8.5%) were determined as run-in failures because of adverse events (AE), non-compliance with study treatment, physician decision, protocol deviation, and subject/guardian decision (Figure 1). Run-in failure solely because of AE occurred in only 2 patients.
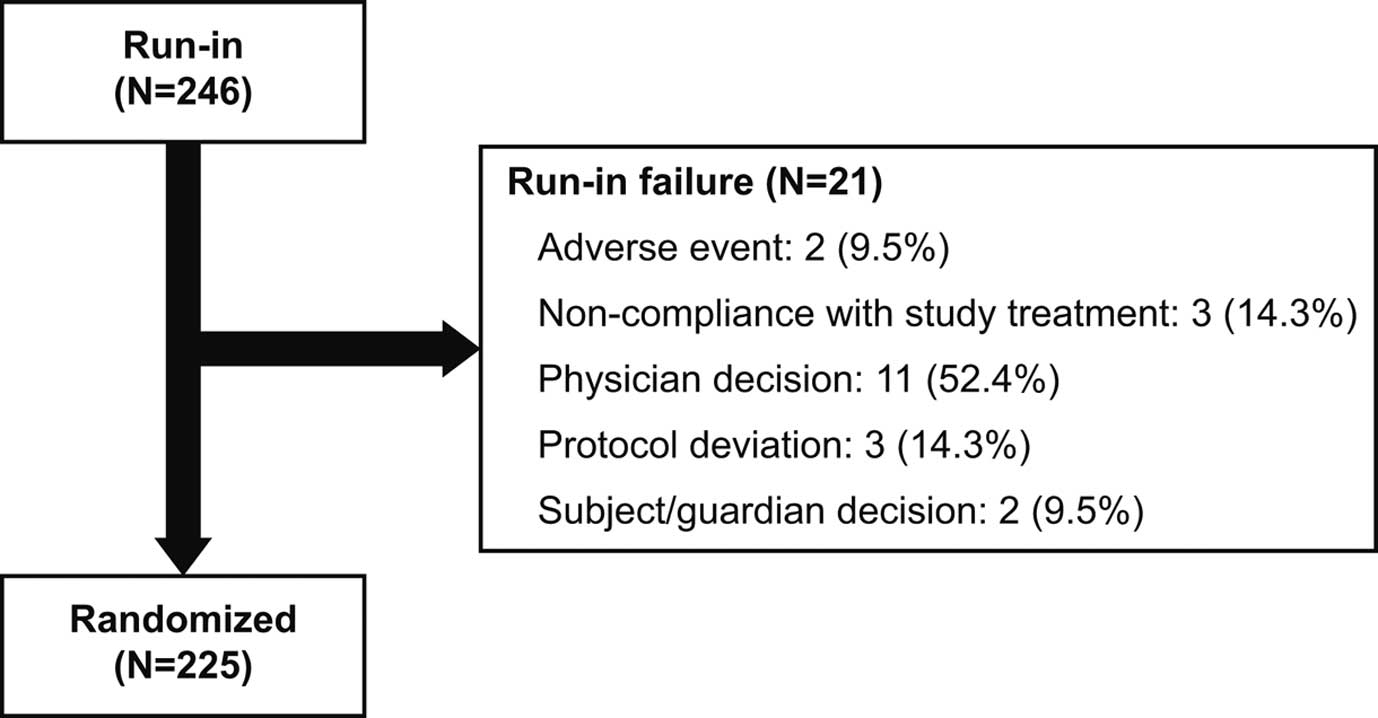
Patient flow in the Prospective comparison of ARNI with ACEI to determine the noveL beneficiaL trEatment vaLue in Japanese Heart Failure patients (PARALLEL-HF) trial. ACEI, angiotensin-converting enzyme inhibitor; ARNI, angiotensin receptor neprilysin inhibitor.
Of the 246 patients who entered the run-in period, AE occurred in 32 patients. The most frequent AE was viral upper respiratory tract infection (4.1%; n=10), followed by pneumonia (0.8%; n=2) and dizziness (0.8%; n=2). No cases of angioedema were reported. All AE are summarized in Table S1.
Effects of Sacubitril/Valsartan on Vital Signs and Laboratory Parameters During the Run-in PeriodChanges in vital signs and laboratory parameters from baseline (screening) to the end of the run-in period are summarized in Table 2. Systolic and diastolic BPs decreased by an average of 4.3 and 2.2 mmHg, respectively, following the 2-week run-in period with sacubitril/valsartan 50 mg b.i.d. Heart rate increased by an average of 1.6 beats/min during the same period of time. All changes were statistically significant. Serum creatinine levels and eGFR did not change. Serum potassium levels significantly decreased from 4.33 to 4.22 mEq/L (P<0.001).
| Baseline (screening) (n=245) |
End of run-in (n=245) |
Change from baseline |
P value | |
|---|---|---|---|---|
| SBP (mmHg) | 121.8±15.9 | 117.5±16.0 | −4.3±12.3 | <0.01 |
| DBP (mmHg) | 72.6±12.4 | 70.4±11.7 | −2.2±11.1 | <0.01 |
| HR (beats/min) | 73.3±12.9 | 74.9±13.5 | 1.6±11.6 | 0.03 |
| Creatinine (mg/dL) | 1.04±0.26 | 1.04±0.28 | 0.01±0.14 | 0.4184 |
| eGFR (mL/min/1.73 m2) | 57.50±16.47 | 57.51±17.24 | 0.01±7.21 | 0.980 |
| Potassium (mEq/L) | 4.33±0.46 | 4.22±0.49 | −0.11±0.44 | <0.001 |
Data are mean±SD. DBP, diastolic blood pressure. Other abbreviations as in Table 1.
Key baseline variables of the 225 patients randomized in the study are summarized in Table 3.
| Clinical trials | Registries | |||||
|---|---|---|---|---|---|---|
| PARALLEL-HF (n=225) |
PARADIGM-HF (n=8,442) |
Ivabradine Japanese Phase II trial11 (n=126) |
J-EMPHASIS-HF10 (n=221) |
CHART 212 LVEF<40% (n=730) |
JCARE-CARD13 LVEF<40% (n=985) |
|
| Age (mean) years | 67.9 | 63.8 | 59 | 68.7 | 66.9 | 66.6 |
| Female sex (%) | 14.2 | 21.9 | 14.3 | 20.4 | 23.3 | 27.8 |
| NYHA class (%) | ||||||
| I | 0 | 0.3 | 0 | 0 | 14.3 | 35.7 |
| II | 93.8 | 64.6 | 92.0 | 82.8 | 67.0 | 55.7 |
| III | 6.2 | 33.5 | 8.0 | 17.2 (III/IV)a | 17.1 | 5.9 |
| IV | 0 | 1.5 | 0 | – | 1.7 | 2.7 |
| Race, Asian (%) | 100b | 17.9 | – | 100b | – | – |
| Heart rate (mean) beats/min |
73.1 | 73.5 | 82.7 | 74.3 | 74.0 | 70.9 |
| SBP (mean) mmHg | 122.3 | 128.4 | 118.2 | 117.6 | 117.9 | 113.2 |
| DBP (mean) mmHg | 72.8 | 77.8 | 71.9 | 69.5 | 69.8 | 66.0 |
| LVEF (mean) % | 28.1 | 29.5 | 28.4 | 26.1 | 31.1 | 27.0 |
| NT-proBNP (median) pg/mL |
1,130 | 1,612.5 | 812 | 2,635.8/2,354.3 | – | – |
| BNP (median) pg/mL | – | – | 143 | 469.3/435.6c | 216.0 | 396 |
| BMI (mean) kg/m2 | 24.5 | 28.1 | 24.9 | 22.6 | 22.7 | 22.7 |
| eGFR (mean) mL/min/1.73 m2 |
74.6 (57.9d) | 68.1 | – | 56.6 | 58.2 | – |
| eGFR <60 mL/min/1.73 m2 (%) |
28.0 | 35.3 | – | 60.4/60.0c | – | – |
| Ischemic etiology (%) | 47.6 | 59.9 | 42.9 | 33.5 | 50.1 | 39.8 |
| Medical history (%) | ||||||
| Hospitalization for HF | 72.9 | 62.8 | – | 76.5 | 77.1 | 100 |
| Hypertension | 67.6 | 70.7 | 50.8 | 58.4 | 84.7 | 50.4 |
| Angina pectoris | 25.8 | 32.9e | 26 | 16.3 | – | – |
| Myocardial infarction | 43.1 | 43.2 | 30.2 | 30.3 | 39.3 | – |
| PCI | 36.9 | 21.4 | – | 28.5 | 29.2 | 20.8 |
| CABG | 16.9 | 15.5 | – | 11.8 | 10.1 | 11.4 |
| Atrial fibrillation | 33.8 | 36.5 | 7.1 | 34.4 | 38.1 | 24.5 |
| Diabetes mellitus | 46.2 | 34.5 | 50 | 39.8 | 38.1 | 33.3 |
| Stroke | 9.3 | 8.6 | – | 14.0 | 18.9 | 14.6 |
| Current smoker | 16.0 | 14.4 | – | 14.0 | – | |
| Treatment (%) | ||||||
| Diuretic | 83.1 | 82.6 | 82 | 85.1 | 76.2 | 88 |
| ACEI | 62.7 | 77.7 | 45.2 | 49.8 | 57.7 | 44.2 |
| ARB | 37.3 | 22.6 | 25.4 | 36.7 | 26.7 | 45.9 |
| ACEI or ARB, or both | 100 | 99.8 | 70.6 | 82.4 | – | 83.5 |
| β-blocker | 94.7 | 94.3 | 92.9 | 86.0 | 69.6 | 65.9 |
| MRA | 59.1 | 58.4 | 54 | – | 43.7 | 45.9f |
| Digoxing | 8.4 | 30.3 | 6.3 | 14.0 | – | 28.7 |
| Anticoagulant | 48.9 | 31.8 | – | 52.5 | – | – |
| Aspirin | 39.1 | 52.0 | – | – | – | 49 |
| ADP antagonist | 22.7 | 15.1 | – | – | – | – |
| Lipid lowering | 56.0 | 55.3 | – | 50.7 | 38.8h | 23.1h |
| CRT | 12.4 | 6.8 | 6.3 | 11.8i | 5.6 | – |
| ICD | 6.7 | 14.8 | 1.6 | 6.8 | 7.0 | – |
| CRT-D | 10.7 | 5.0 | 5.6 | – | – | – |
aIncludes both NYHA class III and IV. bAll Asian patients with HFrEF are Japanese. cEplerenone/placebo. deGFR calculated using formula for Japanese; 194*(serum creatinine [mg/dL])−1.094*(age [years])−0.287*(0.739 if female). eStable/unstable. fSpironolactone only. gIncludes all digitalis derivatives. hStatins only. iIncludes CRT and CRT-D. ADP, adenosine diphosphate receptor; BMI, body mass index; CABG, coronary artery bypass graft; CHART-2, Chronic Heart Failure Analysis and Registry in the Tohoku District-2; CRT, cardiac resynchronization therapy; CRT-D, CRT plus defibrillator therapy; DBP, diastolic blood pressure; HF, heart failure; ICD, implantable cardioverter defibrillator; JCARE-CARD, Japanese Cardiac Registry of Heart Failure in Cardiology; LVEF, left ventricular ejection fraction; PCI, percutaneous coronary intervention. Other abbreviations as in Table 1.
Comparison With PARADIGM-HF In PARALLEL-HF, there was a predominance of male patients (85.8%), and the mean patient age was 67.9 years, which was higher than in PARADIGM-HF (63.8 years). The majority of patients were in NYHA Class II at the screening visit (93.8 vs. 64.6% in PARADIGM-HF), and the remaining patients were in NYHA Class III (6.2 vs. 33.5% of patients in PARADIGM-HF). Although the median NT-proBNP level was also lower in PARALLEL-HF (1,130 vs. 1,612.5 pg/mL in PARADIGM-HF), LVEF was similar across the 2 trials (28.1 vs. 29.5%, respectively). Both SBP and diastolic BP (DBP) were relatively lower in PARALLEL-HF than in PARADIGM-HF (122.3 vs. 128.4 mmHg and 72.8 vs. 77.8 mmHg, respectively) despite similar heart rate (73.1 vs. 73.5 beats/min). As anticipated, mean baseline BMI was lower in PARALLEL-HF (24.5 vs. 28.1 kg/m2). These characteristics of PARALLEL-HF patients were consistent with the findings from randomized clinical trials and registries of Japanese HFrEF patients.
Comparison With Japanese HFrEF Trial and Registry Population The mean patient age in PARALLEL-HF (67.9 years) was similar to that in J-EMPAHASIS-HF (68.7 years) and the HFrEF (LVEF <40%) subgroups of the CHART 2 (66.9 years) and JCARE-CARD (66.6 years) registries (Table 3). In addition, the NYHA class distribution was similar to previous observations of Japanese HFrEF patients, with the majority of patients in NYHA Class II/III in both registries (84.1% in CHART-2 and 61.6% in JCARE-CARD) and randomized studies (100% in Ivabradine J-P2 and >82.8% in J-EMPHASIS-HF). The mean SBP was approximately 118 mmHg across the randomized clinical trials and CHART 2 registry, and was lowest in the JCARE-CARD registry (113.2 mmHg). The mean LVEF, heart rate and BMI values in PARALLEL-HF were largely comparable to those reported in randomized clinical trials and registry HFrEF populations.
Medical and Surgical Histories of Randomized PatientsComparison With PARADIGM-HF The proportion of patients with ischemic etiology of HF was less in PARALLEL-HF (PARALLEL-HF: 47.6% vs. PARADIGM-HF: 59.9%). The majority of patients in PARALLEL-HF (72.9%) had a prior history of hospitalization for HF compared with 62.8% in PARADIGM-HF. The proportions of patients with hypertension, history of coronary artery disease (angina pectoris and MI) and atrial fibrillation were comparable across the 2 trials, but diabetes was reported in 46.2% in PARALLEL-HF compared with 34.5% in PARADIGM-HF (Table 3).
Comparison With Japanese HFrEF Trial and Registry Population In the comparison with other Japanese patients with HFrEF, the rate of ischemic etiology (47.6%) was comparable to that reported in other clinical trials and registry populations (34–50%). A relatively higher proportion of patients had a history of MI and percutaneous coronary intervention (PCI)/CABG in PARALLEL-HF compared with other randomized clinical trials and registry populations. The high prevalence of diabetes in PARALLEL-HF (46.2%) was largely comparable across the clinical trials (40–50%) (Table 3).
Baseline Treatment of Randomized PatientsComparison With PARADIGM-HF Overall, the patients were well treated in PARALLEL-HF, with the majority of patients taking ACEIs or ARBs (100%), diuretics (83.1%), β-blockers (94.7%) and MRAs (59.1%), the rates of which were very similar to those in PARADIGM-HF, although ARB treatment was more frequent in PARALLEL-HF than in PARADIGM-HF (37.3% vs. 22.6%). It was noted that the majority of patients (93.3%) enrolled in PARALLEL-HF were taking ACEI/ARB at a dose equivalent to ≤10 mg enalapril prior to study entry. The only difference was the lower use of digoxin in PARALLEL-HF than in PARADIGM-HF (8.4 vs. 30.3%). The use of device-based therapies such as cardiac resynchronization therapy (CRT) and CRT plus defibrillator therapy (CRT-D) was reported to be 12.4% and 10.7%, respectively, in PARALLEL-HF (Table 3).
Comparison With Japanese HFrEF Trial and Registry Population Compared with PARALLEL-HF, the rate of ACEI/ARB use in other randomized clinical trials and registries ranged from 70.6% to 83.5%, reflecting the higher use of renin-angiotensin system (RAS) blockers in the PARALLEL-HF population as required by the study protocol. In addition, the use of β-blockers and MRAs was relatively less in the HFrEF cohorts of the CHART-2 (β-blockers, 69.6%; MRA, 43.7%) and JCARE-CARD (65.9% and 45.9%) registries. On the other hand, the use of diuretics was quite comparable across PARALLEL-HF and other randomized clinical trials and registries of Japanese patients with HFrEF (Table 3).
Baseline Risk Analysis by MAGGIC and EMPHASIS-HF Risk ScoresAt screening, all 225 randomized patients in PARALLEL-HF had complete data across all of the 13 variables required for calculation of the MAGGIC risk score. Of the overall population in PARADIGM-HF, 8,409 patients had screening visit data for all of the 13 variables required for calculation of the MAGGIC risk score. The median MAGGIC risk score was 23 points (interquartile range [IQR], 19–26 points; range, 6–33 points) in PARALLEL-HF, which was slightly higher than the median scores in PARADIGM-HF (20 points, IQR 16–24 points; range, 5–42 points; Figure 2).
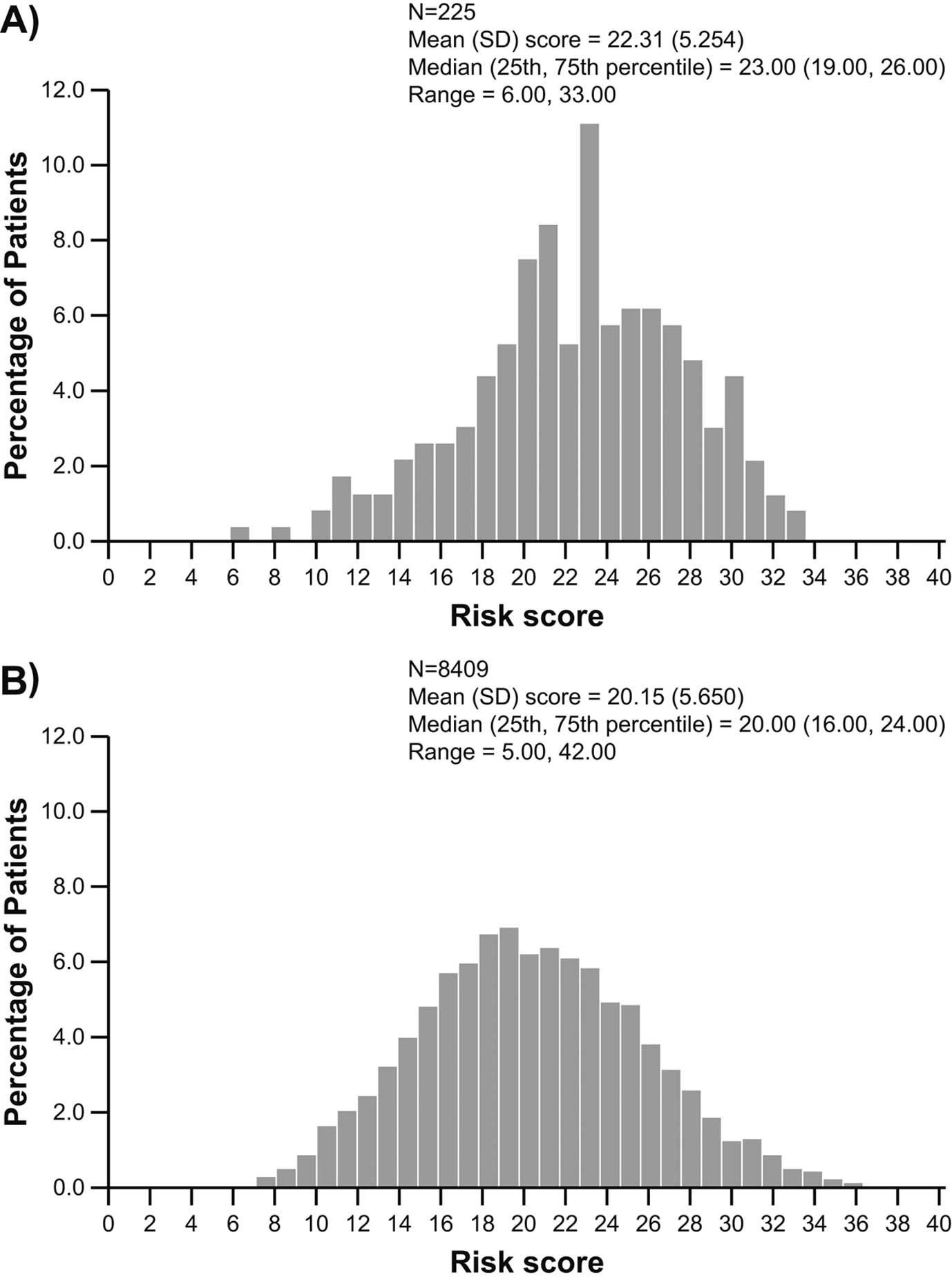
Distribution of MAGGIC risk scores in (A) PARALLEL-HF and (B) PARADIGM-HF. MAGGIC, Meta-Analysis Global Group in Chronic; PARADIGM-HF, Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure. Other abbreviations as in Figure 1.
The EMPHASIS-HF risk score was also calculated for all of the 211 NYHA Class II patients enrolled in PARALLEL-HF. In PARDIGM-HF, a total of 5,378 patients in NYHA Class I/II had complete screening visit data across all of the 10 variables required for calculation of the EMPHASIS-HF risk score. As shown in Figure 3, the median score in PARALLEL-HF was 5 points (IQR, 4–7 points; range, 2–10 points), which was similar to the median score in PARADIGM-HF (5 points, IQR, 3–6 points; range, 0–12 points). However, as with the MAGGIC risk score, the mean EMPHASIS-HF risk score in PARALLEL-HF was also slightly higher than that in PARADIGM-HF (5.44 vs. 4.62). The distribution of the MAGGIC and EMPHASIS-HF risk scores (Figures 2,3) showed a right-side shift towards the higher scores in PARALLEL-HF compared with PARADIGM-HF.
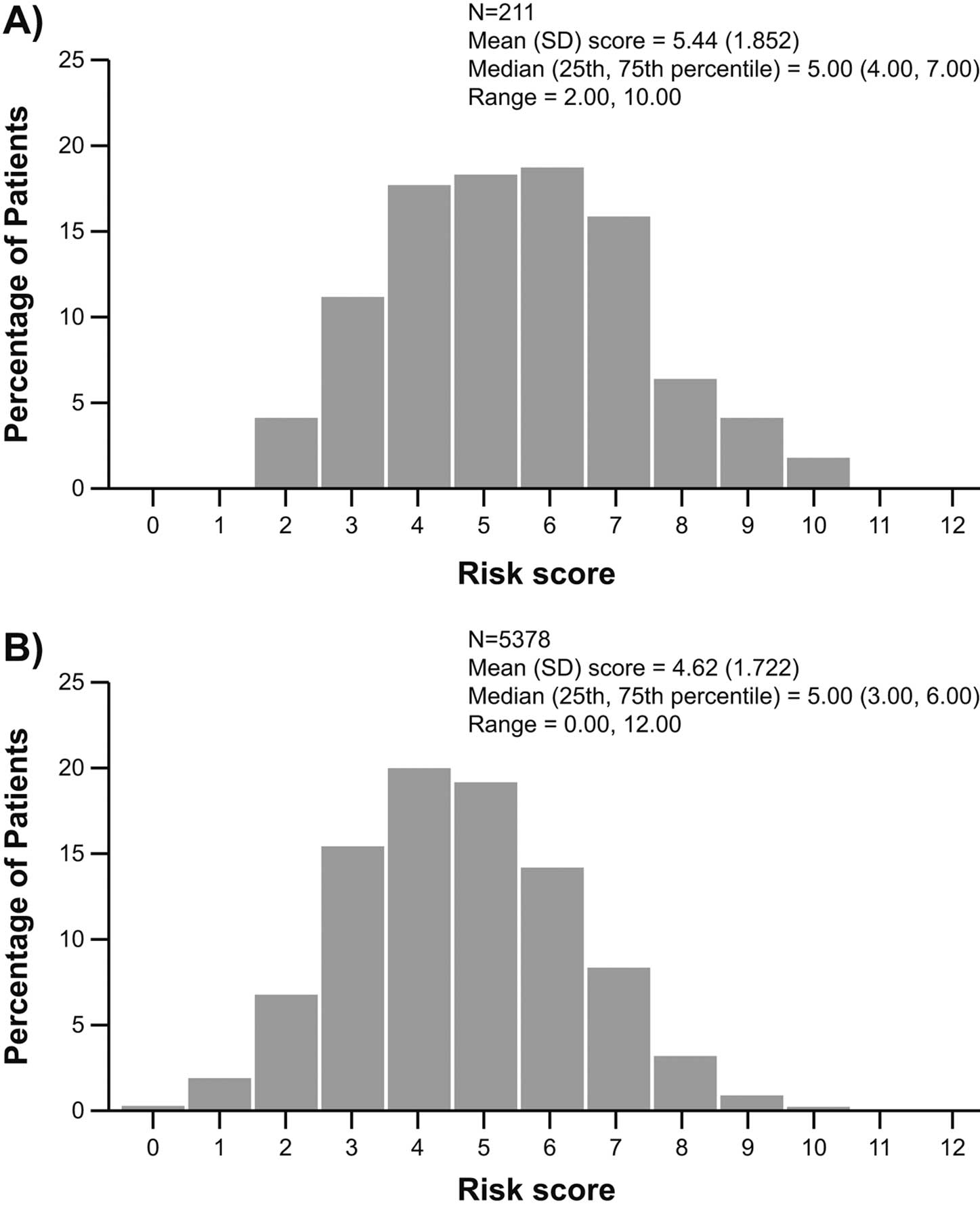
Distribution of EMPHASIS-HF risk scores in (A) PARALLEL-HF and (B) PARADIGM-HF. EMPHASIS-HF, Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure. Other abbreviations as in Figures 1,2.
Comparison of individual variables in MAGGIC risk scores showed differences in a few key patient characteristics, i.e., elderly patients, those with a lower BMI, those with diabetes, and those with a low SBP (<110 mmHg) were higher in PARALLEL-HF compared with PARADIGM-HF trial (Table S2). Similarly, individual variables in EMPHASIS-HF score showed that elderly patients and those with a low SBP (<130 mmHg), diabetes and lower BMI were more in PARALLEL-HF than in PARADIGM-HF (Table S3).
The aim of the PARALLEL-HF study is to demonstrate a similar trend of clinical efficacy of sacubitril/valsartan over enalapril as observed in the PARADIGM-HF study of Japanese patients with HFrEF. The inclusion/exclusion criteria of PARALLEL-HF were largely similar to those of PARADIGM-HF and aimed to enroll a representative population of Japanese patients with HFrEF.
The patients were initiated on sacubitril/valsartan 50 mg b.i.d. in the single-blind active treatment run-in period. During the run-in, patients continued their background HF medications except for ACEIs and ARBs. This enabled maximization of the number of patients who could tolerate at least the lowest doses of sacubitril/valsartan throughout the treatment period with long-term follow-up. The vast majority of patients (>90%) successfully completed the 2-week run-in period.
The 2-week run-in period decreased both SBP and DBP by 4.3 and 2.2 mmHg, respectively, and increased heart rates by 1.6 beats/min (all statistically significant). However, serum creatinine levels and eGFR did not change and, interestingly, serum potassium levels decreased by 0.11 mEq/L (P<0.001). AEs occurred in 32 patients, but only 2 patients discontinued the run-in because of AE, and angioedema was not observed in any case. These preliminary results demonstrated the hemodynamic effects and safety of sacubitril/valsartan in Japanese HFrEF patients also. Unfortunately, data regarding clinical outcomes are not available because the follow-up of the randomized patients is still in progress.
The baseline data presented here indicated that the patients enrolled in PARALLEL-HF were, for the most part, similar to those in the largest randomized global trial, PARADIGM-HF. In addition, patients enrolled in PARALLEL-HF were well treated with conventional evidence-based therapies for HFrEF.
There were few notable differences in the baseline characteristics of the patients enrolled in PARALLEL-HF and PARADIGM-HF, most of which were in line with regional differences reported earlier and further supported by comparison with recent randomized clinical trials in Japanese patients with HFrEF and with regional registry data.8,16 In PARALLEL-HF, most patients were in NYHA Class II, which was relatively higher than those reported in PARADIGM-HF, and the NT-proBNP levels were also relatively lower in PARALLEL-HF. However, it is also important to note that there was no difference in the mean LVEF of patients in the PARALLEL-HF (28.1%) and PARADIGM-HF (29.5%) populations. The most important and interesting finding was that the MAGGIC risk score was slightly higher in PARALLEL-HF compared with PARADIGM-HF, indicating a largely high-risk profile for death among the PARALLEL-HF patients, despite the majority of them being in a lower NYHA class and having low NT-proBNP levels. Similarly, the mean EMPHASIS-HF risk scores, which take into account hematologic and biochemical variables in NYHA Class I/II patients, were also numerically higher in PARALLEL-HF than in PARADIGM-HF trials.
A lower SBP and lower BMI in PARALLEL-HF may be specific to the Japanese/Asian patient population, as demonstrated in the subgroup analysis of PARADIGM-HF study in which patients enrolled in the Asia-Pacific region also had a relatively lower SBP and BMI at baseline compared with patients in Europe and the Americas.16 In addition, a comparison with other randomized clinical trial and registry data for Japanese patients with HFrEF, revealed a similar trend of lower BP while receiving the standard-of-care background HF therapies.12,13 Similarly, the lower rate of ischemic etiology for HF in PARALLEL-HF relative to that reported in PARADIGM-HF was in line with earlier observations from this geographic region.12,13,16 The proportion of patients with diabetes, which is one of the risk factors for poor outcomes in HFrEF patients, was higher in PARALLEL-HF compared with PARADIGM-HF; a similar trend was shown in the clinical trials and registries with Japanese HFrEF patients.
The use of background therapies such as diuretics, β-blockers and MRAs in PARALLEL-HF was very similar to PARADIGM-HF. Although the use of device-based therapy such as CRT/CRT-D or implantable cardioverter defibrillator (ICD) was low, it was generally in line with PARADIGM-HF and other contemporary global trials.9 The majority (93.3%) of patients in PARALLEL-HF were receiving an ACEI/ARB at a dose equivalent to ≤10 mg enalapril before entering the study. Although the target dose of sacubitril/valsartan was well tolerated in most patients receiving low doses of ACEIs/ARBs in the TITRATION study,17 the results from the PARALLEL-HF study will provide further insights into the tolerability of the target dose of sacubitril/valsartan in Japanese HFrEF patients, who are associated with lower SBP and receiving lower maintenance doses of ACEIs/ARBs at study entry.
Overall, patients in PARALLEL-HF were largely representative of contemporary ambulatory patients with chronic HFrEF in Japan who are well treated with evidence-based pharmacological therapies. In addition, enrolled patients were largely similar to those in PARADIGM-HF, including those of the Asia-Pacific subgroup of PARADIGM-HF previously reported in the subgroup analysis by Kristensen et al, which showed that sacubitril/valsartan was equally effective in reducing the risk of CV death and HF hospitalization compared with enalapril.16 The results of PARALLEL-HF are expected by 2019 and will verify whether sacubitril/valsartan provides similar improvements in clinical outcomes in Japanese patients with HFrEF as has been observed in PARADIGM-HF.
This study sponsored by Novartis Pharma AG.
The authors thank Hari Sai Priya Baddela and Syed Abdul Haseeb (Novartis Healthcare Pvt. Ltd., Hyderabad, India) for providing medical writing assistance and editorial support. All authors participated in the development and writing of the paper, reviewed and critically revised the manuscript for content and approved the final version of the manuscript for submission. Ivabradine Phase II trial data were obtained, in part, from ONO Pharmaceutical, Co., Ltd.
H.T. has received speakers’ bureau/honorarium from MSD, Ono Pharmaceutical, Takeda Pharmaceutical, Daiichi-Sankyo, Teijin Pharma, Nippon Boehringer Ingelheim, Bayer Yakuhin, Bristol-Myers Squibb, and research funds from Takeda Pharmaceutical, Bayer Yakuhin, Nippon, Boehringer Ingelheim, Mitsubishi Tanabe Pharma, Sanofi, and Daiichi-Sankyo, honorarium for writing promotional material for Medical Review, and consultation fees from Novartis Pharma K.K.
S.M. has received speakers’ bureau/honorarium from Mitsubishi Tanabe Pharma, Daiichi-Sankyo, Takeda Pharmaceutical, Ono Pharmaceutical, MSD, Pfizer, Bayer Yakuhin, Nippon Boehringer Ingelheim, Kyowa Hakko Kirin, Toa Eiyo, Actelion Pharmaceuticals Japan, Philips Respironics GK, Teijin Pharma, Medtronic Japan, St. Jude Medical, Boston Scientific Japan, and Torii Pharmaceutical, honorarium for writing promotional material for Medtronic Japan, Teijin Pharma, Kyowa Hakko Kirin, research funds from Mitsubishi Tanabe Pharma, Daiichi-Sankyo, Takeda Pharmaceutical, Ono Pharmaceutical, MSD, Pfizer, Kyowa Hakko Kirin, Toa Eiyo, Actelion Pharmaceuticals Japan, Teijin Pharma, Medtronic Japan, St. Jude Medical, Abbott Japan, Nippon Boehringer Ingelheim, and Bayer Yakuhin, and consultation fees from Novartis Pharma K.K.
Y.S. has received research funds from Mitsubishi Tanabe Pharma, Shionogi, Daiichi-Sankyo, Takeda Pharmaceutical, and Otsuka Pharmaceutical, Baxter, consultation fees from Novartis Pharma K.K, and is affiliated with endowed departments sponsored by MSD.
H.I. has received speakers’ bureau/honorarium from Takeda Pharmaceutical, Daiichi-Sankyo, MSD, Mochida Pharmaceutical, Mitsubishi Tanabe Pharma, Kowa Pharmaceutical, Toa Eiyo, Otsuka Pharmaceutical, Medtronic Japan, Astellas Pharma, Bayer Yakuhin, and Ono Pharmaceutical, research funds from Takeda Pharmaceutical, Daiichi-Sankyo, MSD, Mochida Pharmaceutical, Mitsubishi Tanabe Pharma, Kowa Pharmaceutical, Toa Eiyo, Otsuka Pharmaceutical, Medtronic Japan, Astellas Pharma, Bayer Yakuhin, Shionogi, Sumitomo Dainippon Pharma, and Ono Pharmaceutical, honorarium for writing promotional material for Daiichi-Sankyo, consultation fees from Novartis Pharma K.K, and is affiliated with an endowed department sponsored by Medtronic Japan.
K.Y. has received speakers’ bureau/honorarium from Otsuka Pharmaceutical, Ono Pharmaceutical, Mitsubishi Tanabe Pharma, Toa Eiyo, Takeda Pharmaceutical, Medtronic, Bristol-Myers Squibb, Pfizer, research funds from St. Jude Medical Japan, Otsuka Pharmaceutical, Daiichi-Sankyo, Ono Pharmaceutical, Biotronik Japan, Japan Lifeline, Astellas, Sanwa Kagaku Kenkyusho, Boehringer Ingerlheim, Abbott Vascular Japan, Bayer Yakuhin, Teijin Pharma, Mitsubishi Tanabe Pharma, Novartis Pharma K.K., Fukuda Denshi, Taisho Toyama Pharmaceutical, Fukuda Life Tec, and consultation fees from Novartis Pharma K.K.
T.O., N.O., T.K., W.G. were employees of Novartis at the time of the study.
Supplementary File 1
Table S1. Summary of adverse events in patients during run-in period
Table S2. Descriptive screening variables for the calculation of the MAGGIC risk score
Table S3. Descriptive screening variables for the calculation of the EMPHASIS-HF risk score
Please find supplementary file(s);
http://dx.doi.org/10.1253/circj.CJ-17-1424